Abstract
Purpose of Review
Late stage at breast cancer diagnosis is a major contributor to poor survival from breast cancer in many sub-Saharan African countries. Using Ugandan as an example, we discuss barriers along the journey to diagnosis and highlight areas where improvements are needed.
Recent Findings
In Uganda, the majority of breast cancer patients noticed symptoms of their cancer for at least 9 months prior to diagnosis, which is typical of many African countries. For most women, the health provider delay is extensive, owing to barriers related to cost, transport, stigma, provider knowledge, and difficult-to-navigate referral systems.
Summary
Downstaging efforts should focus on reducing health system delay and target low community awareness. Implementation research to strengthen women’s breast cancer knowledge, access to breast health services, and referral pathways provide clear opportunities for downstaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sub-Saharan Africa (SSA) comprises 48 countries with a population of 1.0 billion people [1, 2]. Until recently, the main focus of SSA health systems has been on treating and preventing communicable diseases and improving child and maternal outcomes [3,4,5,6,7,8]. Successful SSA health interventions, particularly for immunization, maternal and child mortality, and HIV therapy have increased life expectancy [9], which, combined with changes in lifestyle, contributes to an increasing incidence of cancer, including breast [10, 11]. In 2013, cancer moved from being the third leading cause of death worldwide to second, only behind cardiovascular disease [12]. The most common cancer among women, in both high and low-income countries, is breast [12, 13]. However, incidence rates vary almost fourfold across the world regions with rates of 27 per 100,000 in Middle Africa, a UN subregion whose largest countries are the DR Congo, Angola, and Cameroon, to 92 per 100,000 in Northern America [13]. The International Agency for Research (IARC) estimates that by 2030, the global incidence burden of breast cancer will grow from 1.7 to 2.4 million with 0.7 million breast cancer deaths [13, 14]. More than 50% of these cases occur in low- and middle-income countries (LMICs) [11].
The variation in population-level mortality rates is not nearly as pronounced as the incidence rates and case fatality rates between high-income countries (HIC) and LMICs, which is secondary to better survival rates in developed countries [15]. For example, 5-year survival from breast cancer in North America and Oceania was 84–89%, but in SSA, ranges from 53% in South Africa, 48% in Uganda (Kagawa) to 12 and 13% in Mali and The Gambia [16]. Overall cancer survival in SSA (all cancer types) is disproportionately low due to an accumulation of barriers and disadvantages along the whole cancer journey, which include limited health care access, weak healthcare infrastructure, a paucity of health care workers with cancer training, advanced stage at diagnosis, few diagnostic and treatment centers, and, in some instances, reliance on traditional therapies and poor compliance with treatment regimens [17].
For breast cancer, drivers of these proximate causes are the many barriers African women experience to overcome access even to be diagnosed, and thereafter to obtain affordable and effective treatment. Amongst these aforementioned barriers, a fundamental milestone in the breast cancer journey is the stage at diagnosis, which is typically late in SSA rendering treatment less likely to be successful. In East Africa, Uganda serves as a prime example, where 64–89% of women with breast cancer are diagnosed with stage III or stage IV disease [18,19,20]. This is not atypical of the stage distribution in sub-Saharan Africa [21]. With this background, the purpose of this chapter is to describe factors contributing to late stage diagnosis in Uganda, as an example of an SSA country. It should be noted that early diagnosis of breast cancer alone is insufficient unless it is linked to treatment. Therefore, for the purposes of this article, we consider any factors that may ultimately contribute to late-stage diagnosis for treatment.
Current Situation in Uganda
Breast cancer incidence rates have increased over the last 20 years in Uganda [22]. The Ugandan population is young, thus the median age of breast cancer diagnosis is between 40 and 50. The limited health care budget and resources in Uganda are directed towards fighting communicable diseases [23]. For breast cancer diagnosis, in 2012, there were 4 mammography units, 2 in government and 2 in private health units, and 42 radiologists [24]. Although the government subsidizes some healthcare, the majority of the population has to self-fund their care. Treatment facilities include surgery (mostly mastectomy), chemotherapy, and radiotherapy are available at the Uganda Cancer Institute in the capital, Kampala, but radiotherapy was not available during 2015–17 [25].
Barriers to Early Diagnosis
Time to Health System Contact
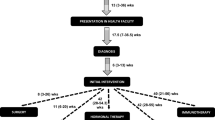
At present, diagnosis of breast cancer in the SSA setting concerns diagnosis of a symptomatic disease, with the majority of cases diagnosed at advanced stages. Currently, the majority of tumors are diagnosed with tumor diameters of 5 cm or larger [21], even though tumor of 2 cm are often palpable and was the mean diameter in pre-screening European countries [26]. In SSA, the vast majority of breast cancers are first appreciated by the woman herself based on breast symptoms and/or a persistent palpable lump or thickening. Cancer diagnosis requires that she then present to the health care system where her provider takes a history, performs a clinical breast examination, and then refers her to a higher level health care facility where imaging and tissue sampling for pathological diagnosis can be performed. This journey consists of two periods, meaning the time between when she first appreciates changes in the breast prior to first contact with the health care system (which when inordinately prolonged is referred to as “patient delay”) and the period between when she first presents for diagnosis and is subsequently diagnosed with cancer (which when inordinately prolonged is referred to as “system or provider delay”). Currently in Uganda, the median time from a woman recognizing a symptom to diagnosis of her breast cancer is 12 months, and for most women, the majority of this journey is after her first contact with the health system [20, 27].
The reasons for increased time to first contact with the health care system are often complex and dependent on where the woman live. At the most basic level, breast cancer awareness and knowledge of its potential cure is also generally low. In Uganda, over 90% of women are aware of breast cancer and approximately 60% know appropriate steps for early detection [28]. However, many women perceive little benefit from early diagnosis [18, 29]. Those who do mostly live in urban settings and perceive the primary benefit to early diagnosis as time afforded to get their estate in order [18, 29]. Espina et al. identified seven factors contributing to patient delay: [1] less education, [2] lack of breast cancer awareness, [3] type of initial symptom (i.e., painless mass), [4] fear, [5] belief in traditional medicine over Western medicine, [6] financial constraints, and [7] poor access to health care [10]. Fear can include fear of presumed inevitable death (fatalism), morbidity from treatment (e.g., disfigurement), being shunned by the community (stigma), and of losing support of loved ones [18, 30, 31]. Finally, SSA women living in rural areas are particularly vulnerable to late-stage diagnosis, partially due to the high cost of transportation and time taken to reach the health system [18, 32]. With the high cost of cancer diagnosis and treatment, fear of the costs of such a diagnosis can also contribute to delays in seeking help.
Time From Health System Contact to Diagnosis
In Uganda, the range of time between when a woman seeks medical care and receives a diagnosis was 7.9 to 15 months, with a mean time of more than 6 months [10]. In a systematic literature review, researchers identified six healthcare factors that contribute to this delay: (1) travel time to health care provider, (2) the number and type of healthcare provider contacted before diagnosis, (3) delay or non-referrals, (4) misdiagnosis, (5) wrong or false reassurance, and (6) delay in obtaining diagnostic work-up [10]. These delays manifested in five or more visits to a health care facility, each being a missed opportunity to shorten this prolonged time to diagnosis [10, 33].
In many SSA countries, particularly in rural communities, providers at primary care clinics—the first point-of-care for most women—are staffed by physician extenders (e.g., nurses or midwives). This is a key first contact point for women, which needs to be strengthened. While many of these physician extenders are likely to know that clinical breast examination (CBE) and history taking is an important step to evaluate symptomatic women, many have received inadequate training and may not recognize the signs of breast cancer, take an appropriate history, or perform a complete CBE. In Uganda, 15% of healthy women reported a history of receiving a CBE in the last 12 months and it is not clear whether this included a history of their symptoms, whether the CBE was complete, or the level of experience of the health provider who performed the examination [34].
When a woman with a suspected breast cancer reaches an appropriate breast cancer diagnostic facility, this is typically a tertiary hospital with imaging and pathology services, often in a major urban center or capital city. In SSA, there are few professionals trained in diagnostic imaging tests. For example, there are < 50 radiologists interpreting medical imaging in Uganda and two thirds practice in the capital, Kampala [35]. In Uganda, 70 % of diagnostic imaging is performed and interpreted by non-radiologists (sonographers); however, most of these health workers have received limited breast-specific training and have not learned an organized approach to interpreting breast imaging, communicating the results and appropriate referral steps [35]. This deficiency is essential to address in SSA because patients are usually responsible for organizing their own follow-up care and bringing their own imaging reports. The cost and limited access to medical imaging makes repeated imaging, secondary to unclear reports, contribute to increased medical expense, loss of confidence in medical care, and further delays diagnosis. The same applies to histological diagnosis, where some patients wait up to 3 months to receive their results from the pathologist. Often times, biopsy services are separated from pathologic interpretation and it is the patients’ responsibility to transport her tissue samples to the pathologist for interpretation and further navigate her follow-up care.
Efforts to Improve Early Diagnosis
A Framework for Early Diagnosis
When addressing the need for downstaging of breast cancer, different strategies are appropriate depending on the distribution of stage and resources, both financial and professional capacity, of a setting. When early diagnosis is shifted to diagnosis of asymptomatic breast cancer, it then becomes “screening,” i.e., diagnosis at a pre-clinical stage. In HICs, screening by mammography is used to detect pre-clinical breast cancer. Such a mammographic screening program is expensive, requires large logistical organization and support from a wide network of trained radiographers, radiologists, and pathologists. It is most effective in populations with high breast cancer incidence rates and in women with less dense breasts, neither of which characterize the lower incidence and younger patient profile—with denser breasts—in Uganda. Thus, mammographic screening is neither feasible, cost-effective, nor appropriate for the SSA setting at present. As a first step, earlier diagnosis of symptomatic disease is needed in SSA and, for these reasons, only other approaches to early detection of palpable lesions are discussed.
In the IARC Handbook of Cancer Prevention on Breast Screening, the expert panel concluded that there is sufficient evidence that screening by CBE alone shifts the stage distribution of tumors to a lower stage, though there was inadequate evidence that CBE alone reduced breast cancer mortality [36]. However, CBE provides opportunities for health providers to educate women about breast cancer and in other populations, it is associated with increased adherence to physician recommendations and a reduction in patient delay [37, 38]. Also, even in HICs, screening and reduction in mortality are strongly associated with strong referral systems. So, while CBE itself may not affect mortality rates, it will have other benefits. For breast self-examination (BSE), there was inadequate evidence that teaching this technique reduces breast cancer mortality, including in women who practice it competently and regularly. However, the prevalence of BSE in LMICs was too low to have any meaningful impact on mortality. Concerning ultrasonography, it was only evaluated in populations with access to mammography screening, thus those results are not relevant for the SSA general population [36, 39].
With this background, the Breast Health Global Initiative’s (BGHI) and American Cancer Society guidelines recommend programs to promote CBE and BSE in LMICs. BGHI identifies public education and awareness raising as the key first steps for cancer early detection in resource-constrained countries [40, 41]. Consequently, the Ugandan Breast Cancer Guidelines also recommend BSE for its practicability and affordability [33]. The benefits of CBE are not restricted to downstaging, nor are they guaranteed by downstaging. Downstaging through CBE will only reduce mortality if followed by a realized referral to treatment. Further, the benefits of CBE do not only concern the examination in question, but extend to future health seeking behaviors.
Policy and Advocacy
Efforts are underway to tackle early diagnosis and treatment of cancer in Uganda. At the national level, policy-level strategies are essential to increase the social acceptance of the disease and encourage women to seek medical care after self-detecting a breast problem. Several politicians have used their high-profile platforms to increase breast cancer awareness. For example, Ugandan parliament implemented an annual Cancer Day to raise awareness. On Cancer Day 2018, Rt. Hon. Rebecca Kadaga (the Speaker of Parliament of Uganda) spoke of “cancer getting the visibility it deserves.” She also commended the efforts of the Uganda Cancer Institute to implement cancer screening centers throughout Uganda by the end of 2019 [42]. However, lack of funding for non-communicable disease persists, as the majority of health funds are allocated to communicable diseases [11, 43]. In 2006, The Ugandan Ministry of Health established the Programme for the Prevention and Control of Non-Communicable Diseases (NCDs) [44]. This program was allocated 0.01% of the total Ministry of Health 2014–15 budget, approximately $27,000 US dollars [44]. An evaluation of progress made to support NCD agenda found continued insufficient funding; only 34.7% of the budgeted money for 2014–2015 was appropriately purposed for NCDs; inadequate manpower, < 70% of human capacity was made available to support NCD-related priorities, and there remained a general lack of accountability [45].
Another important advocacy and support group is the Uganda Women’s Cancer Support Organisation (UWOCASO), a breast cancer survivor group, who act as educators, patient navigators, and organize community events to increase breast cancer awareness [46]. UWOCASO also fund raises to support women with suspected or diagnosed breast cancer. As local survivors, they are uniquely qualified to dispel misconceptions associated with fatalism and support women dealing with stigma. Village health teams, 1–2 members of a community trained by providers at their local community health center to provide health education to other community members, could also learn new health information to increase awareness [47].
Breast Cancer Awareness in the General Population
Increasing breast cancer awareness through education is one facilitator for reducing time to first health system contact. Breast cancer education increases knowledge about the disease (e.g., risk factors, symptoms) and informs women about where to go when they self-detect breast symptoms. Breast cancer education should include teaching women BSE, i.e., to recognize changes in her breasts. In Uganda, 27% of healthy women had ever performed a BSE [34]. Women living in an urban region and participating in breast cancer education were more likely to perform BSE [20, 34]. Fatalism in one’s own breast cancer detection ability contributed to a reluctance of women to participate in BSE [48]. Based on the breast cancer guidelines set forth by Uganda in the 2nd edition (2008), the panel recommends promoting education about BSE. According to these guidelines, BSE should occur on every 10th day of the menstrual cycle. If a patient is symptomatic, then a “triple assessment” is implemented, which includes clinical assessment, breast imaging, and pathologic examination [33]. Providing breast cancer education remains an inexpensive and effective strategy to reduce the time to first presentation at a health care provider.
Population-level cancer education may also improve family support (e.g., time away from family responsibilities, arranging transportation, and financial support) for women seeking medical care. At least two studies partially attributed survivorship to spousal support for seeking medical care early after self-detecting breast symptoms [31, 49].
Health Professionals and the Health System
Achieving shorter times to first presentation with the health provider will only reap benefit if the provider appropriately refers and a diagnosis can be made. Several modes of strengthening these referral and diagnostic pathways have been suggested, and some feasibility studies have already been conducted. Local focal points at health centers and district hospitals have been suggested as key in the early detection and referral process. The local focal point—with dedicated space and fixed time allotment—is a health professional educated in breast cancer awareness, disseminates information to the community, and conducts CBE during regular scheduled clinics. Where needed, this focal point ensures women with suspected tumors are referred and reach the diagnostic centers or the next level health facility, as appropriate. In this way, women and time should not be lost in the current difficult-to-navigate referral systems. With high mobile phone penetration rates in Africa, modern approaches to tighten this navigation pathway using mHealth technologies and linked health system information technology management systems may be a significant game changer in this setting. Currently, such systems are under-developed.
Given the limited healthcare capacity to evaluate symptomatic women, a task shifting option—similar to that adopted for HIV—is to further train non-radiologists to perform, interpret, and make recommendations on breast ultrasound so as to reduce health system delay between presentation and treatment. Some groups in Uganda have trained non-radiologists to perform and interpret breast ultrasound and have performed medical audits to support continued quality improvement and implement a standardized approach for interpreting and reporting breast imaging examinations [35]. A medical audit of breast ultrasound practices at tertiary centers in Uganda showed 50% of biopsies could have been avoided (based on benign findings) using a systematic approach to interpreting and reporting breast ultrasound [32]. Others have piloted strategies, such as moving triage ultrasound to a more local facility so as to remove the time-cost-travel distance barrier when using imaging to evaluate a positive CBE finding [50]. While this pilot study included only a small number of women (N = 212) and had no control group, moving ultrasound to a more local facility reduced late-stage presentation from historical averages of 64–89 to 50%. A similar approach to decentralized tissue sampling and pathological diagnosis may further reduce health system delays, although to sustain this approach, these services may not be available every day. Nevertheless, CBE-based screening programs with locally available ultrasound offer a potential low-cost high availability strategy to improve early detection in SSA.
Finally, while a discussion of Ugandan women’s access to timely and effective treatment is outside the scope of this chapter, approximately 80% of breast cancer will require oncologic surgery and 75% will not get the appropriate treatment in the timeframe required [49, 51]. Improving effective treatment options in coordination with early detection efforts is key in reducing breast cancer mortality. Again, expanding on the 2008 WHO guidelines that focused on management of HIV, task shifting some medical jobs to non-physicians to build capacity and in a similar fashion, task shifting to “strengthen and expand the health workforce” [52] can be applied to breast cancer diagnosis to increase the sustainability of diagnosis efforts [23].
References
The World Bank. World Bank Open Data. 2018 [cited 2018 April 2]; Available from: https://data.worldbank.org/region/sub-saharan-africa?view=chart.
Dingemanse M. Spoken languages of African countries—Nations Online Project. 2018 [cited 2018 March 27]; Available from: http://www.nationsonline.org/oneworld/african_languages.htm.
Jones-Hepler B, Moran K, Griffin J, McClure EM, Rouse D, Barbosa C, et al. Maternal and Neonatal Directed Assessment of Technologies (MANDATE): methods and assumptions for a predictive model for maternal, fetal, and neonatal mortality interventions. Glob Health Sci Pract. 2017;5(4):571–80.
Kawooya MG, Nathan RO, Swanson J, Swanson DL, Namulema E, Ankunda R, et al. Impact of introducing routine antenatal ultrasound services on reproductive health indicators in Mpigi District, Central Uganda. Ultrasound Q. 2015;31(4):285–9.
Nathan R, Swanson JO, Marks W, Goldsmith N, Vance C, Sserwanga NB, et al. Screening obstetric ultrasound training for a 5-country cluster randomized controlled trial. Ultrasound Q. 2014;30(4):262–6.
Swanson JO, Kawooya MG, Swanson DL, Hippe DS, Dungu-Matovu P, Nathan R. The diagnostic impact of limited, screening obstetric ultrasound when performed by midwives in rural Uganda. J Perinatol. 2014;34(7):508–12.
Rice B, Boulle A, Baral S, Egger M, Mee P, Fearon E, et al. Strengthening routine data systems to track the HIV epidemic and guide the response in sub-Saharan Africa. JMIR Public Health Surveill. 2018;4(2):e36.
Lessler J, Moore SM, Luquero FJ, McKay HS, Grais R, Henkens M, et al. Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales. Lancet. 2018;391:1908–15.
Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–5.
Espina C, McKenzie F, Dos-Santos-Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann Epidemiol. 2017;27(10):659–71 e7.
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–60.
Global Burden of Disease Cancer C, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27.
International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012 [cited 2018 March 27]; Available from: http://globocan.iarc.fr/Pages/DataSource_and_methods.aspx.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75.
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010.
Kingham TP, Alatise OI, Vanderpuye V, Casper C, Abantanga FA, Kamara TB, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e158–67.
Meacham E, Orem J, Nakigudde G, Zujewski JA, Rao D. Exploring stigma as a barrier to cancer service engagement with breast cancer survivors in Kampala, Uganda. Psychooncology. 2016;25(10):1206–11.
Hayes Constant TK, Winkler JL, Bishop A, Taboada Palomino LG. Perilous uncertainty: situating women’s breast-health seeking in northern Peru. Qual Health Res. 2014;24(6):811–23.
McKenzie F, Zietsman A, Galukande M, Anele A, Adisa C, Parham G, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer—disparities in outcomes (ABC-DO) study. Int J Cancer. 2018;142(8):1568–79.
Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2016;4(12):e923–e35.
Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991-2010. Int J Cancer. 2014;135(2):432–9.
Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10(1):89–92.
Monu JU, Muyinda Z, Taljanovic M. ISS outreach sub-Saharan Africa insight: Uganda 2011. Skelet Radiol. 2012;41(11):1347–8.
Ministry of Health. Cobalt-60 radiotherapy machine installed—radiotherapy services restored at Uganda Cancer Institute Republic of Uganda 2018 [cited 2018 March 27]; Available from: http://health.go.ug/content/cobalt-60-radiotherapy-machine-installed-–-radiotherapy-services-restored-uganda-cancer.
Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10(3):R41.
Galukande M, Mirembe F, Wabinga H. Patient delay in accessing breast cancer care in a sub Saharan African country: Uganda. Br J Med Med Res. 2014;4(13):2599–610.
Atuhairwe C, Amongin D, Agaba E, Mugarura S, Taremwa IM. The effect of knowledge on uptake of breast cancer prevention modalities among women in Kyadondo County, Uganda. BMC Public Health. 2018;18(1):279.
Muthoni A, Miller AN. An exploration of rural and urban Kenyan women’s knowledge and attitudes regarding breast cancer and breast cancer early detection measures. Health Care Women Int. 2010;31(9):801–16.
Scheel JR, Molina Y, Anderson BO, Patrick DL, Nakigudde G, Gralow JR, et al. Breast cancer beliefs as potential targets for breast cancer awareness efforts to decrease late-stage presentation in Uganda. Journal of Global Oncology. 2018; https://doi.org/10.1200/JGO.2016.008748.
Koon KP, Lehman CD, Gralow JR. The importance of survivors and partners in improving breast cancer outcomes in Uganda. Breast. 2013;22(2):138–41.
Scheel JR, Nealey EM, Orem J, Bugeza S, Muyinda Z, Nathan RO, et al. ACR BI-RADS use in low-income countries: an analysis of diagnostic breast ultrasound practice in Uganda. J Am Coll Radiol. 2016;13(2):163–9.
Gakwaya A, Galukande M, Luwaga A, Jombwe J, Fualal J, Kiguli-Malwadde E, et al. Breast cancer guidelines for Uganda (2nd edition 2008). Afr Health Sci. 2008;8(2):126–32.
Scheel JR, Molina Y, Patrick DL, Anderson BO, Nakigudde G, Lehman CD, et al. Breast cancer downstaging practices and breast health messaging preferences among a community sample of urban and rural Ugandan women. J Glob Oncol. 2017;3(2):105–13.
Scheel JR, Peacock S, Orem J, Bugeza S, Muyinda Z, Porter PL, et al. Improving breast ultrasound interpretation in Uganda using a condensed breast imaging reporting and data system. Acad Radiol. 2016;23(10):1271–7.
Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, et al. Breast-cancer screening—viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–8.
Romanoff A, Constant TH, Johnson KM, Guadiamos MC, Vega AMB, Zunt J, et al. Association of previous clinical breast examination with reduced delays and earlier-stage breast cancer diagnosis among women in Peru. JAMA Oncol. 2017;3(11):1563–7.
Scheel JR, Molina Y, Coronado G, Bishop S, Doty S, Jimenez R, et al. Healthcare factors for obtaining a mammogram in Latinas with a variable mammography history. Oncol Nurs Forum. 2017;44(1):66–76.
International Agency for Research on Cancer. Breast cancer screening. Lyon: IARC; 2014.
Yip CH, Smith RA, Anderson BO, Miller AB, Thomas DB, Ang E, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2009;113(8 Suppl):2244–56.
McTiernan A. Guideline implementation for breast healthcare in low- and middle-income countries. Cancer. 2008;113(S8):2244–56.
Mwebembezi E. Uganda commemorates World Cancer Day 2018. World Health Organisation; 2018 [cited 2018 March 27]; Available from: http://www.afro.who.int/news/uganda-commemorates-world-cancer-day-2018.
Kangmennaang J, Mkandawire P, Luginaah I. Breast cancer screening among women in Namibia: explaining the effect of health insurance coverage and access to information on screening behaviours. Glob Health Promot. 2017; https://doi.org/10.1177/1757975917727017.
Schwartz JI, Guwatudde D, Nugent R, Kiiza CM. Looking at non-communicable diseases in Uganda through a local lens: an analysis using locally derived data. Glob Health. 2014;10:77.
Essue BM, Kapiriri L. The unfunded priorities: an evaluation of priority setting for noncommunicable disease control in Uganda. Glob Health. 2018;14(1):22.
Nakigudde G. The Uganda Women’s Cancer Support Organisation: About Us. 2017 [cited 2018 March 27]; Available from: http://uwocaso.org.ug/about-us/.
Ali N, Njeri A, Ssembatya R, Matovu A, Streeter M, DeStigter K. Using village health teams to disseminate ultrasound education in rural Uganda. JGR. 2015;1(2)
Lukong KE, Ogunbolude Y, Kamdem JP. Breast cancer in Africa: prevalence, treatment options, herbal medicines, and socioeconomic determinants. Breast Cancer Res Treat. 2017;166(2):351–65.
Hulett JM, Armer JM, Stewart BR, Wanchai A. Perspectives of the breast cancer survivorship continuum: diagnosis through 30 months post-treatment. J Pers Med. 2015;5(2):174–90.
Matovu A, Scheel JR, Galukande M, DeStigter KK. Implementation of a resource-appropriate strategy for downstaging breast cancer in rural Uganda. JGR. 2016;2(1):1–6.
Vanderpuye V, Grover S, Hammad N, PoojaPrabhakar, Simonds H, Olopade F, et al. An update on the management of breast cancer in Africa. Infect Agent Cancer. 2017;12:13.
World Health Organization. Task shifting: global recommendations and guidelines. 2008 [cited 2018 March 28]; Available from: http://www.who.int/workforcealliance/knowledge/resources/taskshifting_guidelines/en/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Global Breast Cancer
Rights and permissions
About this article
Cite this article
Scheel, J.R., Anderson, S., Foerster, M. et al. Factors Contributing to Late-Stage Breast Cancer Presentation in sub-Saharan Africa. Curr Breast Cancer Rep 10, 142–147 (2018). https://doi.org/10.1007/s12609-018-0278-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-018-0278-7