Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous disease with different biological characteristics and treatment outcomes compared to other subtypes of breast cancer. Recently, platinum-based chemotherapy has received increased attention in TNBCs based on promising results especially in the neoadjuvant setting. Homologous recombination deficiency (HRD) and tumor infiltrating lymphocytes (TILs) are new emerging biomarkers associated with platinum efficacy. Despite heterogeneity of methods used for HRD assessment, results of published studies showed consistently the predictive value of HRD for efficacy of platinum-based chemotherapy, especially in the neoadjuvant setting. Prognostic value of TILs in TNBCs is consistent across the trials and while the predictive value of TILs for platinum-based therapy in TNBCs is promising, it needs to be confirmed in further trials. Further research should focus on prospective validation of these biomarkers to confirm their clinical utility, while combination of these markers could lead to composite biomarkers that mirror both tumor and microenvironment properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
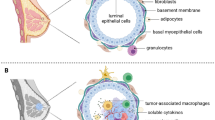
Breast cancer is one of the most common malignancies in women in western countries, with an estimated 231,840 new cases diagnosed in the USA since the beginning of 2015 [1]. Triple-negative breast cancer (TNBC) represents approximately 10–20 % of all breast cancers [2•]. It is defined by immunohistochemistry as a tumor that does not overexpress the human epidermal growth factor receptor 2 (HER2) and that lacks expression of estrogen (ER) and progesterone receptors (PR). TNBC expressing epidermal growth factor receptor and cytokeratin 5/6 constitute tumors with core basal phenotype with significantly worse outcome within TNBC and represents about 53 % of TNBCs [3]. TNBC is a heterogeneous group with different biological characteristics and treatment outcomes in metastatic as well as in adjuvant settings compared to other subtypes of breast cancer [2•]. Therefore, identification of biomarkers should be important to improve treatment selection in this type of breast cancer.
TNBC is associated with special clinical and biological features that affect women of younger age compared to the overall population of breast cancer patients, particularly women of African American or West African ancestry, and women of low socioeconomic status [4, 5]. TNBC is associated with higher grade and tumor stage at the time of presentation. TNBC patients have a higher risk of distal recurrence and death and have a higher likelihood of developing central nervous system and visceral metastases than those with non-TNBC [6–8]. While median survival of metastatic non-TNBC is 28 months, median survival of metastatic TNBC is approximately 10 months [9]. Similarly, among 180 women with primary TNBC, distant recurrence-free survival was about 66 % compared to 80 % of patients with non-TNBC [10].
TNBC represents a heterogeneous disease, despite immunohistochemical definition, this subtype of breast cancer, beyond invasive ductal carcinoma includes several other histological entities like metaplastic, medullary, secretory, and/or apocrine breast cancer [11–15]. Genomic profiling revealed six distinct molecular subtypes of TNBC including basal-like 1 and 2 (BL1 and BL2), an immunomodulatory (IM), a mesenchymal-like (M), a mesenchymal stem-like (MSL), and a luminal androgen receptor (LAR) subtype [16••]. TNBC exhibits a high level of genomic instability and recent data suggest that TNBC is oligoclonal or multiclonal at the time of diagnosis, and this clonality is more prominent than in other breast cancer subtypes [17]. For instance, a recent study showed that only 50 % of cancer cells exhibit p53 or PIK3CA mutations. This phenomenon has important clinical consequences as tumors possess a potential driver mutation in a fraction of subclones with a high probability of overgrowths of different subclones that are not inhibited with targeted therapy [16••].
Standard predictive factors for breast cancer treatment selection are hormone-receptor status for endocrine therapy and HER2 amplification for anti-HER2 therapy [18]. However, due to lack of these therapeutic targets, cytotoxic chemotherapy remains the only standard option of systemic therapy in TNBC [18]. Therefore, a better understanding of this subtype of breast cancer is important to identify new therapeutic targets as well as biomarkers for individualization of treatment.
In recent years, platinum-based chemotherapy received increased attention in TNBCs based on promising results in the neoadjuvant setting, where addition of platinum to the chemotherapeutic regimen in TNBCs was associated with increased likelihood to achieve a pathological complete response (pCR) especially in patients with BRCA1 mutations [19••, 20•, 21•]. As platinum-based chemotherapy is associated with substantial toxicity (mainly hematological toxicity for carboplatin, nephro-, neuro- and ototoxicity for cisplatin), identification of predictive biomarkers is very important. In the last few years, two categories of biomarkers associated with platinum agents had increased attention—markers of homologous recombination deficiency and tumor infiltrating lymphocytes. In this article, we review these biomarkers in TNBCs in the context of platinum-based therapy.
Molecular Profiles of TNBC
In a seminal study, Perou and colleagues identified four intrinsic subtypes of breast cancer using gene expression profiling: luminal, HER2 positive, normal, and basal-like subtype [22]. Claudin-low subtype characterized by decreased expression of genes related to cell-to-cell adhesions and tight junctions were identified later [23]. While, there is a significant overlap between basal-like subtype of breast cancer and TNBC, these categories are not the same. TNBC is defined based on immunohistochemical analysis of ER/PR and HER2 receptors, and basal-like subtype is defined based on the gene expression profile of tumor that is similar to normal basal cells. Initial studies showed that approximately 65 % of TNBC are basal like, while, only 55 % of basal-like breast tumors are triple negative. Therefore, further genomic studies analyzed gene expression profile of TNBC, and six distinct molecular subtypes of TNBCs were revealed (Table 1) [16••].
Basal-like 1 and 2 subtypes of TNBC are characterized by high expression of genes involved in proliferation, cell cycling, and cell division. In the BL1 molecular profile, there is enrichment for DNA damage response genes suggesting an increased sensitivity to DNA-damaging agents including cisplatin and carboplatin while, the BL2 molecular profile is associated with expression of genes related to growth factor signaling. The immunomodulatory subtype is distinguished based on expression of genes involved in innate and adaptive immunity, and this profile is a consequence of lymphocytic infiltration of tumor tissue. Compared to other subtypes, the molecular profile of the IM subtype mirrors gene expression in tumor microenvironment and not directly in cancer cells, while true tumor gene expression profile in the IM subtype likely includes tumors that are biologically of basal-like and mesenchymal-like subtypes [16••]. Recently, lymphocytic infiltration was shown to be prognostic in several types of tumors including TNBC [24, 25]. The mesenchymal and mesenchymal stem-like subtypes are characterized by enrichment of genes involved in epithelial to mesenchymal transition. MSL overlaps with the claudin-low breast cancer subtype and is associated with low proliferation and expression of genes related to cancer stem cell phenotype. Mesenchymal-like subtypes show activation of multiple growth factor signaling pathways like transforming growth factor beta (TGF-β) signaling and high expression of several growth factors like EGFR (epidermal growth factor receptor), PDGFR (platelet-derived growth factor), and FGF2 (fibroblast growth factor 2) with autocrine and paracrine activation [16••]. Metaplastic breast cancer is typically associated with mesenchymal-like molecular profile [14]. The luminal androgen receptor subtype is a distinct subtype characterized by significant overexpression of androgen receptor (AR) and activation of signaling involved in steroid synthesis, porphyrin metabolism, and androgen/estrogen metabolism despite lack of estrogen receptor expression [16••].
Molecular profiling of TNBC is important not only from the biological point of view, but recent studies showed clinical validity/utility of gene expression profiles [26]. Retrospective analysis of 130 TNBC tumors treated with neoadjuvant chemotherapy showed that pathological complete response (pCR) was associated with a specific molecular profile. Patients with a BL1 subtype achieved pCR in 52 % of the time after antracycline and taxane-based chemotherapy, whereas tumors with BL2, MSL and LR subtypes achieved pCR in 0, 23, and 10 %, respectively [27••]. In another trial (GeparTrio phase III trial), women with a LAR subtype had a lower pCR rate after chemotherapy, compared to other subtypes (13 % vs. 25 %) that suggested the relative chemoresistance of the LAR subtype [28•].
Genomic Biomarkers Associated with Efficacy of Platinum-Based Therapy in TNBCs
Germline mutations of BRCA1 and BRCA2 genes are responsible for a considerable proportion of hereditary breast cancer. These genes encode proteins involved in DNA repair via homologous recombination of double-stranded DNA damage [28•]. BRCA1, but not BRCA2-mutated tumors are typically triple negative and have a basal-like molecular profile, suggesting sensitivity of these tumors to DNA-damaging agents including platinum-based chemotherapy regimen [29]. Based on association between genomic instability, BRCA inactivation, and response to DNA-damaging agents, several studies measure genomic instability as a marker of homologous recombination deficiency (HRD) and response to platinum-based chemotherapy in TNBCs. Genomic instability was usually quantified as a score of telomeric allelic imbalance, loss of heterozygosity (LOH), and/or larger scale transition scores, while HRD score is usually significantly associated with BRCA1 and BRCA2 mutations (tmBRCA) [30–35]. Recently, it was showed that the HRD score is highly conserved across multiple repeat biopsies from different regions of the same cancer [36].
Platinum-based regimens were recently studied in several neoadjuvant trials in TNBCs, and these studies as well as meta-analysis suggest the effectivity of platinum in TNBCs [37•, 38–40]. In recently reported I-SPY 2 trial, combination of veliparib (PARP inhibitor) and carboplatin with standard neoadjuvant chemotherapy increased pCR from 26 to 52 % in TNBC patients [37•]. Addition of cisplatin/carboplatin to the neoadjuvant regimen consistently increases the pCR rate in about 15 % [20•, 40, 41•, 42, 43]. Meta-analysis of 11 randomized neoadjuvant trials revealed that pCR rate in carboplatin-containing arms were significantly higher than in control arms (odds ratio = 1.80, 95 % CI 1.39–2.32, p < 0.001). The addition of carboplatin was associated with a pCR rate as high as 51.2 % with an absolute pCR difference of 13.8 % as compared with control regimens [40]. However, despite improvement of pCR, the role of platinum in neoadjuvant setting is still controversial mainly due to unknown impact on relapse-free or overall survival. Therefore, identification of predictive biomarkers related to the response to platinum-based therapy is very important. In recent years, several new biomarkers related to efficacy of platinum-based therapy in TNBCs were evaluated (Table 2) [20•, 41•, 42–44].
The GeparSixto trial was a neoadjuvant study aimed to determine the efficacy of adding carboplatin to neoadjuvant chemotherapy for TNBC and HER2-positive breast cancer. The addition of carboplatin significantly improved the pCR rate in patients with TN, but not HER2-positive breast cancer; pCR rates of 53.2 vs. 36.9 %, p = 0.005 in TNBCs and 32.8 vs. 36.8 %, p = 0.58 in HER2-positive breast cancer, respectively [19••]. Further biomarker analysis aimed to determine biomarkers associated with efficacy of carboplatin. Formalin-fixed, paraffin-embedded (FFPE) tumor tissues were available in 193/315 patients (61.3 %) enrolled into the study. Researchers determined the HRD score and tumor BRCA1/2 mutations (tmBRCA) for each sample and defined HR deficiency as either tmBRCA positive and/or high HRD score (≥42) defined as the unweighted sum of LOH scores [29, 31, 32]. In this analysis, 70.5 % of tumors were HR deficient, 30.3 % exhibit tumor BRCA1/2 mutations, and 19.8 % exhibited germ-line BRCA1/2 mutation. HR-deficient tumors were more likely to achieve pCR as opposed to HR-non-deficient tumors (55.9 vs. 29.8 %, p = 0.001). Addition of carboplatin to neoadjuvant therapy increased the pCR rate in HR-deficient tumors but not in HR-non-deficient tumors (64.9 vs. 45.2 %, p = 0.025). In patients with non-tmBRCA, a high HRD score was associated with a higher pCR rate than a low HRD score (49.4 vs. 30.9 %, p = 0.05), irrespective of the use of carboplatin. Based on these data, authors concluded that HR deficiency in TNBC and high HRD score in non-tmBRCA TNBC are predictors of response to neoadjuvant anthracycline and taxane-containing chemotherapy, irrespective of the use of carboplatin. These data suggest that HR deficiency may be a predictive biomarker to identify patients with the highest likelihood of benefit to DNA-damaging agents [20•].
In another neoadjuvant study, treatment with four cycles of cisplatin was associated with a 61 % pCR rate in a cohort of 107 BRCA1-mutated breast cancer patients [21•]. The probability of achieving a pCR by another chemotherapy regimen in this patient population was significantly lower, supporting the role of BRCA1 mutation as a biomarker of platinum effectivity in neoadjuvant setting [41•].
In a phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancers, the HRD-LOH (loss of heterozygosity) score was significantly associated with pCR [42]. Subsequently, a predictive value of the combined HRD score, defined as an unweighted sum of LOH, telomeric allelic imbalance (TAI), and large-scale state transitions (LST) scores were reported. The study showed that TNBCs with a HRD score of ≥42 were significantly associated with pCR when treated with neoadjuvant platinum-based chemotherapy and moreover identified responders lacking deleterious BRCA1/2 mutation [43].
In another neoadjuvant trial, the number of subchromosomal regions with allelic imbalance extending to the telomere (NtAI) predicted cisplatin sensitivity in vitro and pathologic response to preoperative cisplatin treatment in TNBC patients [30].
In the adjuvant setting, the data are much more limited. In a study by Vollebergh et al., patients with stage III HER2-negative breast cancer with BRCA1 loss as assessed by comparative genomic hybridization (CGH) analysis had substantially improved the overall survival after adjuvant high-dose platinum-based chemotherapy that induced double-strand breaks compared with the standard anthracycline-based chemotherapy [34].
In metastatic TNBCs, single-arm phase II trial evaluated biomarkers that could predict the efficacy of cisplatin/carboplatin. This study showed that BRCA-like genomic instability signature discriminated responding and non-responding tumors. This signature was based on determination of mean homologous recombination deficiency-loss of heterozygosity/homologous recombination deficiency-large-scale state transitions (HRD-LOH/HRD-LST) scores [45]. The TNT trial was a randomized phase III trial of carboplatin compared with docetaxel for patients with metastatic or recurrent locally advanced triple-negative or BRCA1/2 breast cancer. In this trial, there was no difference in objective response (31.4 % in carboplatin arm vs. 35.6 % in docetaxel arm, p = 0.44) nor differences in progression-free and overall survival in unselected study population. However, among the BRCA-positive patients, the difference between arm was significant with objective response rate of 68 % with carboplatin and 33 % with docetaxel (p = 0.03). Opposite to neoadjuvant trials, the HRD score did not select for sensitivity to carboplatin over docetaxel [46].
Immune-Based Biomarkers Associated with Efficacy of Platinum-Based Therapy in TNBCs
Increasing data suggest a prognostic value of immune cells in different types of cancer, including breast cancer. Infiltration of tumor with different subtypes of immune cells is closely associated with prognosis and/or response to anticancer treatment [24, 25].
One of the first studies that assessed the association between tumor-infiltrating lymphocytes (TILs) and prognostic outcome was published more than 60 years ago, and in recent years, emerging data support this association [47]. Tumor infiltrating cells includes T-lymphocytes, B-lymphocytes, macrophages, and dendritic cells. It was shown that a vast majority of TILs are CD3+ T-cells, which can be further divided into CD4+ (T-helper), CD8+ (cytotoxic T-cells), and T-regulatory cells (T-regs). Most data support the prognostic value of cytotoxic T-cells, and studies showed that the total number of these cells, including intratumoral CD8+ T-cells and CD8+ T-cells in proximal and distal tumor stroma are associated with better prognosis [48]. Further studies showed, that this association is mainly related to hormone-receptor negative and TN breast cancer. Lymphocyte-predominant breast cancer (LPBC) is defined as tumors with more than 50–60 % lymphocytic infiltrates and is associated with better prognosis compared to breast cancer with lower lymphocytic infiltrates.
The prognostic value of TILs was shown in numerous retrospective and prospective trials in TNBCs [48–52]. Recent meta-analysis that included 2987 patients with early-stage BC with a median follow-up of 113 months showed that tumors rich in TILs were associated with improved recurrence-free survival (hazard ratio [HR] = 0.70, 95 % CI 0.56–0.87, p = 0.001) as well as the overall survival (HR = 0.66, 95 % CI 0.53–0.83, p = 0.0003). Moreover, every 10 % increment in TILs was associated with a 15–20 % reduction in recurrence or mortality. In addition, tumors rich in TILs were predictive of improved OS, irrespective of the disease phenotype (TNBC or basal phenotype) and/or TILs distribution (intratumoral or stromal), or subtype of TILs (e.g., TILs-non-specified, cytotoxic (CD8+), or regulatory (forkhead box protein 3, FOXP3+) T-cells [53]. In primary breast cancer, patients with LPBC had an increased probability to achieve pCR as opposed to patients without LPBC (40 vs. 4 %, p = 0.01), and this difference is most striking in TNBCs [54].
As we previously discussed, HR deficiency is a potential biomarker associated with efficacy to platinum agents in GeparSixto trial, a neoadjuvant trial aimed to determine the value of addition of carboplatin to antracycline/taxane-based regimen [18]. The authors of this study assessed TILs as another biomarker associated with treatment response to carboplatin. This biomarker analysis substudy showed that stromal TILs predicted pCR, and this was confirmed in multivariate analysis. The pCR was 59.9 % in LPBC compared to 33.8 % in non-LPBC (p = 0.001). In LPBCs patients treated with a carboplatin-based regimen, the pCR rate was 75 %, suggesting that TILs are predictive of pCR especially in TNBCs treated with platinum-based chemotherapy [55••].
Conclusions
Different treatment regimens combining platinum salts with chemotherapies such as taxanes, vinorelbine, or gemcitabine have been evaluated in metastatic as well as neoadjuvant setting in breast cancer. Platinum-based chemotherapy has been postulated to be specifically efficacious in tumors with impaired DNA repair pathways such as in specific subsets of TNBCs. However, no prospective trials have been reported that demonstrate a survival advantage to such regimens compared with non-platinum agents. Therefore, platinum-containing regimens are usually reserved for women with good performance status, but high disease burden, whose disease has progressed on other available chemotherapy agents [56]. Considering substantial toxicity associated with platinum-based chemotherapy identification of predictive biomarkers are very important. TNBC is a heterogeneous disease; therefore, clinical trials assessing efficacy of new drugs should incorporate biomarkers for treatment selection that enrich for population with the highest likelihood of achieving therapeutic response and, at the same time, enable biomarker validation in prospective setting. Several new biomarkers and therapeutic targets are emerging in TNBC including growths factor signaling pathways, microRNAs, circulating tumor cells and/or circulating tumor DNA. Two classes of new predictive biomarkers, homologous recombination deficiency and tumor infiltrating lymphocytes that are associated with platinum effectivity in TNBCs patients, have received increased attention in recent years. Although HRD is directly linked to cancer cell DNA repair ability after DNA damage, TILs are part of the tumor microenvironment that modulates the host response to DNA-damaging agents. Despite heterogeneity of methods used for HRD assessment, results of available studies consistently showed a predictive value of HRD for efficacy of platinum-based chemotherapy in TNBCs. Availability of fresh frozen tissue might be one of the limitation for their routine use outside of clinical trials, and reproducibility across laboratories and prospective validation are another requirements for their application in daily clinical practice. Utility of HRD is limited to a small proportion of TNBC, and therefore, new biomarkers and therapeutic targets should be identified and validated for the other subtypes of TNBC as well. While data on prognostic value of TILs in TNBCs are consistent across the trials, predictive value of TILs for platinum-based therapy in TNBCs remains limited. As TILs are heterogeneous population of immune cells infiltrating tumor tissue, identification of specific subsets of TILs specifically associated with platinum efficacy could further improve their clinical values as biomarkers. Further research should focus on prospective validation of these biomarkers to confirm their clinical utility in treatment selection process, while combination of them could lead to composite marker that mirrors both tumor and microenvironment properties.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7(12):683–92. Comprehensive article summarizing importance of TNBC.
Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–76.
Bauer K, Brown M, Cress R, Parise C, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population based study from the California Cancer Registry. Cancer. 2007;109(9):1721–8.
Rakha E, El-Sayed M, Green A, Lee A, Robertson J, Ellis I. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32.
Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17–22.
Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84.
Hines LM, Risendal B, Byers T, Mengshol S, Lowery J, Singh M. Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. J Womens Health (Larchmt). 2011;20(10):1543–50.
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34.
Wetterskog D, Lopez-Garcia MA, Lambros MB, A'Hern R, Geyer FC, Milanezi F, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol. 2012;226(1):84–96.
Lae M, Freneaux P, Sastre-Garau X, Chouchane O, Sigal-Zafrani B, Vincent-Salomon A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol. 2009;22(2):291–8.
Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, et al. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17(4):605–13.
Hennessy BT, Krishnamurthy S, Giordano S, Buchholz TA, Kau SW, Duan Z, et al. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23(31):7827–35.
Montagna E, Maisonneuve P, Rotmensz N, Cancello G, Iorfida M, Balduzzi A, et al. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer. 2013;13(1):31–9.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. Seminal paper that, for the first time describes molecular subtytes within TNBC.
Hu X, Stern HM, Ge L, O'Brien C, Haydu L, Honchell CD, et al. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol Cancer Res. 2009;7(4):511–22.
Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–52.
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. Important neoadjuvant trial that showed increased pCR in TNBC patients that received carboplatin as a part of neoadjuvant chemotherapy.
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. Another large neoadjuvant trial that suggest effectivity of carboplatin in neodjuvant setting.
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–5. Study that showed striking efficacy of platinum-based chemotherapy in patients with BRCA1-associated breast cancer.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68.
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–605.
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–92.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–50.
Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232(2):142–50. Excellent review article of biomarkers in TNBC with clinical implications.
Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533–40. Study that revalidated molecular subtypes in TNBC.
von Minckwitz G, Fontanella C. Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast. 2013;22 Suppl 2:S149–51.
Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17(5):1082–9.
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2(4):366–75.
Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18(20):5806–15.
Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72(21):5454–62.
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776–82.
Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22(7):1561–70.
Telli M, Jensen KC, Abkevich V, Hartman AR, Vinayak S, Lanchbury J, et al. Homologous recombination deficiency (HRD) score predicts pathologic response following neoadjuvant platinum based therapy in triple-negative and BRCA1/2 mutation associated breast cancer. Cancer Res. 2012;72:suppl; abstr PD09-04.
Timms K, Chagpar AB, Wali V, Bossuyt V, Reid JE, Gutin A, et al. Reproducibility of homologous recombination deficiency (HRD) scores in biopsies of triple negative breast cancer (TNBC) tumors. J Clin Oncol. 2015;33:suppl; abstr 1091. Study that revealed high reproducibility of HRD score in biopsies in TNBC.
Rugo HS, Olopade O, DeMichele A, van 't Veer L, Buxton M, Hylton N, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: first efficacy results from the I-SPY 2 trial. 2013 San Antonio Breast Cancer Symposium. Abstract S5-02
Shinde AM, Zhai J, Yu KW, Frankel P, Yim JH, Luu T, et al. Pathologic complete response rates in triple-negative, HER2-positive, and hormone receptor-positive breast cancers after anthracycline-free neoadjuvant chemotherapy with carboplatin and paclitaxel with or without trastuzumab. Breast. 2015;24(1):18–23.
Chen XS, Yuan Y, Garfield DH, Wu JY, Huang O, Shen KW. Both carboplatin and bevacizumab improve pathological complete remission rate in neoadjuvant treatment of triple negative breast cancer: a meta-analysis. PLoS One. 2014;9(9):e108405.
Von Minckwitz G, Timms K, Untch M, Elkin EP, Fasching PA, Schneeweiss A, et al. Prediction of pathological complete response (pCR) by homologous recombination deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: Results from GeparSixto. J Clin Oncol. 2015;33:suppl; abstr 1004. Important study, that showed predictive value of pathological complete remission by HRD assessment after carboplatinum-based neoadjuvant chemotherapy.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–9.
Telli ML, Jensen KC, Vinayak S, Kurian AW, Lipson JA, Flaherty PJ, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33(17):1895–90.
Telli ML, Timms K, Reid JE, Neff C, Abkevich V, Gutin A, et al. Combined homologous recombination deficiency (HRD) scores and response to neoadjuvant platinum-based chemotherapy in triple-negative and/or BRCA1/2 mutation-associated breast cancer. J Clin Oncol. 2015;33:suppl; abstr 1018.
Kaklamani VG, Jeruss JS, Hughes E, Siziopikou K, Timms KM, Gutin A, et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast Cancer Res Treat. 2015;151(3):629–38.
Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33(17):1902–9.
Tutt A, Ellis P, Kilburn L, Gilett C, Pinder S, Abraham J, et al. The TNT trial. 2014 San Antonio Breast Cancer Symposium. Abstract S3-01.
Moore OS, Foote FW. The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2(4):635–42.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55.
Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer—a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8(12):e79775.
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–8.
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132(3):793–805.
Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115103.
Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467–76.
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. Important large study that showed predictive value of TILs in TNBC patients.
Carrick S, Ghersi D, Wilcken N, Simes J. Platinum containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2004;3:CD003374.
Acknowledgement
This publication is the result of the implementation of project funded by the Slovak Grant Agency VEGA 1/0044/15.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Michal Mego and James M. Reuben declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Biomarkers
Rights and permissions
About this article
Cite this article
Mego, M., Reuben, J.M. Emerging Predictive Biomarkers of Response to Platinum Therapy in Triple-Negative Breast Cancer. Curr Breast Cancer Rep 7, 224–231 (2015). https://doi.org/10.1007/s12609-015-0194-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-015-0194-z