Abstract
Background
As aortic valve (AV) repairs become more sophisticated, surgeons need increasingly detailed information about the structure and function of this valve. Unlike two-dimensional transesophageal echocardiography (2D-TEE), using three-dimensional (3D)-TEE makes it possible to image the entire AV. We hypothesized that measuring coaptation surface area (CoapSA) would be feasible and reproducible, and CoapSA would decrease in patients with aortic insufficiency.
Methods
We developed a new technique to calculate the AV-CoapSA using 3D-TEE. We measured the coaptation surfaces between the right coronary cusp/left coronary cusp, right coronary cusp/non-coronary cusp, and left coronary cusp/non-coronary cusp in ten normal AVs and ten AVs with moderate-severe aortic insufficiency (AI). Since computer models have previously shown that CoapSA is trapezoidal, we used the formula: trapezoid area = length × (medial coaptation height + lateral coaptation height)/2. The total CoapSA was calculated by adding all three areas. To adjust for valve size, we indexed the value to the diameter of the ventricular aortic junction (VAJ). Measurements were performed by two observers.
Results
The intra-observer correlation was 0.84 for one observer (P < 0.0001) and 0.93 for the other (P < 0.0001). The inter-observer correlation was 0.87 (P < 0.0001). In normal valves, the CoapSA [mean total (standard deviation)] was significantly greater than in the insufficient valves [1.61 (0.31) cm2 vs 1.03 (0.22) cm2, respectively; P < 0.001]. After indexing for the VAJ diameter, the total CoapSA remained significantly greater in normal valves than in insufficient valves.
Conclusion
In this proof of concept study, we present a new and innovative technique to measure AV-CoapSA using 3D-TEE. It is reproducible and shows decreased CoapSA in patients with AI. Coaptation surface area may provide insight into mechanisms of AI and may have predictive value following AV repair.
Résumé
Contexte
Alors que les réparations de valve aortique (VA) deviennent de plus en plus perfectionnées, les chirurgiens ont besoin d’informations de plus en plus détaillées concernant la structure et le fonctionnement de cette valve. L’échocardiographie transœsophagienne tridimensionnelle (ÉTO-3D) permet d’obtenir une image de la VA dans son intégralité, ce qui n’est pas le cas avec l’ÉTO bidimensionnelle (2D). Nous avons émis l’hypothèse qu’il serait faisable de mesurer la surface de coaptation (SCoap), que cette mesure pourrait être reproduite, et que la SCoap serait réduite chez les patients souffrant d’insuffisance aortique.
Méthode
Nous avons mis au point une nouvelle technique afin de calculer la SCoap-VA à l’aide de l’ÉTO-3D. Nous avons mesuré les surfaces de coaptation entre le feuillet coronarien droit et le feuillet coronarien gauche, le feuillet coronarien droit et le feuillet non coronarien, et le feuillet coronarien gauche et le feuillet non coronarien de dix VA normales et de dix autres présentant une insuffisance aortique (IA) modérée à grave. Des modèles informatiques ont démontré que la SCoap est de forme trapézoïdale, c’est pourquoi nous avons utilisé la formule suivante : Surface = (longueur × (hauteur de coaptation médiale + hauteur de coaptation latérale)/2). La SCoap totale a été calculée en additionnant les trois surfaces. Afin de tenir compte de la taille de la valve, nous avons indexé la valeur au diamètre de la jonction ventriculo-aortique (JVA). Les mesures ont été prises par deux observateurs.
Résultats
La corrélation intra-observateur était de 0,84 pour un observateur (P < 0,0001) et de 0,93 pour l’autre (P < 0,0001). La corrélation inter-observateur était de 0,87 (P < 0,0001). La SCoap totale moyenne de valves normales (1,61 ± 0,31 cm2) était significativement plus grande que celle de valves en insuffisance (1,03 ± 0,22 cm2, P < 0,001). Après l’indexation pour tenir compte du diamètre JVA, la SCoap totale est demeurée significativement plus grande pour les valves normales que pour les valves en IA.
Conclusion
Dans cette étude visant à valider le concept, nous présentons une technique nouvelle et innovante pour mesurer la SCoap de la VA à l’aide de l’ÉTO-3D. Cette technique est reproductible et démontre une SCoap réduite chez les patients en IA. La SCoap pourrait offrir des pistes de compréhension des mécanismes de l’IA et avoir une valeur prédictive après une réparation de la VA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The aortic valve (AV) is a complex three-dimensional structure, and its competence depends on the dynamic interaction between its three cusps and the aortic annulus in order to ensure adequate coaptation. Aortic valve repair is becoming increasingly popular around the world.1 As repair techniques become more sophisticated, surgeons require increasingly detailed information about the structure and function of the AV.2 One measurement, the length of coaptation between the cusps after AV repair, has been reported to correlate with the durability of the repair.3
Two-dimensional transesophageal echocardiography (2D-TEE) is a standard of care in cardiac surgery, but it cannot show, in their entirety, the full contiguous interactions between the various aspects of the AV.4 Three-dimensional transesophageal echocardiography (3D-TEE) has opened up new avenues to image the AV. Using multiple plane reconstruction (MPR), it is now possible to visualize the three coaptation planes between the AV cusps.4
We developed a new technique to calculate the coaptation surface area (CoapSA) of the AV using 3D-TEE. We hypothesized that the CoapSA could be measured by 3D-TEE with a high degree of reproducibility and that the CoapSA would be reduced in patients with aortic insufficiency (AI).
Methods
The study was approved by the Ottawa Hospital Human Research Ethics Board and written informed consent was waived.
Study population
After developing and refining a technique to measure CoapSA by 3D-TEE (described below), we applied it prospectively to ten consecutive patients with structurally and functionally normal AV. We then applied the technique to a group of ten patients undergoing surgery for moderate to severe AI who met the following criteria: they had a tri-leaflet AV; the quality of the TEE images was adequate for visualization of the aortic cusps, and they had functional aortic annular dilatation as defined by the current American Society of Echocardiography (ASE) guidelines.5 We chose patients with a dilated functional aortic annulus because the primary mechanism of AI in this patient population is a decrease in CoapSA. The valves in these patients are defined in the surgical literature as type 1a, 1b, or 1c.6 The mechanism of AI was found to be type 1a (dilatation of the sinotubular junction [STJ]) in five patients and type 1b (aortic root and STJ dilatation) in five patients. Associated cusp prolapse (type 2) was also found in one of the patients with type 1a disease and in three of the patients with type 1b disease. The AI jets were central in origin and centrally directed in all patients except for the four patients with associated cusp prolapse.
Transesophageal echocardiography
After induction of general anesthesia and during a period of stable hemodynamics, intraoperative 3D-TEE AV images were obtained by one of two operators (B.S. or C.H.) as part of the routine comprehensive intraoperative TEE examination. Images were acquired using a Philips iE33 3D Transesophageal X7-2t probe and platform (Philips Ultrasound, Andover, MD, USA). An electrocardiography-gated full-volume 3D data set of the AV was obtained at the mid-esophageal short-axis AV level, and it was stored digitally for off-line analysis. To optimize image resolution, imaging depth was limited to include only the AV (while keeping the width of the sector screen such that we could visualize the mitral valve), seven cardiac cycles were utilized, and ventilation and electrocautery were interrupted during acquisition.
Off-line measurements and reproducibility
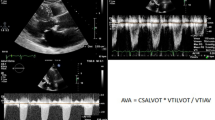
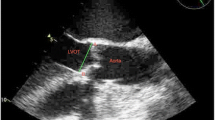
We analyzed the images with Philips QLab software (Philips Ultrasound, Andover, MD, USA). Using MPR, we adjusted the orthogonal imaging planes for optimal visualization of all three aortic coaptation lines (Fig. 1). By moving the red and green planes (Fig. 2), we identified the points where the cusps came together. For each one, we measured the medial and lateral coaptation heights (labelled A and B in Fig. 1) and the coaptation length (labelled C in Fig. 1). We also measured the diameter of the aortic annulus at the level of the ventricular aortic junction (VAJ) in a mid-esophageal long-axis view. All measurements were performed at the end of diastole, which we defined as the 3D frame immediately following closure of the mitral valve.
Images of a normal aortic valve from multiple plane reconstruction (MPR) of the aortic valve. Step 1: The red plane intersects the medial coaptation surface of the non-coronary cusp (NCC) and right coronary cusp (RCC). The yellow line labelled A represents the medial coaptation height. Step 2: The red plane intersects the lateral coaptation surface of the NCC and RCC. The yellow line labelled B represents the lateral coaptation height. Step 3: The two red planes represent the medial and lateral coaptation surfaces, respectively. The yellow line labelled C represents the distance between the two red lines, i.e., the coaptation length
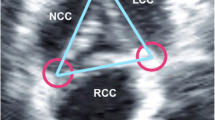
We calculated three CoapSAs for each patient: right coronary cusp/left coronary cusp, right coronary cusp/non-coronary cusp, and left coronary cusp/non-coronary cusp (Fig. 2). Since computer and silicone models have previously shown that the coaptation areas between AV cusps are trapezoidal, we calculated the CoapSA by using the following formula: trapezoid area = coaptation length × (medial coaptation height + lateral coaptation height)/2.7,8 The total CoapSA was calculated by adding all three areas for each valve. To adjust for varying valve sizes, we indexed this value to the diameter of the VAJ.
All measurements were performed independently and in random order by two anesthesiologists (B.S. and C.H.) with National Board of Echocardiography certification and expertise in 3D-TEE. They were blinded to each other’s measurements, but they obviously could not be blinded to the presence of aortic apparatus disease. For each measurement, the 3D data set was loaded into the MPR function in QLab, and the measurements described in Fig. 2 were obtained. In order to optimize pixel identification, gain, brightness, and magnification settings were optimized before obtaining the measurements. Each observer performed the measurements twice for the normal AVs (at different times and in random order to assess intra-observer variability) and once for the AVs with insufficiency.
Statistical analysis
Continuous variables are reported as mean (standard deviation) and categorical variables are reported as percentages. Spearman correlation coefficients were calculated to determine inter- and intra-observer variability. A Bland-Altman analysis was performed for inter-observer measurements by plotting difference vs average for each point measured by the two observers. Mean (standard deviation) of the difference as well as 95% limits of agreement were computed. Comparison of continuous variables between groups was performed using a two-tailed Student’s t test with unequal variances for normally distributed data. All analyses were performed using SAS version 9.2 (Cary, NC, USA), and Fig. 3 was constructed using GraphPad Prism 5.0 (La Jolla, CA, USA). All reported P values are two-sided.
Linear correlation scatter plots. (a) Linear correlation between measurements 1 and measurements 2 for observer 1. (b) Linear correlation between measurements 1 and measurements 2 for observer 2. (c) Linear correlation between observer 1 and observer 2 for all measurements. (d) Bland-Altman analysis for inter-observer variability (solid line represents mean difference; dashed lines represent upper and lower 95% confidence limits)
Results
The 3D data sets were obtained in all 20 patients (Table 1). The intra-observer correlation in normal valves was 0.84 (95% confidence interval [CI] 0.78 to 0.89; P < 0.0001) for observer 1 and 0.93 (95% CI 0.90 to 0.95; P < 0.0001) for observer 2 (Figs 3a and 3b). The inter-observer correlation for all valves was 0.87 (95% CI 0.84 to 0.89; P < 0.0001) (Fig. 3c). The Bland-Altman analysis for intra- and inter-observer variability yielded the following: For observer 1 intra-observer variability, the mean difference was 0.036 (0.11) cm2 (95% limits of agreement: −0.18, +0.25). For observer 2 intra-observer variability, the mean difference was 0.003 (0.08) cm2 (95% limits of agreement: −0.15, +0.16). For inter-observer variability (Fig. 3d), the mean difference was 0.009 (0.08) cm2 (95% limits of agreement: −0.16, +0.17). For all of the above Bland-Altman analyses, more than 95% of the values were within the predicted limits of agreement, indicating good intra- and inter-observer reliability.
In the normal valves, the mean total CoapSA was 1.61 (0.31) cm2 and the average aortic annular diameter was 2.43 (0.25) cm. In patients with moderate to severe AI, the mean CoapSA was significantly smaller [1.03 (0.22) cm2; P < 0.003 vs normal valves]. After indexing for annular diameter, the ratio of the total CoapSA to the aortic annular diameter remained significantly smaller in AI valves than in normal valves [0.37 (0.11) cm vs 0.66 (0.09) cm, respectively; P = 0.0002].
Discussion
Our study shows that 3D-TEE can be used to measure AV CoapSA in patients with normal AV and in patients with moderate to severe AI. There was strong inter- and intra-observer reliability between observers. Of equal interest and as hypothesized, the CoapSA was significantly decreased in incompetent AVs compared with normal competent AVs.
The AV is considered a challenging structure to image with 3D-TEE because of its anterior position and thin cusps.9 Sugeng et al. found that the AV could be reliably visualized with 3D-TEE in only 18-22% of their 211 study patients.10 Interestingly, we were able to obtain adequate data sets for all our patients. This difference could be explained by the fact that we made use of a different 3D imaging modality. Their criterion for adequacy of image was based on 3D zoom while we based adequacy on the ability to identify coaptation lines on MPR. We focused only on MPR image acquisition and optimization. In this study, we performed this work offline. The same software is available on the echo machine in the operating room; therefore, the technique can easily be performed in the operating room in a time frame that makes the measurements clinically useful.
The AV function involves a dynamic interaction between the cusps and the multilevel AV annulus. Unlike the mitral valve, the functional aortic annulus consists of three distinct levels: the ventricular aortic junction proximally, the sinotubular junction distally, and the “surgical” aortic annulus, which is a crown-shaped structure that serves as the insertion point of the valve cusps. Competence of the AV is critically dependent on proper coaptation between adjacent cusps.
The concept of CoapSA dates back roughly forty years during the initial development of AV prostheses by Swanson and Clark.8 Using silicon models, they recognized that the CoapSA was not shaped like a simple rectangle, but because of the coronet shape of the valve, it was shaped more like a trapezoid (Fig. 1) with two coaptation heights (a shorter medial one located near the centre of the valve and a longer lateral one located near the outer circumference of the valve) and a distance/length between the two heights. Fig. 1 illustrates this concept using a computer model. The CoapSA cannot be measured by 2D-TEE because of the anatomical relationship between the esophagus and the heart; consequently, only two of three AV coaptation planes can be seen with 2D-TEE.4 Three-dimensional-TEE collects a wide pyramidal volume of information that can then be cropped in an infinite number of ways. Three-dimensional-TEE removes this anatomical barrier and allows the ability to visualize all three coaptation planes.
Our study has a number of important potential implications. First, it provides a platform on which to build a better understanding of AV coaptation, both in normal and disease states. When ventricular relaxation occurs and aortic pressure exceeds ventricular pressure, the aortic cusps collapse centrally and close the aortic orifice. For a competent seal to occur, the aortic cusps must abut one another to create a sufficient CoapSA. As the AV apparatus dilates, or when there is prolapse of a cusp, the ability for the aortic cusps to coapt decreases, resulting in AI.6 Results of our study showed that the CoapSA in patients with AI also decreases significantly, which intuitively makes perfect sense.
A second important application of this technique is in the immediate postoperative evaluation of AV repair. The current 2D echo predictors of long-term AV competence after repair have been described and include the presence of residual AI, the level of the plane of coaptation in relation to the VAJ, and the length of coaptation of the AV cusps in the mid-esophageal long-axis view.2 Due to the limitations of 2D TEE, this coaptation length, a unidimensional value, can be measured for only two of the three coaptation planes. We consider CoapSA to be a better predictor of the long-term durability of AV repair, and this will be the subject of a future study.
A third application is in the field of virtual surgery. Using high-fidelity simulation models, surgeons are beginning to look at the possibility of evaluating surgical procedures in the virtual environment.11 Using preoperative 3D-TEE measurements, surgeons could evaluate the effectiveness of various procedures before taking a patient to the operating room. Restoring adequate CoapSA may become an important outcome when evaluating a surgical procedure.
Our study shows a difference in CoapSA between normal AV and AI valves. Some of the variability in CoapSA is due to differences in patient size, heart size, and AV size. To mitigate these factors, we considered it appropriate to use some form of CoapSA index. Options for indexing variables included those related to patient size (e.g., height, weight, and body surface area), those related to heart size but unrelated to the AV, and those related to the AV itself. From an anatomical perspective, for any AV to be competent, the surface area of coaptation must be of sufficient size to prevent regurgitation. This is true of valves with small or large VAJ diameters. Indeed, for valves in which the VAJ is dilated, the surface area of the cusps would have to be even larger to provide enough CoapSA. Consequently, indexing to VAJ provides the best measure of CoapSA to obtain AV competence, which is ultimately what we hope this measurement will provide, whether it is used pre- or post-repair.
Our study has several limitations. First, it involved a small number of patients. The choice of ten patients per group was chosen in order to show reproducibility in the measurements taken. Ten measurements were obtained for each valve, resulting in 200 measurements made by each observer, and each observer repeated 100 of these measurements twice. This allowed us to assess the intra- and inter-observer variability and reliability with confidence. The study also suffers from the lack of a current gold standard for the measurement of AV CoapSA. Unlike mitral valve assessment, which can be checked against surgical findings, the AV CoapSA cannot be directly measured by the surgeon. Nevertheless, other such examples exist in echocardiography, for instance, the pressure half-time method to calculate mitral valve area, where empiric data validation has become widely accepted despite the inability to measure it directly.12
To support our measurement rationale, we used patients with AI resulting from annular dilatation. The reason we chose type 1 AI patients (annular dilatation), with or without associated cusp prolapse, was precisely because the mechanism of AI in that patient population is decreased CoapSA. Our measurements support this theory. Finally, indexing to VAJ may be problematic in patients with type 1c disease (isolated VAJ dilatation). Fortunately, this is a rare condition, and the VAJ diameter would be normalized in the context of post AV repair.
In this proof of concept study, we present an innovative technique for measuring AV CoapSA using intraoperative 3D-TEE. The technique is feasible, appears highly reproducible, and seems to correspond with clinical reality in a small number of patients with type 1 AI. The ability to measure CoapSA reliably opens up interesting avenues for the evaluation of AV repair in the operating room and for predicting its durability.
References
David TE. Aortic valve repair for management of aortic insufficiency. Adv Card Surg 1999; 11: 129-59.
Van Dyck MJ, Watremez C, Boodhwani M, Vanoverschelde JL, El Khoury G. Transesophageal echocardiographic evaluation during aortic valve repair surgery. Anesth Analg 2010; 111: 59-70.
le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009; 2: 931-9.
Imai K, Watanabe N, Saito K, et al. Quantitative measurements of aortic valve coaptation by three-dimensional transesophageal echocardiography in patients with aortic regurgitation without primary leaflet disease. J Echocardiogr 2010; 8: 7-13.
Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendation for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003; 16: 777-802.
Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009; 137: 286-94.
Labrosse MR, Boodhwani M, Sohmer B, Beller CJ. Modeling leaflet correction techniques in aortic valve repair: a finite element study. J Biomech 2011; 44: 2292-8.
Swanson WM, Clark RE. Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ Res 1974; 35: 871-82.
Vegas A, Meineri M. Three-dimensional transesophageal echocardiography is a major advance for intraoperative clinical management of patients undergoing cardiac surgery: a core review. Anesth Analg 2010; 110: 1548-73.
Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol 2008; 52: 446-9.
Fabricius AM, Walther T, Falk V, Mohr FW. Three-dimensional echocardiography for planning of mitral valve surgery: current applicability? Ann Thorac Surg 2004; 78: 575-8.
Hatle L, Angelsen B, Tromsdal A. Noninvasive assessment of atrioventricular pressure half-time by Doppler ultrasound. Circulation 1979; 60: 1096-104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Benjamin Sohmer, A. Stéphane Lambert, Michel Labrosse, and Munir Boodhwani were involved in the study design. Benjamin Sohmer, Christopher Hudson, and Juliet Atherstone were involved in conducting the study. Benjamin Sohmer, Christopher Hudson, Juliet Atherstone, A. Stéphane Lambert, and Munir Boodhwani participated in manuscript preparation. Christopher Hudson and Munir Boodhwani participated in the data analysis.
Rights and permissions
About this article
Cite this article
Sohmer, B., Hudson, C., Atherstone, J. et al. Measuring aortic valve coaptation surface area using three-dimensional transesophageal echocardiography. Can J Anesth/J Can Anesth 60, 24–31 (2013). https://doi.org/10.1007/s12630-012-9819-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-012-9819-0