Abstract
Purpose
Chemotherapy-induced painful peripheral neuropathy (CIPPN) affects up to 90% of cancer patients treated with chemotherapy agents. Despite the fact that it is relatively common, the underlying pathophysiology is still unclear and its treatment remains generic. Mechanisms of CIPPN are multifactorial, dependent on the specific chemotherapeutic agent used, and include multiple patient-related factors, including genetic factors that may predispose patients to either develop or not develop CIPPN. The purpose of this article is to review mechanisms, clinical signs and symptoms, diagnosis, treatment options, and prognosis for patients who develop CIPPN. We also offer research considerations for this complex and unpredictable phenomenon.
Principal findings
Chemotherapeutic agents can damage the peripheral nervous system, including the nerve terminals, axons, cell body, and myelin sheath of sensory nerves. Herein, we describe some of the anatomical and functional changes that are thought to take place at various levels of the nervous system. On a clinical level, patients with CIPPN report multiple symptoms. It is essential to obtain an accurate history from the patient and to perform a thorough physical examination in order to obtain the patient’s subjective perspective. Additionally, objective measurements may be needed in order to articulate clearly the effects of this complex syndrome and to ensure an accurate diagnosis, treatment, and prognosis.
Conclusions
The management of CIPPN remains a clinical challenge for pain practitioners. As more research is being carried out to elucidate its pathophysiology and therapy, the innovative use of several non-traditional categories of drugs seems promising in the management of this complex phenomenon. Studies addressing predictability and possible genetic predisposition are necessary not only for preventive measures but also for targeted treatments.
Résumé
Objectif
La neuropathie périphérique douloureuse induite par la chimiothérapie (CIPPN) affecte jusqu’à 90 % des patients cancéreux traités par chimiothérapie. Bien qu’elle soit relativement fréquente, la physiopathologie sous-jacente à cette neuropathie est encore mal connue et son traitement reste générique. Les mécanismes de la CIPPN sont multifactoriels, dépendants des agents spécifiques utilisés pour la chimiothérapie, et incluent de nombreux facteurs liés au patient, notamment des facteurs génétiques pouvant prédisposer ou non au développement de la CIPPN. L’objectif de cet article est de revoir les mécanismes, les signes et symptômes cliniques, le diagnostic, les options thérapeutiques et le pronostic des patients qui développent une CIPPN. Nous proposerons également des axes de recherche sur ce phénomène complexe et imprévisible.
Constatations principales
Les agents chimiothérapeutiques peuvent endommager le système nerveux périphérique, notamment les terminaisons nerveuses, les axones, le corps cellulaire et la gaine de myéline des nerfs sensitifs. Ici, nous décrivons certaines modifications anatomiques et fonctionnelles qui semblent survenir à différents niveaux du système nerveux. Sur le plan clinique, les patients atteints de CIPPN signalent de nombreux symptômes. Il est essentiel d’obtenir un historique précis du patient et d’effectuer un examen physique approfondi afin d’avoir le point de vue subjectif du patient. De plus, des mesures objectives peuvent être nécessaires afin de comprendre clairement les effets de ce syndrome complexe et pour en assurer un diagnostic, traitement et pronostic précis.
Conclusions
La gestion de la CIPPN reste un défi clinique pour les spécialistes de la douleur. Au fur et à mesure que de nouvelles études sont menées pour élucider sa physiopathologie et son traitement, l’utilisation novatrice de plusieurs familles de médicaments non traditionnels semble prometteuse dans la gestion de ce phénomène complexe. Des études portant sur la prévisibilité et la prédisposition génétique sont nécessaires, non seulement pour prendre des mesures préventives, mais aussi pour des traitements ciblés.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
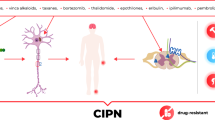
Chemotherapy-induced painful peripheral neuropathy (CIPPN) in patients treated for cancer is difficult to diagnose, treat, and determine a prognosis due to multifactorial mechanisms, the specific chemotherapeutic agent used, and multiple patient-related factors. Estimates indicate CIPPN affects up to 90% of cancer patients treated with chemotherapy agents and from 20-50% of women treated for breast cancer.1 The onset can be immediate or occur well after the end of treatment; however, not all cancer patients treated with chemotherapy develop CIPPN. Furthermore, not all chemotherapy-induced peripheral neuropathy is painful. The duration of CIPPN varies from weeks to months or years and is dependent on the type of chemotherapy used.2-4 Symptoms associated with CIPPN vary in presentation, consistency, and duration, and with the agents used for treatment, further contributing to the complexity of this insidious and elusive pain syndrome.5 The pathophysiology of CIPPN remains elusive at the macro and micro physical nerve structural levels and appears to have a genetic component that needs further exploration.6-8 The purpose of this review is to identify current mechanisms, clinical signs and symptoms, diagnosis, treatment options, and prognosis for CIPPN induced by the taxanes, specifically paclitaxel. Furthermore, we explore future research opportunities at the genetic level in an effort to predict who will and will not develop CIPPN due to paclitaxel and to determine best practice treatment options.
Clinical evidence suggests that treatment for cancer with neurotoxic agents may result in variable degrees of neuropathy.6 Specific classes of chemotherapy agents have been implicated in the development of CIPPN, including platinum-based derivatives, vinca alkaloids, taxanes, and proteasome inhibitors (Table 1).
Mechanisms of CIPPN
Chemotherapeutic agents can damage the peripheral nervous system, including the nerve terminals, axons, cell body, and myelin sheath of sensory nerves. Herein, we describe some of the anatomical and functional changes that take place at various levels of the nervous system.
Skin nerve endings
Studies have shown that taxanes, vinca alkaloids and platinum-based agents all decrease the number of intraepidermal nerve fibres in the rat hind paw.9,10 Interestingly, in one study, pretreatment with minocycline prevented the decrease in nerve fibres.10 The loss of nerve terminals may produce hyperexcitability in the nerve fibres and induce spontaneous discharges.11
Primary sensory neurons and dorsal root ganglia (DRG)
Clinically, CIPPN patients have paresthesias and decreased vibration sensitivity.14 Animal studies have shown that vincristine induces abnormal function of A-beta, A-delta, and C-fibre sensory nerves in the vincristine-induced chronic pain patients12 and in the motor and autonomic nerves.13 Cisplatin, paclitaxel, and bortezomib significantly reduce the velocity of caudal nerve conduction and produce axonal degeneration in the sciatic nerve.14
Platinum drugs bind to DNA strands and induce apoptotic cell death. Particularly, they bind to the cell bodies in the DRG in the peripheral nerves and thus result in peripheral neuropathy. Further, oxaliplatin can damage DNA, leading to chronic neuropathy, and it can also alter the function of voltage-gated sodium channels in the peripheral nerves.15,16 Interestingly, carboplatin produces significant neuropathy less frequently.17
While cisplatin accumulates in the DRG and peripheral nerves,18,19 paclitaxel stabilizes microtubules, decreases epidermal nerve fibres, and activates macrophages and microglia in the DRG, peripheral nerves, and spinal cord.20,21 Additionally, with large cumulative doses, paclitaxel can affect the motor nerves.
Paclitaxel, vincristine, cisplatin, and bortezomib form swollen and vacuolated mitochondria in axons within the mitochondria, which increases their permeability and leak, results in release of intracellular calcium, and consequently activates a caspase-mediated apoptotic pathway.22-24
Paclitaxel and vincristine also increase alpha-2-delta-1 subunits of calcium channels in the DRG and dorsal horn. Additionally, they increase cytosolic calcium from extracellular and intracellular stores of mitochondria.25-27
Paclitaxel, oxaliplatin, and vincristine increase Na+ current in the DRG and make neurons susceptible to paresthesias and fasciculations.28-30 Oxaliplatin decreases the expression of mechano-gated and temperature-sensitive potassium channels (TREK1, TRAAK types) and increases the expression of pro-excitatory channels such as the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels.31 Cisplatin, oxaliplatin, and paclitaxel upregulate transient receptor potential vanilloid 1 (TRPV1), transient receptor potential A1 (TRPA1), transient receptor potential M8 (TRPM8), and transient receptor potential vanilloid 4 (TRPV4) in the DRG neurons, which leads to hyperexcitability of nociceptors.32-34 Oxaliplatin, paclitaxel, vincristine, and bortezomib also increase free radicals in DRG cells secondary to an increase in cytosolic calcium.35-37 Further, paclitaxel, vincristine, and oxaliplatin activate calcium-dependent proteases, calpains and caspases in DRG cells and thus initiate neuronal apoptosis.38,39 Paclitaxel and cisplatin increase neuropeptide Y, substance P, and calcitonin gene-related peptide (CGRP) in DRG neurons.40,41 Vincristine increases 5-hydroxytryptamine 2A receptors of 5-hydroxytryptamine on the DRG neurons and dorsal horn of the spinal cord and sensitizes both peripheral nociceptive fibres and spinal dorsal horn neurons.42,43
In summary, anti-cancer agents activate ion channels, including sodium, calcium, potassium of DRG, and glutamate-activated N-methyl-D-aspartate (NMDA) receptors to alter cytosolic ionic milieu, particularly intracellular calcium that triggers secondary changes such as release of free radicals to induce neuropathic pain. Damage to mitochondria leads to an increase in permeability and release of intracellular calcium, activation of protein kinase C, phosphorylation of TRPV, activation of capases/calpains, and generation of nitric oxide and free radicals, resulting in cytotoxicity to axons and neuronal cell bodies.
Spinal cord and dorsal horn and the brain
Paclitaxel increases spontaneous activity and after-discharges of deep spinal lamina neurons to noxious mechanical thermal stimuli of the skin. In addition, paclitaxel increases after-discharge and abnormal windup to transcutaneous electrical stimuli and decreases the expression of glutamate transporter proteins in the dorsal horn.44
Vincristine may cause increased sciatic nerve excitability and induce a state of glutamate excitotoxicity through enhancing NMDA receptor expression and diminishing CGRP expression, thus resulting in axonal degeneration.45 Finally, oxaliplatin increases protein kinase C activity and upregulates gamma/epsilon isoforms of protein kinase C in the thalamus and periaqueductal areas of the brain.46,47
Immune cells
Chemotherapeutic agents can penetrate the blood-nerve barrier and bind to the peripheral axons and the DRG. In addition, they affect macrophage, epidermal Langerhans cells, and glial cells. Paclitaxel and vincristine increase the number of protein gene product 9.5-positive Langerhans cells in the skin.9 Further, taxanes induce upregulation of matrix metalloproteinases (MMPs) in Schwann cells, DRGs, and peripheral nerves. Especially, MMP-1 in the Schwann cells, neutrophils, and neurons degrades myelin and activates macrophage in the DRGs and peripheral nerves. In addition, MMPs induce inflammatory cytokines (e.g., interleukin [IL]-1, IL-6, IL-8), tumour necrosis factor-α, and nitric oxide from satellite cells, myelin, macrophages, and glial cells. In summary, they directly or indirectly act on primary neurons to induce hypersensitivity of peripheral nerves, which is a hallmark of neuropathic pain syndrome.48-50
Clinical signs and symptoms
Chemotherapy-induced painful peripheral neuropathy is caused by several classes of neurotoxic chemotherapy agents, including vinca alkaloids, taxanes, platinum-based compounds, and proteasome inhibitors.2-4 Neuropathic pain is considered part of the dysfunctional pain syndromes and neither protects nor supports healing and repair.8 The clinical signs and symptoms, severity, and duration vary depending on the type of neurotoxic chemotherapeutic agent administered and the frequency and dosages. Neuropathic pain may appear after the first treatment or weeks/months later and is more common in patients with preexisting nerve damage from CIPPN or other diseases (e.g., diabetes).5,51-54 Use of neurotoxic chemotherapeutic agents results in similar and different clinical signs and symptoms, including pain and numbness, motor impairment, and decreased pinprick and vibration perceptions.6 Paclitaxel induces sensory impairment and pain, whereas vincristine is more often associated with peripheral sensorimotor effects, including motor dysfunction such as foot drop. Reconciliation of the types of neurotoxic chemotherapeutic agents received by the patient is an important factor in determining what signs and symptoms to expect. Effects of neurotoxic chemotherapeutic agents generally involve the distal extremities symmetrically, characterized by a “stocking and glove” phenomenon with paresthesia or dysesthesia, numbness, and tingling originating in the hands and feet.2,5 Numbness and tingling in the hands and feet may be an early sign of impending development of CIPPN.
Symptoms are also dependent on the sensory fibres affected. For example, damage of small nerve fibres (SNF) is more likely to present as neuropathic pain and allodynia.52,55 Both sides can be affected and symptoms may be more predominant on one side of the body. Alteration in sensory, motor, and autonomic functioning appears to be the primary pattern for CIPPN.1,5,7,51 Sensory symptoms are dose-related and cumulative; they can be delayed in appearing and include both positive and negative signs. Abnormal sensation such as paresthesia and dysesthesia are common clinical symptoms. Other symptoms include loss of proprioception, ataxia, loss of balance, and decreased sensation of vibration. Motor symptoms range from minor motor dysfunction to paralysis and tend to begin primarily in the distal extremities. Examples include hypotonia, as a result of involvement of the lower peripheral motor neurons and muscles, and hyporeflexia, often caused by damage to unmyelinated nerve fibres and resulting in hypotension, cardiac conduction irregularities, impotence, bowel and bladder dysfunction, constipation, paralytic ileus, and urinary retention.1,2,5,51,53,56,57
There are multiple instruments used to evaluate chemotherapy-induced symptoms, side effects, and adverse events. The most common scales used are those from the World Health Organization (WHO), Eastern Cooperative Oncology Group (ECOG), National Cancer Institute of Canada - Common Toxicity Criteria, and the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.53,54 The WHO scale grades peripheral neuropathy from 0 (none) to 4 (paralysis), follows progression of sensory function and motor function, but does not address deep tendon reflexes. Vague and obscure terms are used to grade neurotoxicity, e.g., “mild weakness” and “marked motor loss”, which lack specific criteria for grading. The ECOG includes more objective assessment of motor (deep tendon reflexes) and sensory (paresthesia) function and includes an autonomic component (constipation). Unfortunately, limits of the ECOG scale include its single-item approach when denoting the presence or absence of neuropathy and suffers from the same deficit as the WHO by using vague terms, e.g., “disabling sensory loss”.1,51,53
Recent efforts suggest evidence that subgroups of patients can be clustered by the severity of their symptoms, especially those treated with taxanes, vinca alkaloids, and platinum classes of neurotoxic chemotherapeutic agents.2,5,58,59 In a study of 40 breast cancer patients, five different symptom scales was used to identify symptom clusters, showing that a comprehensive assessment of symptoms may lead to more accurate identification of those patients receiving neurotoxic chemotherapeutic agents who may develop CIPPN.5
In summary, patients with CIPPN report multiple symptoms. Eliciting an accurate picture from the patient is most important in order to obtain their subjective perspective. Nevertheless, objective measurements are also needed in order to articulate clearly the effects of this complex phenomenon and to ensure an accurate diagnosis, resulting treatment, and prognosis. Thus, the importance of a comprehensive history and physical examination cannot be overemphasized and must include input from patients and their significant others. Assessment of the various symptoms that each patient experiences is vital in determining the type, level of severity, and diagnosis of CIPPN.
Diagnosis
The diagnosis of CIPPN is generally made based on case history and clinical presentation. Occasionally, electrophysiological studies can be beneficial to confirm the diagnosis. The symptom history/assessment includes pain, loss of deep tendon reflexes, or the presence of symmetrical “stocking-glove” numbness or paresthesia after neurotoxic chemotherapy.4,6,52,53,57 History and physical examination are essential for an accurate diagnosis of CIPPN. A specific knowledge of the pattern of neuropathy associated with specific neurotoxic chemotherapy agents is also essential.53,54 Loss of dexterity in the hands is often perceived as being clumsy; in addition, smell and taste changes and hearing loss have been associated with certain neurotoxic chemotherapy agents.
The National Comprehensive Cancer Network recommends using a comprehensive pain assessment algorithm to assess neuropathic pain.60 It is vital to quantify the intensity and characteristics of the pain experienced by the patient through the patient interview. Also, it is helpful for patients to keep a pain diary in which they record the intensity, location, and quality of pain as it occurs. Unidimensional pain scales, such as a numerical pain rating scale and the Wong-Baker FACES® Pain Rating Scale are used with adult and pediatric patients, respectively.61-63 The Brief Pain Inventory (BPI) and McGill Pain Questionnaire are examples of multidimensional instruments to measure pain. The McGill Pain Questionnaire evaluates the “sensory, affective and evaluative” nature of pain in addition to pain intensity.60,64 The BPI records pain location, intensity, and interference with daily activities. It can be completed quickly and is easily used in the outpatient setting.65,66
Use of quantitative sensory testing (QST) is an objective means to assess and quantitate impairments in motor skills, touch, warmth, and heat. In a recent study, 14 patients and 18 healthy controls developed neuropathy after paclitaxel and vincristine and received QST 18 months apart. Test results showed impairments in motor skills, touch deficits, and impaired warmth/heat deficits.6
Common neurophysiological tests used to confirm the diagnosis of CIPPN include nerve conduction velocity measurements of sensory nerve action potential, motor nerve conduction velocity, compound muscle action potential, and needle electromyography. Nerve biopsies are rarely indicated for CIPPN.54
The role of skin biopsy is evolving, and guidelines have been established for quantification of linear density of intraepidermal nerve fibres as a reliable method to assess the diagnosis of small fibre neuropathies—if agreed upon counting rules are used.54,67 Results of a recent study to investigate changes in the density of Meissner’s corpuscles and epidermal nerve fibres (ENFs) thought to contribute to multiple forms of neuropathy among 14 CIPPN patients and 18 healthy controls have identified deficiencies in both Meissner’s corpuscles and ENFs.6
The National Cancer Institute’s Common Terminology Criteria for Adverse Events scoring of grade 1 or higher sensory neuropathy and at least 4 on a numerical pain rating scale of 0-10 serve as an average representation of chemotherapy-induced pain and are commonly used as baseline criteria for identifying patients with CIPPN.3,6,52
In summary, the diagnosis of CIPPN is complex and requires a thorough history and physical examination. While electrophysiological studies could be somewhat beneficial, laboratory studies (blood and serologic testing) and magnetic resonance imaging are seldom used.2,3,52,53,56,57,68 Other factors to consider in assisting with the diagnosis of CIPPN include analysis of motor, sensory, and autonomic functions, and attention to gait and quality of life is also recommended.
Management of CIPPN
Pharmacological treatment
The management of neuropathic pain is a challenge for clinicians. Commonly used drugs, including anticonvulsants, antidepressants, and opioids, have only modest analgesic effects at best. Those drugs are administered as a mono or combination therapy. Table 2 summarizes current published guidelines for the treatment of neuropathic pain, including CIPPN.
Anticonvulsants
Any of the old generation or new generation anticonvulsants can be used for treating CIPPN; however, gabapentin and pregabalin are most frequently used. The starting dose of gabapentin is 100-300 mg at bedtime, which is gradually increased every three to seven days to twice daily and then to three times daily. The maximal dose is 3,600 mg daily in three to four divided doses. The starting dose of pregabalin is 25-75 mg at bedtime, which is gradually increased every three to five days to twice daily and then to three times daily. The maximal dose is 600 mg daily in three divided doses. Their common side effects are dizziness and sedation.69-71 The chemical structure of gabapentin is similar to gamma-aminobutyric acid, which is an inhibitory neurotransmitter in the central nervous system. Nevertheless, the main mechanism of action is binding to voltage-gated calcium channels, blocking the alpha-2-delta subunit of calcium channels, and thus producing hyperpolarization.70
Antidepressants
Tricyclic antidepressants (TCAs), such as amitriptyline (tertiary amine) and nortriptyline (secondary amine) are widely used as adjuvant analgesics to treat CIPPN. Less commonly, the selective serotonin norepinephrine reuptake inhibitors (SNRIs) are used. The starting dose of amitriptyline and nortriptyline is 10-25 mg daily at bedtime, and the maximal dose is 100-150 mg·day−1. The dose is gradually increased by 10-25 mg about every seven days.69 The side effects of TCAs are sedation, dry mouth, blurred vision, constipation, urinary retention, orthostatic hypotension, and hypertension. Tricyclic antidepressants non-selectively block the reuptake of serotonin and norepinephrine in the central nervous system (CNS).
Selective serotonin norepinephrine reuptake inhibitors
The selective SNRI (duloxetine, venlafaxine) inhibits the reuptake of serotonin and norepinephrine in the CNS and has been reported to have an analgesic effect in neuropathic pain. Duloxetine has shown efficacy in clinical trials of chemotherapy-induced peripheral neuropathy. The recommended dose is 30-60 mg once a day. The most common side effect is nausea; others include somnolence, dizziness, constipation, and sexual dysfunction.72 Citalopram, a selective serotonin reuptake inhibitor, is started at a dose of 20 mg·day−1 and is increased to a dose of 40 mg·day−1,73 however, its efficacy is controversial.
Topical agents (capsaicin, lidocaine)
Lidocaine inhibits the transport of ions across nerve membranes and prevents initiation and conduction of impulses. Applying the 5% lidocaine patch to painful areas has analgesic effects in the mixed peripheral neuropathy. The most common adverse effect is a mild local reaction.69
Capsaicin may release substance P from primary afferent nociceptors via the TRPV1 receptor and then deplete substance P. It blocks the action potential from peripheral sites to the CNS. The most common side effect is erythema.74
Opioids
In general, the use of opioids alone or in combination with other drugs for managing any type of neuropathic pain (including CIPPN) has been controversial. Nevertheless, based on our clinical experience, some patients do benefit from using these agents despite their side effects. That said, the use of opioids should be closely monitored to minimize their overuse and/or abuse.
Others
Commonly used medications, such as nonsteroidal anti-inflammatory drugs (e.g., acetylsalicylic acid and indomethacin) do not have analgesic effects in neuropathic pain patients.75
Non-pharmacological treatment: acupuncture, physical therapy/occupational therapy, massage
The non-pharmacological treatments, such as acupuncture, physical therapy/occupational therapy, and massage therapy may reduce cancer-related neuropathic pain (including CIPPN) and are relatively safe with very few side effects.76 Further, learning coping mechanisms and cognitive behavioural therapy could be very helpful in selected groups of patients.
Prevention
Early detection, monitoring, preemptive pain management, and possibly adequate post chemotherapy pain management are thought to prevent CIPPN.1,5,77,78 All the same, it is not clear if early physical therapy can prevent CIPPN. Several neuroprotective drugs have been considered for the prevention of CIPPN and have shown promise in preliminary reports, but they are not for general use due to insufficient evidence supporting their efficacy. The list of these drugs includes amifostine, glutathione, glutamine, erythropoietin, and acetyl-L-carnitine, acetylcysteine, adrenocorticotropic hormone (ACTH), BNP7787, calcium and magnesium, diethyldithiocarbamate, Org 2766, oxcarbazepine, and vitamin E.53,54,79-86
Prognosis
The prognosis for patients experiencing CIPPN is multifactorial; it is dependent on the specific chemotherapeutic agent used and includes multiple patient-related factors. Severity increases with duration of treatment; it is dose-related and progression may cease when treatments are completed. Exceptions include the platinum-based compounds whereby the sensory loss may progress for several months after cessation of treatment, commonly described as “coasting”.6,52,53,80 Preexisting neuropathy from other disease processes, such as diabetes, must also be considered in the prognosis of CIPPN patients. On the other hand, there are patients with preexisting neuropathies who do not develop CIPPN. Therefore, more research is needed to elicit optimal prognoses for those who develop CIPPN.
Conclusions
Chemotherapy-induced painful peripheral neuropathy continues to be a challenge to clinicians and the patients whom we serve. A thorough medical history and physical examination are critical in making the correct diagnosis and in guiding appropriate therapy. In addition, a better understanding of the pathophysiology of CIPPN can be fostered through further clinical trials evaluating neuropathic medications and the underutilized neuroprotective agents that will bring us a step further in the management CIPPN. Studies focusing on the genetic predisposition to CIPPN are necessary, not only to establish preventive measures but also to develop targeted treatments.
References
Bokhari F, Sawatzky JA. Chronic neuropathic pain in women after breast cancer treatment. Pain Manag Nurs 2009; 10: 197-205.
Polomano RC, Farrar JT. Pain and neuropathy in cancer survivors. Cancer Nurs 2006; 29(2 Suppl): 39-47.
Reyes-Gibby C, Morrow PK, Bennett MI, Jensen MP, Shete S. Neuropathic pain in breast cancer survivors: using the ID pain as a screening tool. J Pain Symptom Manage 2010; 39: 882-9.
Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309: 1359-67.
Golan-Vered Y, Pud D. Chemotherapy-induced neuropathic pain and its relation to cluster symptoms in breast cancer patients treated with paclitaxel. Pain Pract 2013; 13: 46-52.
Boyette-Davis JA, Cata JP, Driver LC, et al. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol 2013; 71: 619-26.
Cooney MA, Culleton-Quinn E, Stokes E. Current knowledge of pain after breast cancer treatment: a systematic review. Pain Manag Nurs 2013; 14: 110-23.
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Ann Rev Neurosci 2009; 32: 1-32.
Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 2006; 201: 507-14.
Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp Neurol 2011; 229: 353-7.
Seltzer Z, Devor M. Ephaptic transmission in chronically damaged peripheral nerves. Neurology 1979; 29: 1061-4.
Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage 2007; 33: 166-79.
Pal PK. Clinical and electrophysiological studies in vincristine induced neuropathy. Electromyogr Clin Neurophysiol 1999; 39: 323-30.
Carozzi VA, Canta A, Oggioni N, et al. Neurophysiological and neuropathological characterization of new murine models of chemotherapy-induced chronic peripheral neuropathies. Exp Neurol 2010; 226: 301-9.
Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol 2000; 406: 25-32.
Grolleau F, Gamelin L, Boisdron-Celle M, Lapied B, Pelhate M, Gamelin E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. J Neurophysiol 2001; 85: 2293-7.
Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 2008; 15: 3081-94.
Meijer C, de Vries EG, Marmiroli P, Tredici G, Frattola L, Cavaletti G. Cisplatin-induced DNA-platination in experimental dorsal root ganglia neuronopathy. Neurotoxicology 1999; 20: 883-7.
Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 1992; 10: 795-803.
Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 2008; 66: 218-28.
Peters CM, Jimenez-Andrade JM, Jonas BM, et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 2007; 203: 42-54.
Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006; 122: 245-57.
Melli G, Taiana M, Camozzi F, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol 2008; 214: 276-84.
Broyl A, Corthals SL, Jongen JL, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol 2010; 11: 1057-65.
Sun X, Windebank AJ. Calcium in suramin-induced rat sensory neuron toxicity in vitro. Brain Res 1996; 742: 149-56.
Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg 2006; 102: 1485-90.
Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience 2007; 144: 714-20.
Matsumoto G, Ichikawa M, Tasaki A, Murofushi H, Sakai H. Axonal microtubules necessary for generation of sodium current in squid giant axons: I. Pharmacological study on sodium current and restoration of sodium current by microtubule proteins and 260 K protein. J Membr Biol 1984; 77: 77-91.
Nieto FR, Entrena JM, Cendan CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008; 137: 520-31.
Ghelardini C, Desaphy JF, Muraglia M, et al. Effects of a new potent analog of tocainide on hNav1.7 sodium channels and in vivo neuropathic pain models. Neuroscience 2010; 169: 863-73.
Descoeur J, Pereira V, Pizzoccaro A, et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 2011; 3: 266-78.
Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 2008; 28: 1046-57.
Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain 2010; 6: 15.
Anand U, Otto WR, Anand P. Sensitization of capsaicin and icilin responses in oxaliplatin treated adult rat DRG neurons. Mol Pain 2010; 6: 82.
Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain 2008; 9: 463-72.
Kim HK, Zhang YP, Gwak YS, Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology 2010; 112: 432-9.
Muthuraman A, Jaggi AS, Singh N, Singh D. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol 2008; 587: 104-11.
Joseph EK, Levine JD. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur J Neurosci 2004; 20: 2896-902.
Boehmerle W, Zhang K, Sivula M, et al. Chronic exposure to paclitaxel diminishes phosphoinositide signaling by calpain-mediated neuronal calcium sensor-1 degradation. Proc Natl Acad Sci U S A 2007; 104: 11103-8.
Horvath P, Szilvassy J, Nemeth J, Peitl B, Szilasi M, Szilvassy Z. Decreased sensory neuropeptide release in isolated bronchi of rats with cisplatin-induced neuropathy. Eur J Pharmacol 2005; 507: 247-52.
Jamieson SM, Liu JJ, Connor B, Dragunow M, McKeage MJ. Nucleolar enlargement, nuclear eccentricity and altered cell body immunostaining characteristics of large-sized sensory neurons following treatment of rats with paclitaxel. Neurotoxicology 2007; 28: 1092-8.
Thibault K, Van Steenwinckel J, Brisorgueil MJ, et al. Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. Pain 2008; 140: 305-22.
Hansen N, Uceyler N, Palm F, et al. Serotonin transporter deficiency protects mice from mechanical allodynia and heat hyperalgesia in vincristine neuropathy. Neurosci Lett 2011; 495: 93-7.
Cata JP, Weng HR, Chen JH, Dougherty PM. Altered discharges of spinal wide dynamic range neurons and down-regulation of glutamate transporter expression in rats with paclitaxel-induced hyperalgesia. Neuroscience 2006; 138: 329-38.
Kassem LA, Gamal El-Din MM, Yassin NA. Mechanisms of vincristine-induced neurotoxicity: possible reversal by erythropoietin. Drug Discov Ther 2011; 5: 136-43.
Norcini M, Vivoli E, Galeotti N, Bianchi E, Bartolini A, Ghelardini C. Supraspinal role of protein kinase C in oxaliplatin-induced neuropathy in rat. Pain 2009; 146: 141-7.
Galeotti N, Vivoli E, Bilia AR, Vincieri FF, Ghelardini C. St. John’s Wort reduces neuropathic pain through a hypericin-mediated inhibition of the protein kinase Cgamma and epsilon activity. Biochem Pharmacol 2010; 79: 1327-36.
Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 2007; 21: 686-98.
Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs 2010; 28: 703-13.
Mangiacavalli S, Corso A, De Amici M, et al. Emergent T-helper 2 profile with high interleukin-6 levels correlates with the appearance of bortezomib-induced neuropathic pain. Br J J Haematol 2010; 149: 916-8.
Visovsky C. Chemotherapy-induced peripheral neuropathy. Cancer Invest 2003; 21: 439-51.
Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care 2011; 5: 1-7.
Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerve Syst 2008; 13: 27-46.
Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol 2012; 14 Suppl 4: iv45-54.
Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer 2004; 12: 619-25.
Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 2013; 22: 2787-99.
Urch CE, Dickenson AH. Neuropathic pain in cancer. Eur J Cancer 2008; 44: 1091-6.
Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2001; 2: 8-14.
Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain 2008; 135: 262-70.
Burton AW, Fine PG, Passik SD. Transformation of acute cancer pain to chronic cancer pain syndromes. J Support Oncol 2012; 10: 89-95.
Caraceni A, Brunelli C, Martini C, Zecca E, De Conno F. Cancer pain assessment in clinical trials. A review of the literature (1999-2002). J Pain Sympt Manage 2005; 29: 507-19.
De Conno F, Caraceni A, Gamba A, et al. Pain measurement in cancer patients: a comparison of six methods. Pain 1994; 57: 161-6.
Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988; 14: 9-17.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1: 277-99.
Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330: 592-6.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994; 23: 129-38.
Lauria G, Hsieh ST, Johansson O, et al; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010; 17(903-12): e44-9.
Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 2003; 60: 1524-34.
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237-51.
Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010; 85(3 Suppl): S3-14.
Tsavaris N, Kopterides P, Kosmas C, et al. Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: a pilot study. Pain Med 2008; 9: 1209-16.
Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010; 150: 573-81.
Corbett CF. Practical management of patients with painful diabetic neuropathy. Diabetes Educ 2005; 31: 523-4.
Wallace M, Pappagallo M. Qutenza®: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother 2011; 11: 15-27.
Galluzzi KE. Management of neuropathic pain. J Am Osteopath Assoc 2005; 105(9 Suppl 4): S12-9.
Cassileth BR, Keefe FJ. Integrative and behavioral approaches to the treatment of cancer-related neuropathic pain. Oncologist 2010; 15(Suppl 2): 19-23.
Jung BF, Herrmann D, Griggs J, Oaklander AL, Dworkin RH. Neuropathic pain associated with non-surgical treatment of breast cancer. Pain 2005; 118: 10-4.
Argyriou AA, Polychronopoulos P, Koutras A, et al. Peripheral neuropathy induced by administration of cisplatin- and paclitaxel-based chemotherapy. Could it be predicted? Support Care Cancer 2005; 13: 647-51.
Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev 2011; (2): CD005228.
Argyriou AA, Chroni E, Koutras A, et al. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology 2005; 64: 26-31.
Kemp G, Rose P, Lurain J, et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol 1996; 14: 2101-12.
Smyth JF, Bowman A, Perren T, et al. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol 1997; 8: 569-73.
Gamelin L, Boisdron-Celle M, Morel A, et al. Oxaliplatin-related neurotoxicity: interest of calcium-magnesium infusion and no impact on its efficacy. J Clin Oncol 2008; 26: 1188-9; author reply 1189-90.
Wang WS, Lin JK, Lin TC, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 2007; 12: 312-9.
Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Support Care Cancer 2011; 19: 1769-77.
Bianchi G, Vitali G, Caraceni A, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer 2005; 41: 1746-50.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Salahadin Abdi contributed substantially to the conception and design of the article. Robert L. Massey and Hee Kee Kim contributed substantially to the acquisition of data. Robert L. Massey, Hee Kee Kim, and Salahadin Abdi contributed substantially to the analysis and interpretation of data and to the draft of the article.
Rights and permissions
About this article
Cite this article
Massey, R.L., Kim, H.K. & Abdi, S. Brief review: Chemotherapy-induced painful peripheral neuropathy (CIPPN): current status and future directions. Can J Anesth/J Can Anesth 61, 754–762 (2014). https://doi.org/10.1007/s12630-014-0171-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0171-4