Abstract
Purpose
Intraoperative cell salvage (ICS) is used as an alternative to allogeneic blood transfusion in an attempt to avoid or minimize the risks associated with allogeneic blood. Intraoperative cell salvage is generally avoided in surgeries where malignancy is confirmed or suspected due to concern for potential metastasis or cancer recurrence. The application of post-processing methods for ICS is hypothesized to eliminate this potential risk. The purpose of this narrative review is to examine the in vitro experimental evidence as it pertains to the removal of tumour cells from ICS blood and to review the clinical studies where ICS blood has been used in patients with malignancy.
Source
A search of the English literature for relevant articles published from 1973 to 2012 was undertaken using MEDLINE and Cochrane databases. Bibliographies were cross-referenced to locate further studies.
Principal findings
Leukoreduction filters are an effective method for removal of malignant cells from ICS blood. Small non-randomized clinical studies to date do not show evidence of an increased rate of metastasis or cancer recurrence. Although a theoretical risk of disease recurrence persists, the decision to use autologous ICS blood must be weighed against the known risks of allogeneic blood transfusion.
Conclusion
Transfusion of autologous blood harvested via ICS should be considered a viable option for reduction or avoidance of allogeneic product during many oncologic surgeries and may be a lifesaving option for those patients who refuse allogeneic blood products.
Résumé
Objectif
L’épargne peropératoire de cellules sanguines (ICS) est utilisée comme méthode de remplacement des transfusions de sang allogène pour éviter ou minimiser les risques qui lui sont associés. L’épargne peropératoire des cellules sanguines est généralement évitée au cours des interventions chirurgicales lorsqu’un processus malin est confirmé ou suspecté en raison de la crainte de métastases potentielles ou de récidive du cancer. L’hypothèse d’une utilisation des méthodes de traitement post-ICS est formulée pour éliminer ce risque. Cet article a pour objectif d’analyser les données probantes expérimentales in vitro concernant la suppression de cellules tumorales de sang d’ICS et de passer en revue les études cliniques au cours desquelles du sang d’ICS a été utilisé chez des patients souffrant de processus malins.
Source
Une recherche d’articles pertinents publiés entre 1973 et 2012 a été effectuée dans la documentation en langue anglaise des bases de données PubMed et Cochrane. Les références bibliographiques de chaque article ont été également croisées à la recherche de nouvelles références.
Constatations principales
Les filtres de réduction leucocytaire constituent une méthode efficace pour la suppression des cellules malignes dans le sang d’ICS. À ce jour, de petites études cliniques non randomisées n’ont pas fourni de données probantes sur une augmentation du taux de métastases ou de récidive cancéreuse. Bien qu’un risque théorique de récidive de la maladie persiste, la décision d’utilisation du sang autologue d’ICS doit être évaluée contre les riques connus d’une transfusion de sang allogène.
Conclusion
La transfusion de sang autologue récupéré grâce à l’ICS doit être envisagé comme une option viable pour réduire ou éviter le recours à un produit allogène au cours de nombreuses chirurgies oncologiques; cela pourrait être également une option pour sauver la vie des patients qui refusent les produits sanguins allogènes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraoperative cell salvage (ICS) has traditionally been avoided in patients with known or suspected malignancy due to fear of tumour recurrence or metastasis.1 Consideration of the risks and benefits of both autologous cell-salvaged blood and allogeneic blood must motivate each intraoperative transfusion. An intimate understanding of the risks and benefits associated with transfusion of ICS blood becomes particularly important in patients who will not accept allogeneic blood products, at which time ICS may be the only acceptable option for replacement of red blood cells. In this review, we examine the literature as it pertains to transfusion of autologous blood from ICS in oncologic surgery. Herein, we discuss this evolving field and its management implications for the perioperative physician in the context of the Canadian blood system.
Methods
The literature search for this narrative review was conducted from April 2011 to March 2012 using the MEDLINE and Cochrane databases and was limited to English language papers published in peer-reviewed journals from 1973 to 2012. The following MeSH terms were searched in PubMed: “operative blood salvage” and “bloodless medical and surgical procedures”. These terms were also searched as keywords. Bibliographies of papers were hand-searched and cross-referenced to locate further studies. We included any in vitro study that investigated the efficacy of tumour cell removal following ICS processing and subsequent filtration or irradiation. In addition, all clinical studies where patients had received ICS blood intraoperatively during malignancy surgery were included (without exclusion). To identify materials in the grey literature, Google Scholar and the Web of Science were consulted for additional references.
Perioperative transfusion of allogeneic blood products
Oncologic surgery is associated with a high rate of blood transfusions.2 There are many complications associated with the perioperative transfusion of allogeneic blood, including administration of the wrong blood product, acute hemolytic transfusion reaction, transmission of infection, and transfusion-associated acute lung injury (TRALI).2 In addition, there are risks associated with transfusion of allogeneic blood specific to the perioperative period. A large number of studies have shown that perioperative transfusion of allogeneic blood is associated with increased morbidity and mortality. Poorer outcomes are ascribed to an increased risk of postoperative infection,3-10 pulmonary complications,8,11 greater number of ventilator days,9,12 renal dysfunction requiring dialysis,9 multi-organ dysfunction,13 intensive care unit days,10 increased length of stay,14 and short-term mortality.9,10 Risk-adjusted models have attempted to control for allogeneic transfusion as simply a marker for illness severity or complicated perioperative course.9 Although this possibility remains, these analyses have identified allogeneic transfusion as a dose-dependent independent predictor of postoperative morbidity and mortality.9
The morbidity and mortality associated with transfusion of allogeneic blood is attributed to a complex, poorly understood interaction between host tissue and transfused allogeneic blood components termed transfusion-related immunomodulation (TRIM).15,16 The immunomodulatory properties of allogeneic blood were first recognized following observations of improved renal allograft survival in patients who had received perioperative blood transfusion.17,18 Leukocytes and their soluble and cellular breakdown products are thought to contribute significantly to TRIM.15 Subsequent leukoreduction (LR) of select blood components was initially undertaken to decrease the incidence of adverse events that were known to be related to the leukocyte component of donor products (febrile non-hemolytic transfusion reaction, transmission of cytomegalovirus, and human leukocyte antigen alloimmunization). Universal prestorage LR was implemented to minimize these complications. In prestorage LR, filtration is done by the blood supplier at the time of whole blood separation into components. In 1999, Canada, along with the United Kingdom, France, Portugal, and Ireland instituted universal prestorage LR for all blood products.19
Complications ascribed to allogeneic blood transfusion derive largely from studies that used non-leukoreduced blood products, either because the study was performed prior to the introduction of prestorage LR, or because the study was performed in a region that does not employ a method of LR. If TRIM is responsible for a measurable component of the morbidity and mortality that is seen as a result of transfusion of allogeneic blood products, a decreased complication rate should be seen following the implementation of a universal prestorage LR program. Randomized controlled trials in cardiac surgery patients have shown that transfusion of leukoreduced allogeneic blood improves short-term20,21 and in-hospital22 mortality and infection rates22 when compared with transfusion of a non-leukoreduced product. Similarly, decreased infection rates were seen in colorectal surgery patients23 and in a meta-analysis of mixed surgical patients24 following transfusion of leukoreduced blood. The effectiveness of universal prestorage LR programs is controversial and continues to be debated.15,19,25
An additional consideration affecting the patient undergoing malignancy resection is whether allogeneic blood transfusion causes immunosuppression that contributes to an increased risk of subsequent cancer recurrence, an observation first described in 1982.26 Impaired function of natural killer cells, decreased T cell production, alteration of T cell ratios, and defective antigen-presenting cell number and function have all been associated with transfusion of allogeneic blood and are thought to be an important contributor to TRIM. Together, these immunological changes may decrease the effectiveness of tumour surveillance in the host.27 Over 100 publications and several meta-analyses28-30 have attempted to address this complex issue, with approximately half of these studies suggesting that allogeneic blood transfusion has a detrimental effect on cancer recurrence. In keeping with previous findings, a recent meta-analysis in patients undergoing curative resection for colorectal cancer found a modest dose-dependent effect of perioperative allogeneic blood transfusion on rate of recurrence (overall odds ratio of 1.42 for recurrence rate following transfusion; 95% confidence interval 1.20 to 1.67).30 Data were insufficient to determine whether recurrence rates were altered by institution of prestorage LR.
Ultimately, the risk to benefit profile of allogeneic blood transfusion in the patient undergoing surgery for tumour resection has several considerations. The decision to transfuse is a complex interplay of dynamic factors that include the patient’s own wishes, their underlying physiologic status, ongoing blood loss, and coagulation status. Based on our current understanding, it is likely that some impact secondary to TRIM will occur. It is currently difficult to know whether a perioperative allogeneic transfusion will contribute to a subsequent complication or eventual recurrence of cancer.
Blood conservation and ICS
Concerns regarding complications associated with perioperative allogeneic blood transfusion coupled with resource limitations have refocused attention on strategies to avoid its use, and the concept of “bloodless surgery” has been popularized.31 In his recommendations in the wake of Canada’s inquiry into the tainted blood scandal, Justice Horace Krever identified alternatives to blood transfusion a priority after thousands of blood transfusion recipients developed HIV and tens of thousands developed hepatitis C.32 Bloodless surgery represents a combination of techniques throughout the perioperative period, with the common goal of minimizing or avoiding allogeneic blood transfusion. Strategies are employed preoperatively (autologous blood donation, iron and erythropoietin supplementation), intraoperatively (ICS, acute normovolemic hemodilution, antifibrinolytic drugs, surgical technique), and postoperatively (minimization of phlebotomy, conservative transfusion trigger, antifibrinolytic drugs, return of shed blood). These strategies are particularly important for those patients that refuse blood products. There are no studies that directly compare the efficacy of various blood conservation techniques for avoidance of allogeneic blood transfusion. In our experience, amongst the most effective of these practices is the use of ICS. The majority of patients that refuse allogeneic blood products are Jehovah’s Witnesses (members of the Watchtower Bible and Tract Society). They will not accept transfusion of whole blood or its primary components, namely red blood cells, white blood cells, plasma, or platelets, nor will they accept stored or banked blood.33 As ICS involves autologous blood, its acceptance is at the discretion of each individual patient.33
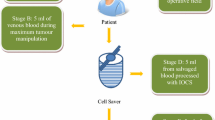
During ICS, blood loss is suctioned and collected, filtered (pore diameter between 120 and 180 μm), and washed for subsequent re-transfusion of autologous red blood cells (RBCs). Contaminants, such as cell debris, fat globules, and bone chips are removed, and a final wash results in transfusion-ready plasma-depleted RBCs suspended in saline (hematocrit 0.6-0.8). By Canadian standards, autologous RBCs processed by ICS can be stored at room temperature and safely transfused within six hours of collection (or stored at 4°C for 24 hr).34 Autologous transfusion of ICS blood has many advantages: the risk of transfusion reaction is reduced; alloimmunization and immunosuppression associated with allogeneic blood are avoided; the quality of the red cell is excellent; and ICS is available regardless of the patient’s starting hematocrit.35 When blood loss is > 800 mL, ICS is an effective means of reducing or avoiding allogeneic transfusion.35
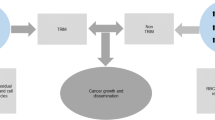
The primary objection to the use of ICS in oncologic surgery is the unproven but theoretical possibility that malignant cells from the surgical field will be re-transfused into the patient and result in subsequent tumour recurrence and metastases.1 This is based on the belief that oncologic surgery often violates the tumour, resulting in “spillage” of the cancer cells into the operative field. This potential controversy was first aired in 1975 when malignant cells were documented in harvested shed blood after processing through the cell saver.36 At that time, it was unknown whether these cells were viable, if they would survive upon transfusion, or if they had proliferative or metastatic potential. More than 35 years later, many of these unknowns remain, and the use of ICS in oncologic surgery remains controversial.
Tumour cells harvested from the operative site—do they pose a risk?
It is reasonable to conclude that the risk associated with autotransfusion of a small number of potentially tumourigenic cells from the operative field is negligible, as many cancer patients already have a significant number of circulating cancer cells prior to surgery.37-41 It is thought that tumour cells are released continuously in a regulated balance between tumour and host. Indeed, the median number of tumour cells released into the renal vein of patients with primary renal cancer has been estimated at 37 million per day.42 These cells are felt to be viable and potentially tumourigenic as they are freshly released into a major blood vessel from the tumour site. However, the ability of circulating tumour cells to form metastases is low, a concept that has been termed “metastatic inefficiency”.43 Based on animal studies, it has been estimated that as few as 0.01-0.000001% of disseminated cancer cells have the ability to form metastatic lesions.43
Despite acknowledgement of circulating tumour cells in most patients, concern persists regarding transfusion of harvested tumour cells from the operative site, as many studies have established that tumour cells remain in blood after collection and processing using ICS.36,37,44-48 Quantification of remaining tumour cells is variable amongst studies, but the literature is in agreement that ICS alone does not effectively remove or destroy tumour cells that have been collected from the operative field. Advanced stage of disease and intraoperative tumour rupture have been identified as risk factors for gross tumour contamination of blood in the surgical field and the subsequent difficulty in removing these cells with ICS processing alone.49 Tumour cells that are harvested at the time of resection are unlikely to be exclusively from the pool of circulating cells (from tumour draining veins for example), and as shown in an elegant study by Hansen et al., they are more likely a different subset of tumour cells, potentially with different proliferative and metastatic potential.37 In 93% of 61 patients with 15 different tumour types studied, they were able to show the presence of tumour cells in salvaged blood, often in numbers that could not be explained by the percentage of circulating cells.37 Potential sources of these cells include tumour cells that remain at the resection margin or suture line, inadvertent tumour rupture, pressurization of the tumour from handling, presence of peritoneal disease or occult microscopic disease, and lymphatic spillage. In a third of the cases examined, they were able to show that the recovered tumour cells had proliferative capacity by forming cell colonies in vitro. One of these cell lines was capable of inducing tumour growth upon injection into nude (athymic) mice.37
Methods for removal of tumour cells from ICS blood
Two methods aimed at eliminating tumour cells from the red cell concentrate of ICS blood have been described and studied: filtration through a LR filter, and gamma irradiation.
Filtration
Leukoreduction filters have been studied extensively for their ability to remove tumour cells from ICS blood. Those currently in use are third and fourth generation filters made of tightly packed small-pore microfibre webs. The mechanism of tumour cell removal by LR filters is not definitely known, but is presumed to be due to interdependent physical and biological processes. As with white blood cell filtration of whole blood, several different mechanisms are likely responsible.50 Large tumour cell clusters will be readily trapped (barrier retention), while small clusters or single cells may be removed by barrier retention or via cell adhesion of charged surface molecules (retention by adsorption). Filtration speed has historically limited the applicability of LR filters, but it has improved dramatically with newer generation models that can process a unit of RBC in less than two minutes.51 The use of a standard blood filter or microaggregate filter is unnecessary when a LR filter is used.
Several studies have investigated whether it is possible to eliminate tumour cells from the red cell concentrate of blood collected with ICS using LR filtration (Table 1). These are ex vivo studies using oncologic cell lines that are seeded in banked or fresh blood45,51-56 or primary tumour cells collected at the time of tumour resection48,49,57-59 and then processed via ICS and subsequent LR filtration. Together, these studies show that advanced generation LR filters are very effective in the removal of tumour cells after ICS processing (the results of experiments where tumour cells were NOT completely removed are recorded in bold print in Table 1). Although one could argue the clinical relevance of studies looking at oncologic cell lines rather than primary tumour cells, it does serve proof of principle that the addition of LR filtration is effective for removal of tumour cells. Furthermore, oncologic cell lines are more robust than primary tumour cells and would be expected to “outperform” primary tumour cells through the filtration process, and thus, they are a good test.45 Using a sensitive polymerase chain reaction (PCR)-based assay and increasing numbers of cells (hepatocellular carcinoma (HCC) cell line), Gwak et al. showed that tumour cell load may affect the performance of LR filtration. Once they reached 1 × 105 cells·mL−1, they were no longer able to filter out tumour cells consistently and effectively,56 though other studies have used tumour cell lines in similar numbers and were able to remove tumour cells completely.45,51 It is uncertain whether this is a property of the HCC cell line or the exquisite sensitivity of the assay used by Gwak et al. One of the criticisms of using a PCR-based assay for this type of study is that a positive result can still be obtained from cell fragments and recovered DNA that have no viability or proliferative potential. Similarly, in the study by Liang et al., tumour cell burden was hypothesized to contribute to ineffective LR filtration.49 In patients undergoing resection of HCC, tumour cells were effectively removed with LR filtration unless there was intraoperative tumour rupture (2/15 patients). The authors hypothesized that the tumour burden in these two patients exceeded the filtering capacity of the LR filter, and in one patient, they were able to remove all remaining tumour cells with a second LR filter.
Hansen et al. argue that the studies examining LR filter efficacy for removal of tumour cells from ICS blood use methodology that does not possess sufficient sensitivity to detect residual malignant cells and, as a result, show a reduction rather than an elimination of tumour cells.60 In studying nine different LR filters (type and generation not specified), they found reduction rates of 4-5 log for tumour cell lines and 3 log for solid tumour cells. With up to 107 tumour cells shed during oncologic surgery, they concluded that re-transfusion of ICS blood following LR filtration was not safe practice.60 However, in various oncologic surgeries, they have also shown that the number of tumour cells shed was much lower and ranged from 0.2-4,000 cells·mL−1.37 Using similar methodology and assays with comparable and very sensitive limits of detection, multiple studies have now shown efficacy with advanced generation LR filters in cell concentrations up to 1 × 105 cells·mL−1 (Table 1). From all of the available studies, it seems that filtration capacity of the LR filter becomes a realistic concern only at such a tumour cell burden that occurs with either very advanced disease or intraoperative tumour rupture. Within the limits of comparing in vitro work with in vivo conditions, fears of undetected potentially malignant cells post-LR processing can be allayed by the inability to propagate these cells further in vitro.
Irradiation
Gamma irradiation is known to render sufficiently exposed cells mitotically inactive while preserving the quality of the red blood cell product.61 It is the basis for prophylactic irradiation of cellular allogeneic blood products in immunocompromised recipients for prevention of transfusion-associated graft vs host disease. Although irradiation of a solid tumour in situ is often unsuccessful or incomplete, it is thought that the well-oxygenated single-cell suspension that results from ICS processing is optimally suited for tumour cell elimination by irradiation.62
Studies have shown that tumour cells remaining in the red cell concentrate of blood collected and processed with ICS can be effectively eliminated with gamma irradiation (25-50 Gy), as they are rendered mitotically inactive. This was shown both immediately post irradiation58,62 and following a period of in vitro culture to assess for any occult viable cells62 (Table 2). However, a subsequent study comparing the efficacy of irradiation with LR filters found irradiation to be less effective.52
The chief limitation of irradiation of ICS blood is the availability of a gamma irradiator on-site. Relatively few hospital transfusion laboratories house an irradiator because of the high cost, significant maintenance, quality assurance requirements, and limited need outside of specialized transplant and cancer care centres. Further, even if they were available in-house, there is additional risk with moving autologous blood untested for transmissible disease from the operating room area to the transfusion medicine laboratory. This is a risk with respect to wrong blood to wrong patient errors that is borne not only by the surgical patient but also by other patients served by the transfusion service. For this reason, secondary removal of tumour cells by an LR filter prior to re-infusion would likely be the preferred option for most centres.
It is known that the constellation of factors required for a circulating cancer cell to cause metastatic disease in a host is complex and inefficient.43,63 Consider the steps required for an intraoperatively shed cancer cell to form metastatic disease upon re-transfusion following ICS and its requisite processing. A cell would have to survive ICS processing, maintain proliferative capacity, and then subsequently find a suitable milieu for formation of disseminated disease. There is evidence that cancer cells lose integrity and proliferative capacity following ICS processing.44,46 If a small subset of cells were to survive ICS processing and subsequent LR filtration or irradiation, what is the likelihood that it would engender metastatic disease upon re-transfusion to the patient? Ultimately, the answer to this question is unknown, but the previously described in vitro experiments herein provide good evidence that tumour cells are effectively removed prior to autotransfusion. The in vivo component of this question, i.e., whether patients who receive autotransfused blood are at higher risk of hematogenous metastases, can be answered only through clinical studies.
Clinical experience
Reports of autotransfusion of ICS blood collected during surgery for resection of malignancy date back to 1986.64 Malignancy recurrence or metastases secondary to perioperative transfusion practices is a difficult field of study given the heterogeneity of the clinical circumstance, the type and stage of cancer, and the long follow-up times required. Nonetheless, 15 clinical studies have been published reporting outcomes on patients who received transfusion of ICS blood intraoperatively during malignancy surgery64-78 (Table 3). The majority of these studies were single-centre non-randomized small studies where the outcome of patients who received intraoperative ICS blood was compared with historic or case controls. Four of these were controlled studies comparing intraoperative transfusion of ICS blood with transfusion of preoperatively donated autologous blood70,71 or transfusion of allogeneic blood or no blood.73,78 It is critical to point out that the majority of studies (13/15) involved ICS blood that was transfused without the benefit of subsequent LR filtration or irradiation. The notion that autotransfusion of ICS blood increases a patient’s risk of disease recurrence through hematogenous dissemination is not supported by any of these studies. A very recent meta-analysis supported this conclusion.79 A ten-year follow-up study of patients who underwent resection of HCC showed that avoiding allogeneic blood with the use of ICS improved outcomes72 (Table 3). Critics will rightfully argue that these studies lack the power and the follow-up time to discern a meaningful difference62; however, an analysis of these data collectively is the best evidence to date.
Evolution of practice
In 1986, at a time when autologous transfusion programs in the United States were becoming increasingly available, the Council on Scientific Affairs published a report endorsing autologous blood transfusions in the form of cell salvage or preoperative autologous donation.80 It cited patient safety via avoidance of infection and alloimmunization and the sparing of blood bank resources as the primary advantages. Without any supporting in vivo evidence (at the time or in the intervening years), the presence or suspicion of malignant cells within the surgical field was introduced as a contraindication to the use of ICS.
This view has influenced the practice of autologous transfusion since, and the intervening years have been dominated largely by guidelines citing malignancy as a contraindication to ICS. During this time, however, a large research effort has been invested to prove or disprove this theory. Multiple surgical programs have continued to recognize both the potential benefit of autologous transfusion and the potential harm of allogeneic transfusion, and they have persisted in offering ICS (± perioperative autologous donation) to their patients undergoing resection of malignancy.66,69,70,72,74,76 This practice has afforded more than 20 years of experience from some of these teams who clearly have experienced benefit rather than detriment to their patients. The current body of in vitro evidence together with a growing clinical experience suggest that ICS should not be withheld from patients with malignancy. In contrast, there is a substantial body of literature showing increased morbidity and complication in patients who receive allogeneic blood transfusion in this setting. Recent practice guidelines, including those from the National Institute for Health and Clinical Excellence (NICE) and from the Society of Thoracic Surgeons and Cardiovascular Anesthesiologists are now recommending the use of ICS with subsequent LR filtration for some malignancy surgeries, suggesting that in many circumstances the known risks of ICS blood are less than those of allogeneic blood.81-83
Conclusion
Although transfusion of allogeneic blood is considerably safer than it was several decades ago, it still poses significant risk to the patient. The impact of TRIM, particularly in the patient with cancer, has yet to be completely understood. Pertinent to Canadian practice, the effect of prestorage LR on reducing TRIM-related adverse events has not been completely elucidated. Nevertheless, there is some evidence to support the observation that patients who receive allogeneic blood suffer more infections, a higher incidence of tumour recurrence, and increased morbidity as compared with patients who receive only autologous blood. Universal prestorage LR, as is practised in Canada, likely has some benefit with respect to the reduction in immunomodulation associated with allogeneic transfusion in the cancer patient, but this has yet to be fully understood.
When ICS is used for oncologic surgery, harvested red blood cell concentrates do contain a significant number of malignant cells. Despite this, the evidence to date does not show an increased risk of tumour recurrence or metastasis as a result of using ICS during oncologic surgery. A large body of evidence suggests that the safety of ICS blood can be improved further when used for cancer surgery with the subsequent use of an LR filter or irradiation. The majority of evidence, together with ease of use, has resulted in a recommendation for LR filters (over irradiation) in new practice guidelines.
A high-quality randomized controlled trial would be a difficult undertaking due to the heterogeneity of patients and their oncologic disease, the complexity of the decision to transfuse blood (autologous, allogeneic, or both), the number of patients required to detect a clinically meaningful difference, and the follow-up time necessary. In the current climate of increasing acceptance of ICS use in oncologic surgery, it is conceivable that such a trial may be realized.84 Given the difficulty of these studies, the suggestion of establishing local, national, or international registries to monitor tumour recurrence and patient survival deserves attention and has been suggested previously in the literature.85
Avoidance of allogeneic blood products is not possible for all patients undergoing resection of malignancy. Nevertheless, transfusion of autologous blood harvested via ICS should be considered a viable option for reduction or avoidance of allogeneic product and may be a lifesaving option for those patients who refuse allogeneic blood products.
References
Waters JH. Indications and contraindications of cell salvage. Transfusion 2004; 44(12 Suppl): 40S-4S.
Weber RS, Jabbour N, Martin RC 2nd. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol 2008; 15: 34-45.
Heiss MM, Mempel W, Jauch KW, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet 1993; 342: 1328-33.
Tartter PI. Blood transfusion and infectious complications following colorectal cancer surgery. Br J Surg 1988; 75: 789-92.
Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion 1992; 32: 517-24.
Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med 2002; 30: 2249-54.
Chelemer SB, Prato BS, Cox PM Jr, O’Connor GT, Morton JR. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann Thorac Surg 2002; 73: 138-42.
Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011; 114: 283-92.
Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006; 34: 1608-16.
Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001; 119: 1461-8.
Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005; 33: 1191-8.
Vamvakas EC, Carven JH. Allogeneic blood transfusion and postoperative duration of mechanical ventilation: effects of red cell supernatant, platelet supernatant, plasma components and total transfused fluid. Vox Sang 2002; 82: 141-9.
Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg 1997; 132: 620-5.
Weber EW, Slappendel R, Hemon Y, et al. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST). Eur J Anaesthesiol 2005; 22: 249-57.
Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007; 21: 327-48.
Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology 2005; 10(Suppl 1): 208-14.
Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc 1973; 5: 253-9.
Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med 1978; 299: 799-803.
Vamvakas EC, Blajchman MA. Universal WBC reduction: the case for and against. Transfusion 2001; 41: 691-712.
van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation 1998; 97: 562-8.
Boshkov LK, Furnary A, Morris C, Chien G, VanWinkle D, Reik R. Prestorage leukoreduction of red cells in elective cardiac surgery: results of a double blind randomized controlled trial. Blood 2004; 104: 112a (abstract).
Bilgin YM, van de Watering LM, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation 2004; 109: 2755-60.
Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet 1996; 348: 841-5.
Fergusson D, Khanna MP, Tinmouth A, Hebert PC. Transfusion of leukoreduced red blood cells may decrease postoperative infections: Two meta-analyses of randomized controlled trials. Can J Anesth/Journal canadien d’anesthésie 2004; 51: 417-24.
Blumberg N, Zhao H, Wang H, Messing S, Heal JM, Lyman GH. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion 2007; 47: 573-81.
Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet 1982; 2: 662.
Hellings S, Blajchman MA. Transfusion-related immunosuppression. Anaesth Intensive Care Med 2009; 10: 231-4.
Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg 1993; 80: 427-32.
Vamvakas EC. Perioperative blood transfusion and cancer recurrence: meta-analysis for explanation. Transfusion 1995; 35: 760-8.
Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev 2006; (1): CD005033.
Nagarsheth NP, Sasan F. Bloodless surgery in gynecologic oncology. Mt Sinai J Med 2009; 76: 589-97.
Justice Horace Krever. Royal Commission of Inquiry on the Blood System in Canada. Final Report. Available from URL: http://epe.lac-bac.gc.ca/003/008/099/003008-disclaimer.html?orig=/100/200/301/hcan-scan/commission_blood_final_rep-e/ (accessed June 2012).
Our Kingdom Ministry. How Do I View Blood Fractions and Medical Procedures Involving my Own Blood? November, 2006. Available from URL: http://aggelia.be/km_nov2006.pdf (accessed June, 2012).
Kraegel J. CSA Z902-10 Blood and blood components. Canadian Standards Association. Mississauga, Ontario, 2010. Available from URL: http://shop.csa.ca/en/canada/invt/27020812010 (accessed August 2012).
Pape A, Habler O. Alternatives to allogeneic blood transfusions. Best Pract Res Clin Anaesthesiol 2007; 21: 221-39.
Yaw PB, Sentany M, Link WJ, Wahle WM, Glover JL. Tumor cells carried through autotransfusion. Contraindication to intraoperative blood recovery? JAMA 1975; 231: 490-1.
Hansen E, Wolff N, Knuechel R, Ruschoff J, Hofstaedter F, Taeger K. Tumor cells in blood shed from the surgical field. Arch Surg 1995; 130: 387-93.
Salsbury AJ. The significance of the circulating cancer cell. Cancer Treat Rev 1975; 2: 55-72.
Kar S, Carr BI. Detection of liver cells in peripheral blood of patients with advanced-stage hepatocellular carcinoma. Hepatology 1995; 21: 403-7.
Mori M, Mimori K, Ueo H, et al. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer 1996; 68: 739-43.
Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 2000; 232: 58-65.
Glaves D, Huben RP, Weiss L. Haematogenous dissemination of cells from human renal adenocarcinomas. Br J Cancer 1988; 57: 32-5.
Weiss L. Metastatic inefficiency. Adv Cancer Res 1990; 54: 159-211.
Homann B, Zenner HP, Schauber J, Ackermann R. Tumor cells carried through autotransfusion. Are these cells still malignant? Acta Anaesthesiol Belg 1984; 35(Suppl): 51-9.
Miller GV, Ramsden CW, Primrose JN. Autologous transfusion: An alternative to transfusion with banked blood during surgery for cancer. Br J Surg 1991; 78: 713-5.
Karczewski DM, Lema MJ, Glaves D. The efficiency of an autotransfusion system for tumor cell removal from blood salvaged during cancer surgery. Anesth Analg 1994; 78: 1131-5.
Dale RF, Kipling RM, Smith MF, Collier DS, Smith PJ. Separation of malignant cells during autotransfusion. Br J Surg 1988; 75: 581.
Perseghin P, Vigano M, Rocco G, Della Pona C, Buscemi A, Rizzi A. Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang 1997; 72: 221-4.
Liang TB, Li DL, Liang L, et al. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: Efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation 2008; 85: 863-9.
Dzik S. Leukodepletion blood filters: filter design and mechanisms of leukocyte removal. Transfus Med Rev 1993; 7: 65-77.
Edelman MJ, Potter P, Mahaffey KG, Frink R, Leidich RB. The potential for reintroduction of tumor cells during intraoperative blood salvage: reduction of risk with use of the RC-400 leukocyte depletion filter. Urology 1996; 47: 179-81.
Futamura N, Nakanishi H, Hirose H, Nakamura S, Tatematsu M. The effect of storage on the survival of cancer cells in blood and efficient elimination of contaminating cancer cells by a leukocyte depletion filter. Am Surg 2005; 71: 585-90.
Yamada T, Terai Y, Yamashita Y, Ueki M. Removal of malignant cells through a leukocyte-depletion filter for autologous blood transfusion. Int J Clin Oncol 1997; 2: 143-6.
Fruhauf NR, Dumpich O, Kaudel CP, Kasimir-Bauer S, Oldhafer KJ. Filtration of malignant cells: Tumour cell depletion in an ex vivo model using a leukocyte adhesion filter. Perfusion 2001; 16(Suppl): 51-5.
Kongsgaard U, Wang MY, Kvalheim G. Leucocyte depletion filter removes cancer cells in human blood. Acta Anaesthesiol Scand 1996; 40: 118-20.
Gwak MS, Lee KW, Kim SY, et al. Can a leukocyte depletion filter (LDF) reduce the risk of reintroduction of hepatocellular carcinoma cells? Liver Transpl 2005; 11: 331-5.
Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia 2008; 63: 1332-8.
Poli M, Camargo A, Villa L, Moura R, Colella R, Deheinzelin D. Intraoperative autologous blood recovery in prostate cancer surgery: in vivo validation using a tumour marker. Vox Sang 2008; 95: 308-12.
Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM. Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol 2005; 12: 1017-24.
Hansen E, Bechmann V, Altmeppen J. Intraoperative blood salvage in cancer surgery: Safe and effective? Transfus Apher Sci 2002; 27: 153-7.
Button LN, DeWolf WC, Newburger PE, Jacobson MS, Kevy SV. The effects of irradiation on blood components. Transfusion 1981; 21: 419-26.
Hansen E, Knuechel R, Altmeppen J, Taeger K. Blood irradiation for intraoperative autotransfusion in cancer surgery: demonstration of efficient elimination of contaminating tumor cells. Transfusion 1999; 39: 608-15.
Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 1984; 44: 3584-92.
Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology. Arch Surg 1986; 121: 1326-9.
Hart OJ 3rd, Klimberg IW, Wajsman Z, Baker J. Intraoperative autotransfusion in radical cystectomy for carcinoma of the bladder. Surg Gynecol Obstet 1989; 168: 302-6.
Fujimoto J, Okamoto E, Yamanaka N, et al. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg 1993; 128: 1065-9.
Zulim RA, Rocco M, Goodnight JE Jr, Smith GJ, Krag DN, Schneider PD. Intraoperative autotransfusion in hepatic resection for malignancy. Is it safe? Arch Surg 1993; 128: 206-11.
Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE. Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol 1995; 86: 373-8.
Mirhashemi R, Averette HE, Deepika K, et al. The impact of intraoperative autologous blood transfusion during type III radical hysterectomy for early-stage cervical cancer. Am J Obstet Gynecol 1999; 181: 1310-6.
Gray CL, Amling CL, Polston GR, Powell CR, Kane CJ. Intraoperative cell salvage in radical retropubic prostatectomy. Urology 2001; 58: 740-5.
Davis M, Sofer M, Gomez-Marin O, Bruck D, Soloway MS. The use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? BJU Int 2003; 91: 474-6.
Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today 2005; 35: 1042-6.
Muscari F, Suc B, Vigouroux D, et al. Blood salvage autotransfusion during transplantation for hepatocarcinoma: Does it increase the risk of neoplastic recurrence? Transplant Int 2005; 18: 1236-9.
Nieder AM, Carmack AJ, Sved PD, Kim SS, Manoharan M, Soloway MS. Intraoperative cell salvage during radical prostatectomy is not associated with greater biochemical recurrence rate. Urology 2005; 65: 730-4.
Stoffel JT, Topjian L, Libertino JA. Analysis of peripheral blood for prostate cells after autologous transfusion given during radical prostatectomy. BJU Int 2005; 96: 313-5.
Nieder AM, Manoharan M, Yang Y, Soloway MS. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology 2007; 69: 881-4.
MacIvor D, Nelson J, Triulzi D. Impact of intraoperative red blood cell salvage on transfusion requirements and outcomes in radical prostatectomy. Transfusion 2009; 49: 1431-4.
Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Surg Oncol 2011; 18: 166-73.
Waters JH, Yazer M, Chen YF, Kloke J. Blood salvage and cancer surgery: a meta‐analysis of available studies. Transfusion 2012; epub 10 Feb 2012. DOI: 10.1111/j.1537-2995.2011.03555.x.
Anonymous. Autologous blood transfusions. Council on Scientific Affairs. JAMA 1986; 256: 2378-80.
National Institute for Health and Clinical Excellence. Intraoperative Red Blood Cell Salvage During Radical Prostatectomy or Radical Cystectomy. April 2008. Available from URL: http://www.nice.org.uk/nicemedia/live/11891/40380/40380.pdf (accessed June, 2012).
Ferraris VA, Brown JR, Despotis GJ, et al. 2011 Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann Thorac Surg 2011; 91: 944-82.
Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus 2011; 9: 189-217.
Blajchman MA, Glynn SA, Josephson CD, Kleinman SH. Clinical trial opportunities in transfusion medicine: proceedings of a National Heart, Lung, and Blood Institute State-of-the-Science Symposium. Transfus Med Rev 2010; 24: 259-85.
Elias D, Billard V, Lapierre V. Use of the cell saver in oncologic surgery. Transfus Alternatives Transfus Med 2001; 3: 25-8.
Martin A, Braye A, Wellhausen S, Periper S. Detection of metastatic breast cancer cells in bone marrow: Flow cytometry vs automated image analysis. Am J Clin Pathol 2000; 114: 635 (abstract).
Acknowledgements
The authors sincerely thank Dr. Don Griesdale for critical appraisal of the manuscript.
Disclosures
None.
Funding sources/affiliations
None.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Jacqueline D. Trudeau was the primary author of this review and was responsible for the conception and design of the manuscript. Terrence Waters contributed to manuscript preparation and revision. Kate Chipperfield made substantial contributions to the conception, preparation, and revision of the manuscript.
Rights and permissions
About this article
Cite this article
Trudeau, J.D., Waters, T. & Chipperfield, K. Should intraoperative cell-salvaged blood be used in patients with suspected or known malignancy?. Can J Anesth/J Can Anesth 59, 1058–1070 (2012). https://doi.org/10.1007/s12630-012-9781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-012-9781-x