Abstract
Objectives
Although serum uric acid (sUA) is not a criterion for diagnosing metabolic syndrome (MetS), many studies have identified a positive association between sUA and MetS in patients of various ages and ethnicities. This association has not been fully established in the very elderly.
Design
Cross-sectional and longitudinal study.
Setting and participants
A total of 18,906 Chinese elderly aged 65 and older undergoing routine health checkups in Taiwan were enrolled.
Measurements
Modified Adult Treatment Panel III criteria were used to define MetS. All participants were further divided into nine groups with gender specification according to age (the young-old, 65 to 74; old-old, 75 to 84; and oldest-old, 85 and over) and sUA concentration tertile (males: sUAG1, <5.7 mg/dL; sUAG2, 5.7–6.7 mg/dL; and sUAG3, > 6.7 mg/dL; females: sUAG1, <4.9 mg/dL; sUAG2, 4.9–5.9 mg/dL; and sUAG3, > 5.9 mg/dL). A cross-sectional study was first performed to determine the correlation between sUA and MetS and its components. A longitudinal study then excluded subjects with MetS at baseline to explore the risk of MetS according to sUA levels in 3 age groups.
Results
In the cross-sectional study, we observed a graded, positive association between sUA and MetS components that diminished after age 75. Subjects with higher sUA levels had higher odds ratios (OR) for the occurrence of MetS in the young-old and old-old groups of both sexes (P <0.001) except sUAG2 males in the old-old group. However, the association diminished with age and only a higher OR was observed in sUAG2 males in the oldestold group (OR, 3.38; 95% CI, 1.11–10.30; P = 0.032). In the longitudinal study, the Kaplan–Meier plot showed that higher sUA levels were associated with a higher risk of MetS in the young-old group of both genders (P < 0.001 sUAG3 vs. sUAG1 and sUAG2). Cox regression analysis further confirmed these results (young-old group: sUAG3 HR, 1.90; 95% CI, 1.42-2.54; P < 0.001; old-old group males: HR, 2.20; 95% CI, 1.04-4.65; P = 0.039; young-old females: HR, 1.83; 95% CI, 1.38 - 2.43; P < 0.001).
Conclusions
Higher sUA levels in the young-old group of Chinese elderly were associated with a higher risk of developing MetS. sUA levels are thus regarded as a potential tool for early diagnosis of MetS. However, this association diminished in those over 75 years of age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a constellation of metabolic abnormalities that includes obesity, hypertension, hyperglycemia, and dyslipidemia. MetS is associated with increased risk of cardiovascular disease (CVD), type 2 diabetes (T2D), chronic kidney disease, and all-cause and CVD mortality (1-3). The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) has established diagnostic criteria for MetS, including central obesity, elevated triglyceride levels, low high-density lipoprotein cholesterol (HDL-C) levels, impaired fasting glucose, and elevated blood pressure (4). The paramount purpose of clarifying MetS criteria is to allow early identification of high-risk individuals such that appropriate interventions or treatments can be started early enough to reduce MetS-related disease burden.
Uric acid is a byproduct of purine metabolism in humans; excess accumulation of serum uric acid (sUA) is associated with hypertension, atherosclerosis, insulin resistance, and T2D (5) and is an independent risk factor for all-cause and CVD mortality (6). Although sUA is not among the established MetS criteria, many epidemiological investigations indicate that hyperuricemia is positively associated with MetS (7-12) and MetS components (13) among different ethnic and age populations. A review by Richette et al. concluded that elevated sUA is associated with and predictive of MetS (14). Most of these studies have been conducted on cohorts of older adults or adolescents.
As in most developed countries, the aging of the Taiwanese population due to progress in public health and medical care has become a major issue. The percentage of the Taiwanese population aged 65 and above had increased to 11.6% by the end of 2013 (15). The prevalence of MetS in the overall population in Taiwan is about 20%. MetS prevalence increases significantly with age, affecting more than half of the elderly population (16). Similarly, the prevalence of hyperuricemia increases with age (17). In our previous retrospective study of 27,553 Chinese subjects over 65 years of age, higher sUA levels were associated with a higher risk of MetS (18). A cross-sectional survey conducted by Liu et al. presented similar findings among a cohort of elderly Chinese (19). A recent longitudinal study also showed that increased sUA concentrations are associated with an increased prevalence and incidence of MetS in elderly Europeans (20). These finding indicates that sUA monitoring is important for early detection of subjects at risks for MetS, especially in the elderly. However, only limited data are available regarding the relationship between sUA and MetS in sub-populations of the elderly, commonly referred to as the ‘young-old’, the ‘old-old’, and the ‘oldest-old’ populations. This study aims to shed light on the relationship between sUA levels and MetS in the very elderly.
Methods
Study Population
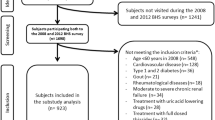
MJ Health Screening Centers are a privately owned chain of clinics located throughout Taiwan that provide regular health examinations to their members. The participants in this study were gathered from these clinics during their routine health checkups. All study participants were enrolled anonymously and gave informed consent. The study protocol was approved by the institutional review board of the MJ Health Screening Center, and the data were provided by MJ Health Screening Center for research purposes only. We randomly selected 36,169 subjects who were over 65 years old during the sampling period of 1999– 2008. We excluded 3,347 subjects who visited only once during the sampling period. Subjects with a past history of hypertension, T2D, cardiovascular events, or were taking medications known to affect MetS components or sUA level were excluded (n = 11,562). In addition, we excluded subjects who were missing data regarding MetS components, sUA, or other general data (n = 2,354). Finally, a total of 18,906 subjects were eligible for further analysis (Stage I). All study participants were further divided into sub-groups according to age and sUA level, with gender specification. We classified subjects by age as young-old (65–74 years old), old-old (75–84 years old), and oldest-old (85 plus) according to the terms defined by Creating Healthy Communities for An Aging Population Work Group (21). Similarly, we divided the subjects into 3 groups based on sUA tertiles (males: sUAG1, <5.7 mg/dL; sUAG2, 5.7–6.7 mg/dL; and sUAG3, > 6.7 mg/ dL; females: sUAG1, <4.9 mg/dL; sUAG2, 4.9–5.9 mg/dL; and sUAG3, > 5.9 mg/dL).
In stage II of the study, after excluding 7,750 subjects with MetS at baseline, a total of 11,157 subjects were eligible for longitudinal follow up. The follow-up period was from 2 –10 years, with the median of 4.8 years. This longitudinal followup analysis investigated the association between sUA levels and the development of MetS in the different-aged population subgroups.
Anthropometric Measurements and General Data
A standard protocol was followed for the health checkup at the MJ clinic. The senior nursing staff in the clinic used a questionnaire to obtain each subject’s medical history, including any current medications. Complete physical examinations were then performed. Waist circumference (WC) was measured horizontally at the level of the natural waist, identified as the location of hollow molding of the trunk when the trunk was laterally concave. Body mass index (BMI) was calculated as the subject’s body weight (kg) divided by the square of the subject’s height (m). Both systolic (SBP) and diastolic blood pressure (DBP) were measured by the nursing staff using a standard mercury sphygmomanometer fitted on the right arm of the subject while seated. After fasting for 10 hours, blood samples were drawn from the antecubital vein for biochemical analysis. Plasma was separated from the blood within 1 hour of drawing, stored at -30°C, and analyzed for fasting plasma glucose (FPG) and lipid profiles. The FPG was detected using the glucose oxidase method (YSI 203 glucose analyzer; Scientific Division, Yellow Springs Instruments, Yellow Springs, OH, USA). Triglycerides (TG) were measured using the dry, multilayer analytical slide method with a Fuji Dri-Chem 3000 analyzer (Fuji Photo Film, Minato-Ku, Tokyo, Japan). Serum high-density lipoprotein cholesterol (HDL-C) concentrations were analyzed using an enzymatic cholesterol assay following dextran sulfate precipitation. sUA levels were measured using s uricase-based method with the Hitachi 7150 automatic biochemical analyzer (Hitachi, Tokyo, Japan).
Definition of Metabolic syndrome
We used the most recent criteria for MetS available in 2009, with some modification of the obesity criteria (22). The definition of obesity was WC = 90 and 80 cm in Taiwanese men and women, respectively (23). The other criteria used are as follows: SBP =130 mmHg or DBP =85 mmHg; TG =150 mg/dL; FPG =100 mg/dL; HDL-C = 40 (men) and 50 mg/dL (women), and taking related medications. Subjects had to meet at least 3 of these criteria to be diagnosed with MetS.
Statistical Analysis
Data are presented as the mean ± standard deviation. All data were tested for normal distribution using the Kolmogorov– Smirnov test and for homogeneity of variances using Levene’s test. Stage I is a cross-sectional designed observation. One-way ANOVA was used to compare differences in metabolic traits and demographic characteristics between the 3 age groups. The odds ratio (OR) was calculated to compare the chance of having MetS between age groups using sUAG1 as a reference. Stage II is a longitudinal analysis using Kaplan–Meier plots and the log rank test to evaluate the effect of time on the occurrence of MetS between different age groups. Finally, multiple cox regression was performed to determine the hazard ratio (HR) of the future MetS occurrence in each age group using sUAG1 as a reference during the follow-up period. All analyses were performed with gender specification within groupings by age and sUA levels. P < 0.05 (two-sided) was considered significant. All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL).
Results
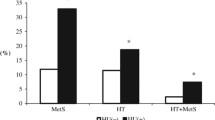
Demographic characteristics of the sUA tertile groups are shown inTable 1. Not surprisingly, all of the MetS components except FPG in females in sUAG1 differed significantly from those of sUAG2 and sUAG3 for both sexes. Interestingly, FPG decreased with increasing sUA levels in the young-old male group. Furthermore, we found a positive association between sUA and MetS components that diminished with age.
Ordinal logistic regression analysis was used to evaluate the risk of MetS with respect to sUA level using sUAG1 as a reference (Table 2). Subjects with higher sUA levels had higher ORs of occurrence of MetS in the young-old and oldold groups of both sexes (P<0.001) except sUAG2 males in the old-old group. In addition, a higher OR was observed in sUAG2 males in the oldest-old group (OR, 3.38; 95% CI, 1.11–10.30; P = 0.032).
In stage II of the study, the Kaplan–Meier plot further showed that the association between high sUA levels and MetS risk was only observed in the young-old group of both sexes (P < 0.001) (Figure 1). A Kaplan–Meier plot could not be constructed for the oldest-old group of females due to insufficient data.
Cox regression analysis showed that sUAGr3 had a greater risk of MetS than did those in sUAG1 of young-old age sub-population (males: HR, 1.90; 95% CI 1.42-2.54; P < 0.001; females: HR, 1.83; 95% CI 1.38–2.43; P < 0.001). Furthermore, in old-old age sub-population, only sUAG3 males had a higher incidence of MetS than did those in sUAG1 (HR, 2.20; 95% CI, 1.04–4.65; P = 0.039) (Table 3).
Discussion
This is the first study to date that combines cross-sectional and longitudinal data to evaluate the relationship between sUA levels and MetS and its components in very elderly people. We found a positive association between sUA and the risk of MetS and its components, but this association diminished with increasing age. Longitudinal data reaffirmed that higher sUA levels are associated with a higher risk of MetS in both sexes but only in the population aged 65–74 years.
Our 2-stage study confirms that hyperuricemia status is an independent risk factor for future development of MetS. These results are consistent with most of the previous prospective studies assessing populations of adults of different ethnicities (24-27). Several studies have focused on the relationship between MetS and sUA levels in the elderly population (18-20). Our previous study and that of Liu et al. found that higher sUA levels, even within the normal range, are positively associated with MetS among the elderly (18-19). Babio et al. conducted a similar 2-stage study to evaluate the role the sUA levels on MetS in elderly European individuals with a mean age of 67 years and a median follow-up period of 3.8 years (20). Concordant with our results, they found that increased sUA concentrations were associated with an increased prevalence and incidence of MetS. They further stated that the associations were stronger in participants older than 67 years, but no further sub-group analysis was available. Furthermore, similar to our cross-sectional findings, these studies observed that the association between sUA levels and MetS is stronger in women than men (19, 20). In contrast, our longitudinal analysis indicates a similar risk for MetS with high sUA in young-old populations of both sexes. However, a higher risk for MetS with high sUA was noted in males in the old-old group. The possible reasons for this discrepancy in results may be attributed to the different ages and demographic characteristics of the study populations and differences in sUA cut-off concentrations between studies. The average age of our study cohort is 70.5 years, which is older than that of the other studies. In addition, unlike other study populations, our cohort excluded subjects with CVD risk such as T2D. The observed gender difference indicates that sex hormones may be an important factor in the interrelationships between hyperuricemia, MetS, and age, although the underlying mechanism is not clear.
The observed association between sUA concentration and FPG is interesting but conflicts with a few of the previous reports. This study shows an inverse correlation between sUA and FPG levels in the young-old male population. These results are concordant with most of the previous studies (18, 28, 29). However, Lin et al. (30) found that sUA did not correlate with FPG. In addition, Choi et al. (31) showed a bellshaped association between sUA and FPG. The discrepancy in these results could be explained by several factors. First, sex differences may affect these outcomes. Several studies, including ours, have suggested divergent associations between sUA levels and hyperglycemia between the sexes (32-36). Similar to our findings, Dai et al. and Nagahama et al. reported a negative association between sUA levels and diabetes in Taiwanese and Japanese men but not in women (32, 33). In contrast, Kawamoto et al. reported that hyperuricemia was more prevalent among women with impaired fasting glucose and newly detected diabetes, which are strongly associated with insulin resistance (34). Chou et al. (35) and Lin et al. (36) investigated sex differences in the relationship between sUA and FPG in non-diabetic Chinese populations, reporting that FPG levels increased significantly with increasing sUA levels only in women. While this evidence clearly indicates sex differences in the association between sUA and FPG, the underlying mechanism is unclear. Second, genetic factors may affect the association between sUA and FPG. Sun et al. genotyped 15 single nucleotide polymorphisms (SNPs) of 11 UA-associated genes to investigate their effect on glucose metabolism. Their results indicate that various SNPs of the UA-associated genes have an impact on the risk of T2D, glucose metabolism, and insulin secretion in a Chinese population (37). In addition, Kolz et al. found that the minor allele for rs734553 in SLC2A9 has a greater association with low sUA levels in women than in men, while the minor allele of rs2231142 in ABCG2 is more strongly associated with higher sUA levels in men than in women. These observations suggest a genetic basis for these sex differences (38). Third, the uricosuric effect of hyperglycemia may contribute to the difference in results. Hyperglycemia enhances uric acid excretion, possibly by impairing tubular reabsorption of uric acid (39, 40). Nan et al. observed a rise in sUA with increasing FPG levels up to 126 mg FPG/dL, followed by a significant decrease in sUA levels with further increases in FPG. Moreover, a declining trend in sUA concentration was observed in patients of both genders with 2-hour plasma glucose of 180 mg/dL; fasting sUA levels were higher in the pre-diabetic population but lower in people with diabetes than in non-diabetic people (41).
Our investigation is unique in that we focused on the relationship between sUA and MetS in elderly sub-populations. Our results suggest a positive correlation between sUA levels and MetS in the ‘young’ elderly but not in the very old. The reason for this phenomenon is unclear but could be explained by the concept of successful aging (SA). Rowe and Kahn first differentiated between usual aging, i.e. functioning well but with a high risk of disease and disability, and SA, demonstrating a high level of functioning across several domains. This concept acknowledges substantial heterogeneity in health trajectories later in life (42). While the authors outlined the framework for an SA model, they did not operationally define SA. Depp and Jeste proposed operational definitions of SA that included the absence of disability and lesser inclusion of psychosocial variables (43). As our enrolled subjects did not take medications related to sUA or MetS components, they may exhibit SA, even during the longitudinal follow-up period. Therefore, the effect of higher sUA on the incidence of MetS may decrease with increasing age in our cohort. Elucidation of the mechanism underlying the associations between SA, sUA, and MetS will require further investigation.
This study has several limitations. First, the enrolled subjects were selected from one private health-screening center. These subjects were in good health, with a better economic status than that of the general population in Taiwan. Thus, our cohort is not representative of this age group. Second, caution should be used in interpreting the results owing to the small numbers of participants in the old-old and oldest-old groups. Lastly, demographic information regarding alcohol intake and dietary factors was not included in this study. These factors might affect the sUA level, resulting in some degree of bias in the results.
In conclusion, Chinese elderly with higher sUA levels were observed to be at higher risk for developing MetS, but this association diminished in those older than 75 years. Strategies for preventing and treating elevated sUA in the very elderly population will require further investigation.
Author contributions: DP designed the study. YLC, CHH, JSL, TJC, HWC, CLH, and PFL conducted the research and performed the statistical analyses. JHC wrote the manuscript. All authors read and approved the final manuscript. Competing interests: We report no potential conflicts of interest relevant to this article.
Disclosure statement: All authors have nothing to declare.
References
Mottillo S, Filion KB, Genest J et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–1132
Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: Current state of the evidence. Diabetes Care 2008;31:1898–1904
Chen J, Muntner P, Hamm LL et al. The Metabolic Syndrome and Chronic Kidney Disease in U.S. Adults. Ann Intern Med 2004;140:167–174
Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III Update: Adjustments and Options. Am J Cardiol 2005;96:53E–59E
So A, Thorens B. Uric acid transport and disease. J Clin Invest 2010;120:1791–1799
Chen JH, Chuang SY, Chen HJ et al. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: A Chinese cohort study. Arthritis Rheum 2009;61:225–232
Ebrahimpour P, Fakhrzadeh H, Heshmat R et al. Serum Uric Acid Levels and Risk of Metabolic Syndrome in Healthy Adults. Endocr Pract 2008;14:298–304
Lohsoonthorn V, Dhanamun B, Williams MA. Prevalence of Hyperuricemia and its Relationship with Metabolic Syndrome in Thai Adults Receiving Annual Health Exams. Arch Med Res 2006;37:883–889
Choi HK, Ford ES. Prevalence of the Metabolic Syndrome in Individuals with Hyperuricemia. Am J Med 2007;120:442–447
Lin SD, Tsai DH, Hsu SR. Association Between Serum Uric Acid Level and Components of the Metabolic Syndrome. J Chin Med Assoc 2006;69:512–516
Ford ES, Li C, Cook S et al. Serum Concentrations of Uric Acid and the Metabolic Syndrome Among US Children and Adolescents. Circulation 2007;15;115:2526–2532
Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 2013;25:210–216
Wu HW, Yu KH, Lin SR et al. Positive Association between Hyperuricemia and Metabolic Syndrome: A Large Health Check-up Population in Taiwan. Chung Shan Medical Journal 2012;123:11–20
Richette P, Perez-Ruiz F. Serum uric acid and metabolic risk. Curr Med Res Opin 2013;29 Suppl 3:9–15
Department of Health. Nutrition and Health Survey in Taiwan: The status of metabolic syndrome in 2005–2008. http://nahsit.nhri.org.tw/node/21. Accessed 22 February 2010.
Council for Economic Planning and Development. 2008-2056 Population Projection in Taiwan ROC. Accessed September 2008
Lee MS, Lin SC, Chang HY, Lyu, LC. High prevalence of hyperuricemia in elderly Taiwanese. Asia Pac J Clin Nutr 2005;14:285–292
Wei SH, Lin JD, Hsu CH et al. Higher uric acid is associated with higher rate of metabolic syndrome in Chinese elderly. Eur Geriatr Med 2014;5: 26–30
Liu M, He Y, Jiang B. Association between Serum Uric Acid Level and Metabolic Syndrome and Its Sex Difference in a Chinese Community Elderly Population. Int J Endocrinol 2014:754678
Babio N, Martínez-González MA, Estruch R et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis 2015;25:173–180
A Report of a Joint Rural Health Advisory Committee and State Community Health Services Advisory Committee Work Group. Creating Healthy Communities for an Aging Population. http://www.health.state.mn.us/divs/orhpc/pubs/healthyaging/hareportnofs.pdf Accessed May 2006
Alberti KG, Eckel RH, Grundy SM et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645
Health Promotion Administration, M.o.H.a.W., R.O.C. http://www.hpa.gov.tw/BHPNet/Web/HealthTopic/TopicArticle.aspx?No=200712250123&parentid=200712250023 Accessed May 2007
Yang T, Chu CH, Bai CH et al. Uric acid level as a risk marker for metabolic syndrome: A Chinese cohort study. Atherosclerosis 2012;220:525–531
Oda E. Serum uric acid is an independent predictor of metabolic syndrome in a Japanese health screening population. Heart Vessels 2014;29:496–503
Gonçalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine 2012;41:450–457
Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008;57:845–852
Liu PW, Chang TY, Chen JD. Serum uric acid and metabolic syndrome in Taiwanese adults. Metabolism 2010;59:802–807
Chiou WK, Huang DH, Wang MH, Lee YJ, Lin JD. Significance and association of serum uric acid (UA) levels with components of metabolic syndrome (MS) in the elderly. Arch Gerontol Geriatr 2012;55:724–728
Lin JD, Chiou WK, Chang HY Liu FH, Weng HF. Serum uric acid and leptin levels in metabolic syndrome: a quandary over the role of uric acid. Metabolism 2007;56:751–756
Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels—the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford) 2008;47:713–717
Dai CY, Chuang WL, Ho CK, Ou TT, Huang JF, Hsieh MY, Yu ML. High Serum Uric Acid As A Novel Risk Factor for Type 2 Diabetes: Response to Dehghan et al. Diabetes Care 2008;31: e67
Nagahama K, Iseki K, Inoue T, Touma T, Ikemiya Y, Takishita S. Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa, Japan. Hypertens Res 2004;27:227–233
Kawamoto R, Tabara Y, Kohara K, Kusunoki T, Abe M, Miki T. Serum Uric Acid Is More Strongly Associated with Impaired Fasting Glucose in Women than in Men from a Community-Dwelling Population. PLoS One 2013;13;8:e65886
Chou P, Lin KC, Lin HY et al. Gender differences in the relationships of serum uric acid with fasting serum insulin and plasma glucose in patients without diabetes. J Rheumatol 2001;28:571–576
Lin KC, Tsai ST, Lin HY, Chou P. Different progressions of hyperglycemia and diabetes among hyperuricemic men and women in the kinmen study. J Rheumatol 2004;31:1159–1165
Sun X, Zhang R, Jiang F. Common Variants Related to Serum Uric Acid Concentrations are Associated with Glucose Metabolism and Insulin Secretion in a Chinese Population. PLoS One 2015;24;10:e0116714.
Kolz M, Johnson T, Sanna S et al. Meta-analysis of 28,141 Individuals Identifies Common Variants within Five New Loci That Influence Uric Acid Concentrations. PLoS Genet 5:e1000504
Christensen PJ, Steestrup O. Uric Acid Excretion with Increasing Plasma Glucose Concentration (Pregnant and Nonpregnant Cases). Scand J Clin Lab Invest 1958;10:182–185
Herman JB, Keynan A. Hyperglycemia and uric acid. Isr J Med Sci 1969;5:1048–1052
Hairong Nan, Zengchang Pang, Shaojie Wang et al. Serum uric acid, plasma glucose and diabetes. Diab Vasc Dis Res 2010;7:40–46.
Rowe JW, Kahn RL. Human aging: usual and successful. Science 1987;10;237:143–149
Depp CA, Jeste DV. Definitions and Predictors of Successful Aging: A Comprehensive Review of Larger Quantitative Studies. Am J Geriatr Psychiatry 2006;14:6–20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JH., Hsieh, CH., Liu, JS. et al. The power of serum uric acid in predicting metabolic syndrome diminishes with age in an elderly Chinese population. J Nutr Health Aging 20, 912–917 (2016). https://doi.org/10.1007/s12603-015-0633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-015-0633-6