Abstract
During the survey of freshwater snail hosts and their digenean larval trematode parasites, a rare cercaria larva belonging to family Transversotrematidae and subclass Digenea (Trematoda) was recovered from the snail species Melanoides striatella tuberculata inhabiting perennial Som river of Udaipur district, Rajasthan, India. More than 28 % mature specimens of these snails were found to be infected with transversotrematid cercaria larvae in the spring season. Body of this cercaria is large, bowl-shaped, biocellate, spinose, transparent and laterally extended having two pigmented eye spots, two hold fast organs extended from the junction of body and tail, large tail with two foliated furcal rami, and cyclocoel intestinal caeca. As far as the authors are aware, this is the first record of a transversotrematid larva from Rajasthan, India. Simultaneously, other forms of cercariae viz., amphistome, echinostome, monostome, gymnocephalous, furcocercous and xiphidiocercous cercariae were also recovered from fifteen species of pulmonate and operculate snails including Lymnaea acuminata f. patula, L. acuminata f. chlamys, L. acuminata f. typica, L. acuminata f. rufescens, L. luteola f. australis, L. luteola f. typica, L. luteola f. impura, Planorbis (Indoplanorbis) exustus, and Anisus (Gyraulus) convexiusculus, Faunus ater, Melania (Plotia) scabra, Thiara (Tarebia) lineata, Melanoides striatella tuberculata, Vivipara bengalensis race gigantica and V. bengalensis race mandiensis. The seasonal occurrence and host-specificity of diverse trematode cercaria larvae are also discussed besides the first record of a rare transversotrematid cercaria larva from Rajasthan, India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that freshwater molluscs serve as intermediate hosts that facilitate completion of the life cycle of majority of digenetic trematode parasites. Indeed these molluscs harbor various developmental stages as sporocysts, rediae and cercariae of adult trematodes (Erasmus 1972). In India, these intra-molluscan larval stages have been well studied by several workers (Ganpati and Rao 1969; Singh 1959; Mukherjee 1966; Murty 1973, Mohandas 1974; Jain 1976; Pandey and Agarwal 1978; Choubisa and Sharma 1983a, Choubisa 1991a, 2010; Janardanan and Shiny 1989; Rajendran and Janardanan 1993; Sanil and Janardanan 2016) and different kinds of cercariae (amphistome, echinostome, furcocercous, gymnocephalous, monostome, xiphidiocercous etc.) and metacercariae (aspidogaster, echinostome, opisthorchid, plagiorchiid, strigeid etc.) from different geographical provinces have been reported.
In India, Rajasthan is the largest state where number of freshwater perennial lentic (ponds, reservoirs, dams etc.) and lotic (rivers. streams, canals etc.) habitats are found. Although, freshwater and terrestrial molluscan fauna have been studied and reported from almost every region of Rajasthan (Ray and Mukherjee 1963; Rathore and Bohra 1987; Choubisa 1992a; Choubisa and Sheikh 2013a, b, c). But studies on larval trematode parasites of freshwater snails are still too scanty (Choubisa 2008a). However, few excellent studies on nutrition and digestion (Sharma and Choubisa 1985; Choubisa 1988a, 2008b), neuroanatomy (Choubisa and Sharma 1983b; Choubisa 1986, 1988b, 1989), histopathogenesis (Choubisa1990a, 1998; Choubisa et al. 2012), parasitic castration (Choubisa and Sheikh 2013d), behaviors and seasonal infection (Choubisa 1991b, 1997, 2002; Choubisa and Sharma 1986) of diverse trematode larvae as well as on their in vitro culture (Choubisa 1988c) have been conducted earlier. Besides the new records of larval digeneans, few new species of cercariae have also been reported from Rajasthan (Sharma and Choubisa 1983; Choubisa 1985, 1990b, 1992b; Choubisa and Sharma 1985). Recently, from evolutionary point of view, a new morph of sporocyst larva has also been reported from Rajasthan (Choubisa and Sheikh 2013e). Nevertheless, works on freshwater snail fauna and their larval trematode parasites are still meager in Rajasthan; despite of its number of perennial freshwater habitats. Therefore, a survey was undertaken to ascertain the pulmonate and operculate snail host species inhabiting diverse lentic and lotic freshwater ecosystems of Rajasthan and their different kinds of cercaria larvae. Simultaneously, a focus was also made on their seasonal occurrence and host—specificity.
During the survey, in spring season, a rare transversotrematid cercaria larva was recovered from the bottom dwelling operculate snail hosts, Melanoides striatella tuberculata inhabiting the perennial Som river of Udaipur district of southern Rajasthan, India which is discussed in detail as a first record from Rajasthan.
Materials and methods
A survey was performed (2011–2012) for diverse forms of cercaria larvae infecting various snail species of freshwater perennial lentic (ponds, reservoirs, dams etc.) and lotic (rivers, streams, canals etc.) habitats of Rajasthan, India. For this purpose, mature living snail specimens 50 to 100 in numbers of Lymnaea acuminata f. patula, L. acuminata f. chlamys, L. acuminata f. typica, L. acuminata f. rufescens, L. luteola f. australis, L. luteola f. typica, L. luteola f. impura, Planorbis (Indoplanorbis) exustus, Anisus (Gyraulus) convexiusculus, Faunus ater, Melania (Plotia) scabra, Thiara (Tarebia) lineata, Melanoides striatella tuberculata, Vivipara bengalensis race gigantica and V. bengalensis race mandiensis (Fig. 1) were collected seasonally from almost each water body by hand or by hand net. These snails were then brought to the departmental laboratory and maintained in separate aquaria containing tap water and a few aquatic plants. Snails were also collected from aquatic weeds spread in water bodies. A record was also prepared for collected snails and their habitats. These snail species were identified morphologically or by their shell structure as described earlier (Tonapi 1980; Subba 1989).
Snail species collected from diverse freshwater lentic and lotic habitats of southern Rajasthan. (1) Lymnaea acuminata f. patula (2) L. acuminata f. chlamys (3) L. acuminata f. typica (4) L. acuminata f. rufescens (5) L. luteola f. australis (6) L. luteola f. typica (7) L. luteola f. impura (8) Planorbis (Indoplanorbis) exustus (9) Faunus ater (10) Melania (Plotia) scabra (11) Thiara (Tarebia) lineata (12) Melanoides striatella tuberculata (13) Vivipara bengalensis race gigantica and (14) V. bengalensis race mandiensis
Snail species of lentic or lotic habitat, of bottom- dwelling and surface-dwelling habits were maintained separately in laboratory aquaria. For examination, the snails were kept in 500 ml glass beaker containing clean tap water and then exposed to natural or artificial light for cercarial emergence and they were also dissected out for the presence of larval trematodes if needed. Cercaria larvae were collected with fine dropper and examined according to the methods reported (Mukherjee 1980). The cercariae and other developmental stages were studied under light cover-slip pressure, both alive as well as in stained mounts. They were then identified following the standard key (Erasmus 1972).
Results and discussion
Transversotrematid cercaria larva
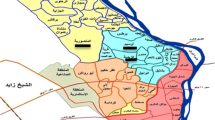
In survey of freshwater snail hosts and their digenean larval trematode parasites, a rare cercaria larva (Fig. 2a, b) belonging to family Transversotrematidae and subclass Digenea (Trematoda) was recovered from the snail host, M. striatella tuberculata (Fig. 1) belonging to family Melaniidae (Thiariidae) inhabiting perennial Som river of Udaipur district of Rajasthan, India (Fig. 3). More than 28 % of these snail species were found to be infected with transversotrematid cercarial larvae in the spring season. Interestingly, another snail species, Melania (Plotia) scabra (Fig. 1) was also recovered from the same habitat (Som river) which also belongs to the same family of snail M. striatella tuberculata but none of them was found to be infected with transversotrematid cercariae. It may be possible due to the difference in their niche. Although, emission of transversotrematid cercarial larvae from snail species, M. scabra and M. striatella tuberculata has been reported earlier from India (Nadakal et al. 1969), but this is the first report from Rajasthan.
A rare transversotrematid cercaria larva stained with Gower’s carmine stain (Fig. 2a; 150x) and its diagrammatic sketch showing different organ systems as seen in alive cercaria (Fig. 2b). AO, adhesive organ; CED, caudal excretory duct, EP, excretory pore; ES, eye spot; EV, excretory vesicle; F, furca; GD, genital ducts; GP, genital pore; IA, intestinal arch; M, mouth; MB, main body; P, pharynx; T, tail; TE, testis; VS, ventral sucker
The present transversotrematid cercariae were found strongly phototrophic, negatively geotactic and active swimmer. Although, they were diurnal but their maximum emergence was found in morning hours. Hence, these larvae are well adapted morphologically as they had two photosensitive pigmented eyes and tail bifurcated at the end region.
Moreover, transversotrematid cercariae exhibited large bowl-shaped, biocellate, spinose, transparent and laterally extended body, two hold fast organs/appendages extending from the junction of body and large tail with two foliated/spatulated furcal rami, and cyclocoel intestinal caeca. Other details of cercaria have been shown Fig. 2b. We neither took any body measurements nor counted the flame cells for flame cell formula. These tactics are generally used in identification of cercarial species besides the morphological features. However, in the present scenario, study of trematode larvae at genetic or DNA level is probably an authentic way or technique for the confirmation as well as identification of cercarial species due to the innumerous variety of cercariae. Molecular approaches for sensitive and specific detection of the parasite species in the snail host is not being widely used in India. Various PCR assays have been developed to detect DNA in fecal matter, definitive hosts and intermediate hosts outside India (Kozak and Wedrychowicz 2010; Caron et al. 2011). Such techniques will also check the chances of duplication of cercarial species or fake work on taxonomy of trematode parasites and provide a key for future studies.
In India, transversotrematid cercarial larva (Cercaria patialensis) was identified and reported for the first time from Punjab state (Soparkar 1924). Subsequently, Anantaraman (1948) from Nellore, south India, Nadakal et al. (1969) from Trivandrum, Kerala and Pandey (1971) from Lucknow, Uttar Pradesh have also reported it. From Rajasthan, this cercaria was detected for the first time. The presence of this trematode cercaria indicates that final hosts that are mostly fishes (Worawit and Kazuya 2012) in Som river are infected with adult transversotrematid parasites.
Seasonal occurrence and behavior of diverse cercariae
In the present survey study a total of seven types or forms of cercariae viz., amphistomes, echinostomes, furcocercous, gymnocephalous, monostomes, transversotrematid, and xiphidiocercous were recovered from fifteen species of snail hosts viz., L. acuminata f. patula, L. acuminata f. chlamys, L. acuminata f. typica, L. acuminata f. rufescens, L. luteola f. australis, L. luteola f. typica, L. luteola f. impura, P. (Indoplanorbis) exustus, A. (Gyraulus) convexiusculus, F. ater, M. (Plotia) scabra, T. (Tarebia) lineata, M. striatella tuberculata, V. bengalensis race gigantica and V. bengalensis race mandiensis (Table 1).
Furcocercous, monostomes and transversotrematid cercariae were found to be relatively more active swimmers and exhibited strongly negative geotactic behavior and aggregated towards light source (highly phototactic).These larvae were not found to encyst on wall of container. Although, amphistome, echinostome and gymnocephalous cercariae were also phototropic but these were passive swimmers and got encysted within 5–10 min after emergence. Those cercariae bearing eye–spots were found to be strongly phototropic as compared to their counter parts. Such behaviors have also been observed by many workers (Erasmus 1972; Pandey and Agrawal 1977, 1978; Choubisa and Sharma 1986). The seasonal emergence or occurrences of different forms of cercariae from their snail-hosts have been depicted in Table 2. All kinds of cercariae were perfectly adapted morphologically to behaviors which help in successful completion of life cycle of digenetic trematode parasites.
Moreover, usually a single snail is infected with one type of larval trematode parasites at a time; however, in some cases the snails shed two types of cercariae exhibiting double infection. Snail species, V. bengalensis, L. accuminata, M. striatella tuberculata and P. exustus, revealed double infection. Not a single case of triple infection ever came across. Such findings have also been observed and reported by many investigators (Mukherjee 1966; Jain 1970; Pandey and Agrawal 1978; Choubisa 1984).
Host-specificity of cercarial larvae
It is interesting to note that monostome and transversotrematid species are restricted to snail host, M. striatella tuberculata whereas amphistome, echinostome and gymnocephalous cercariae have been found to infect P. exustus and A. convexiusculus, L. acuminata and genera of Lymnaeidae family, respectively. Other cercariae, furcocercous and xiphidiocercous have been found to infect a number of snail species (Table 1). It appears that host-specificity and non-specificity is related to selection mechanism exhibited by miracidium, feeding behavior of the final host and part of the ecology of the intermediate hosts. Common genes of different molluscan host genera of a family are responsible for the infection with same larval forms whereas the difference in the host genes among the genera of the same family appear to be responsible for the difference in larval population. This view lends support from the work on gene based parasitic infection (Kennedy 1976) and from the definition given to parasitism by Mac Innis (1974) that parasitism is defined as the case in which one partner, the parasite of a pair of interacting species is dependent upon a minimum of one gene or its product from the other interacting species defined as the host for survival.
In conclusion, to the best of our knowledge, a rare transversotrematid cercarial recovered from M. striatella tuberculata is the first record from Rajasthan (India).The findings of present study are highly significant and add to existing knowledge of modern parasitology. Authors suggest that cercariae should be identified by their genetic study instead of using of flame cell formula.
References
Anantaraman M (1948) Observations on Cercaria patialensis Soparkar, 1924, and its relationships. Indian J Helm 1:11–22
Caron Y, Righi S, Lempereur L, Saegerman C, Losson B (2011) An optimized DNA extraction and multiplex PCR for the detection of Fasciola spp. in lymnaeid snails. Vet Parasitol 178(1–2):93–99
Choubisa SL (1984) The biology of certain larval trematodes infecting freshwater snails of lakes of Udaipur. Ph. D. thesis, Mohanlal Sukhadia University, Udaipur, Rajasthan, India
Choubisa SL (1985) A gymnocephalous cercaria, Cercaria Johrii n. sp. from fresh water snail, Melanoides tuberculatus (Muller) of Fateh Sagar Lake, Udaipur (Rajasthan). Indian J Parasit 9(2): 245–247
Choubisa SL (1986) Histochemical demonstration of esterase in certain fresh water larval trematodes with a note on neuroanatomy. Proc Indian Acad Sci (Anim Sci) 95(5):623–628
Choubisa SL (1988a) Histological and histochemical observations on the digestive gland of Melanoides tuberculatus (Gastropoda) infected with certain larval trematodes and focus on their mode of nutrition. Proc Indian Acad Sci (Animl Sci) 97(3):251–262
Choubisa SL (1988b) Neuroanatomy of furcocercous, Cercaria milleri. Curr Sci 57(7):402–404
Choubisa SL (1988c) In-vitro culture of echinostome cercaria Cercaria tewarii (Choubisa and Sharma 1985) from the metacercaria to vitellogenous stage. Indian J Parasit 12(1):123–128
Choubisa SL (1989) Distribution of non-specific esterase in certain larval digeneans with a note on morphology of nervous system. Indian J Exp Biol 27(1):32–57
Choubisa SL (1990a) Histopathological observations on the digestive gland of Lymnaea auricularia infected with the larval trematodes. Proc Indian Acad Sci (Anim Sci) 99(5):363–368
Choubisa SL (1990b) Cercaria gurayai, n. sp. (furcocercaria) from the fresh water snail Faunus atter (Linnaeus). Records Zool Surv India 87(4):267-271
Choubisa SL (1991a) Snail hosts of larval trematodes in Southern Rajasthan. Indian J Parasit 15(1):49–51
Choubisa SL (1991b) Comparative study on cercarial behaviours and their host specificity. Indian J Parasit 15(2):125–128
Choubisa SL (1992a) Mollusc as bio-indicators for the trophic stages of lakes and lotic environments. Bull Pur Appl Sci 11A(1–2):35–40
Choubisa SL (1992b) On a rare cercaria, Cercaria udaipuriensis II n. sp. from the fresh water snail, Melanoides tuberculatus (Muller). Bio-Sci Res Bull 8(1–2): 13–16
Choubisa SL (1997) Seasonal variation of amphistome cercarial infection in snails of Dungarpur district (Rajasthan). J Parasit Dis 21(2):197–198
Choubisa SL (1998) Focus on histopathogenesis of trematode larvae. J Parasit Dis 22(1):57–59
Choubisa SL (2002) Focus on seasonal occurrence of larval trematode (cercarial) parasites and their host specificity. J Parasit Dis 26(2):72–74
Choubisa SL (2008a) Focus on pathogenic trematode cercariae infecting fresh water snails (Mollusca: Gastropoda) of tribal region of southern Rajasthan (India). J Parasit Dis 32(1):47–55
Choubisa SL (2008b) Mode of nutrition in pathogenic trematode larvae (redia and cercaria) which infect hepatopancreas of fresh water snails (Mollusca: Gastropoda). J Parasit Dis 32(1):68–73
Choubisa SL (2010) Snails as bio-indicators for dreaded trematodiasis diseases. J Commun Dis 42(3):223–226
Choubisa SL, Sharma PN (1983a) Seasonal variations of cercarial infection in snails of Fateh Sagar Lake of Udaipur. Indian J Parasit 7(1):111–113
Choubisa SL, Sharma PN (1983b) Histochemical demonstration of cholinesterase in the nervous system of strigeoid metacercaria. Tetracotyle lymnaei. Indian J Parasit 7(2):217–219
Choubisa SL, Sharma PN (1985) Cercaria tewarii n. sp. (Echinostomatid cercaria) from fresh water snail, Indoplanorbis exustus (Deshayes). Bio-Sci Res Bull 1(1-2):50–53
Choubisa SL, Sharma PN (1986) Incidence of larval trematodes infection and their seasonal variation in the fresh water molluscs of southern Rajasthan. Records Zool Surv India 83(1&2):69–80
Choubisa SL, Sheikh Z (2013a) Freshwater snails (Mollusca: Gastropoda) as bio-indicators for diverse ecological aquatic habitats. Cibtech J Zool 2(3):22–26
Choubisa SL, Sheikh Z (2013b) A new variety of freshwater snail, Thiara scabra var. choubisai from Rajasthan, India. Cibtech. J Zool 3(3):44–46
Choubisa SL, Sheikh Z (2013c) Giant African land snail, Achatina fulica in Udaipur, Rajasthan: a threat to biodiversity and ecosystem. Asian J Biol Life Sci 2(3):279–281
Choubisa SL, Sheikh Z (2013d) Parasitic castration in freshwater snail Melanoides tuberculatus (Mollusca: Gastropoda). Proc Natl Acad Sci India Sect B: Biol Sci 83(2):193–197. doi:10.1007/s40011-012-0133-y
Choubisa SL, Sheikh Z (2013e) A rare trematode sporocyst from freshwater snail, Melanoides tuberculatus (Miller 1722). Cibtech J Zool 2(3):6–9
Choubisa SL, Sheikh Z, Jaroli VJ (2012) Histopathological effects of larval trematodes on the digestive gland of freshwater snail species, Vivipara bengalensis and Lymnaea acuminata. J Parasit Dis 36(2):283–286. doi:10.1007/s12639-012-0116-1
Erasmus DA (1972). The biology of trematodes, Printed in Great Britain at the Belfast: University Press, Belfast
Ganpati PN, Rao KH (1969) On anomalous emission of echinostome larval stages and their intra-redial encystment of cercariae in the snail, Pila globosa Swainson. Curr Sci 37:19–20
Jain SP (1970) Double infection of larval trematodes in molluscs. Agra Univ J Res (Sci) 21:47–48
Jain SP (1976) Studies on amphistomes II. A survey of incidence and nature of amphistome infection in aquatic snail. Agra Univ J Res (Sci) 25:81–89
Janardanan KP, Shiny AC (1989) Two new species of xiphidiocercariae from the apple snail, Pila globosa (Swainson) of Kerala. Riv Parasitol 50:47–52
Kennedy CR (1976) Ecological aspects of parasitology. North-Holland, Amsterdam, pp 246–268
Kozak M, Wedrychowicz H (2010) The performance of a PCR assay for field studies on the prevalence of Fasciola hepatica infection in Galba truncatula intermediate host snails. Vet Parasitol 168(1–2):25–30
Mac Innis AJ (1974) A general theory of parasitism. In: Proceedings of 3rd International Congress of Parasitology, 3, pp. 1511–1512
Mohandas A (1974) Studies on freshwater cercariae of Kerala. I. Incidence of infection and seasonal variation. Folia Parasit 21:311–317
Mukherjee RP (1966) Seasonal variation of cercarial infection in snails. J Zool Soc India 18:39–45
Mukherjee RP (1980) Collection and study of larval trematodes (Platyhelminthes). In: Proc Workshop Tech Parasit Zool Surv India 23–26
Murty AS (1973) Life cycle of Pseudodiplodiscoides pilai (Trematoda: Diplodiscidae) from the gut of the apple snail, Pila globosa (Swainson). J Parasitol 59(2):323–326
Nadakal AM, Mohandas A, Sunderaraman V (1969) Cercaria chackai sp. n. (Transversotrematidae) from Kerala India. J Parasitol 55:1187–1190
Pandey KC (1971) On a rare cercaria, Cercaria soparkari n.sp. (Transversotrematidae) from Lucknow, India. J Helm XLV 4:321–326
Pandey KC, Agrawal N (1977) Studies on cercarial fauna of Kathauta Tal. Lucknow. Indian J Zool 18(1):1–50
Pandey KC, Agrawal N (1978) Larval trematodes and seasonal variations in snails of Kathauta Tal. Lucknow. Indian J Parasit 2(2):139–143
Rajendran KV, Janardanan KP (1993) Studies on the life cycle of Tremiorchis ranarum Mehra and Negi, 1926. J Helminthol 67:95–101
Rathore NS, Bohra P (1987) Molluscan fauna of Lake Kailana (Jodhpur). India. Oikoassay 4(1):11–20
Ray HC, Mukherjee I (1963) Fauna of Rajasthan, India. Part 3, Mollusca. Rec Zool Surv India 61(1 & 4): 403–436
Sanil NK, Janardanan KP (2016) Two new species of xiphidiocercariae from apple snail Pila virens in Malabar. J Parasit Dis, Kerala. doi:10.1007/s12639-015-0741-6
Sharma PN, Choubisa SL (1983) Cercaria udaipuriensis n. sp. from fresh water snails, Vivipara bengalensis from Fateh Sagar Lake. Indian J Parasit 7(2): 209–212
Sharma PN, Choubisa SL (1985) Histochemical demonstration of hydrolytic enzymes in two species of cercariae and in redia. Indian J Parasit 9(2):153–154
Singh RN (1959) Seasonal infestation of Indoplanorbis exustus (Deshayes) with furcocercous cercariae. Proc Natl Acad Sci India 29:61–72
Soparkar MB (1924) A new cercaria from northern India, Cercaria patialensis nov.sp. Indian J Med Res 11:933–942
Subba RNV (1989) Freshwater molluscs of India. The zoological survey of India, Calcutta, p 289
Tonapi GT (1980) Freshwater animals of India. Oxford and IBH Publishing Company, New Delhi
Worawit M, Kazuya N (2012) First record of the fish parasite Transversotrema patialense (Trematoda: Digenea: Transversotrematidae) from Japan. Biogeography 14:121–125
Acknowledgments
The authors are thankful to the University Grants Commission, New Delhi, India for financial assistance (F. No. 39-658/2010, SR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choubisa, S.L., Jaroli, V.J. & Sheikh, Z. First record of a rare transversotrematid cercaria larva (Trematoda: Digenea) from Rajasthan, India: focus on seasonal occurrence and host-specificity of diverse cercariae. J Parasit Dis 41, 496–502 (2017). https://doi.org/10.1007/s12639-016-0837-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-016-0837-7