Abstract
Multidrug-resistant Escherichia coli (MDR-E. coli) is a global health concern. Lactic acid bacteria (LAB) are important probiotics that have beneficial effects on health, and in recent years, their influences in preventing foodborne pathogens-induced colitis have attracted much attention. Therefore, this study aimed to investigate the oral administration of Lactiplantibacillus plantarum NWAFU-BIO-BS29 as an emerging approach to alleviate MDR-E. coli–induced colitis in BALB/c mice model. To illustrate the mode of action of NWAFU-BIO-BS29 interventions with the gut microbiota and immune responses, the changes on the colonic mucosal barrier, regulatory of the gene expressions of inflammatory cytokines, re-modulating the intestinal microflora, and changes in physiological parameters were studied. The results indicated that daily supplementation of 200 µL fresh bacteria for 7 days had ameliorated the associated colitis and partially prevented the infection. The modes of action by ameliorating the inflammatory response, which destructed villous and then affected the intestinal barrier integrity, reducing the secretion of interleukins (6 and β) and tumor necrosis factor (TNF-α) in serum by 87.88–89.93%, 30.73–35.98%, and 19.14–22.32%, respectively, enhancing the expressions of some epithelial integrity-related proteins in the mouse mucous layer of mucins 2 and 3, Claudin-1, and Occludin by 130.00–661.85%, 27.64–57.35%, 75.52–162.51%, and 139.36–177.73%, respectively, and 56.09–73.58% for toll-like receptor (TLR4) in colon tissues. Notably, the mouse gut microbiota analysis showed an increase in the relative abundance of beneficial bacteria, including Lactobacillus, Bacteriodales bacterium, Candidatus Saccharimonas, Enterorhabdus, and Bacilli. Furthermore, the probiotic promoted the proliferation of epithelia and goblet cells by increasing short-chain fatty acids (SCFAs) levels by 19.23–31.39%. In conclusion, L. plantarum NWAFU-BIO-BS29 has potential applications and can be considered a safe dietary supplement to ameliorate the colitis inflammation symptoms of MDR-E. coli infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, pathogenic of multidrug-resistant Escherichia coli has become one of the most dangerous chronic pathogens to human and animal [1]. It can cause many complex chronic inflammatory bowel diseases (IBD), which include ulcerative colitis (UC) or Crohn’s disease (CD) and other forms such as diarrhea, colonic or rectal inflammation, hematochezia, ulcers, and weight loss [2, 3]. The extensive usage of a wide-range of antibiotics has decreased the efficiency of drugs to inhibit MDR-pathogens, which increased the infection rates among people and the difficulty of finding a suitable cure method and caused a crisis globally [4, 5]. Overall, Gram-negative bacteria, including E. coli, are responsible for more than 30% of all hospital-acquired gastrointestinal tract infections [6]. Many reports from different countries in Europe and Asia highlighted MDR-E. coli as a concerning problem. In India, Hussain et al. [7] reported that MDR-E. coli infections in the retail poultry market were 68% in broilers and 64% in chickens. Likewise, in Ugandan hospitals, there were many patients in the intensive care unit (ICU) due to a high occurrence of MDR-pathogen [8]. The increasing incidence of MDR-E. coli infections demonstrated that they might be carried by contaminated foods such as meats, vegetables, and water, thus infected humans via diet [9].
The commensal microbes in the intestinal tract play an essential role in intestinal homeostasis and regulate the work of the immune system. The invasion of pathogens can trigger the over-releasing of pro-inflammatory cytokines, hence disrupting mucosal barrier functions and reducing intestinal permeability [10]. The microbial invasion is started by infecting the intestinal barrier (IB) which consists of mucus, epithelial cells, and tight junction structures. IB works as a barrier to the body from the external environment and also has other functions that include protecting from intestinal disorders such as IBD and UC and regulating the interaction among intestinal contents. Intestinal barrier perturbations could increase epithelial permeability which leads to inadequate protection against microbial invasion. Furthermore, the imbalance between the microbes and the defense systems of the host might enable them to contact directly with the epithelial cells and mucosal immune system, which may result in a chronic intestinal inflammation [11]. Many previous studies have proven that IBD mice demonstrated increased barrier permeability due to prone damage to the intestinal paracellular [4, 10, 12]. The tight junctions (TJ) consist of barrier-forming proteins named zonula occludens protein 1 (ZO-1) and transmembrane proteins named Claudin-1 and Occludin which are pore-forming proteins. Besides, other proteins that cover the surface of epithelial cells and the internal and external mucous layer are named mucins (MUC2-3). The gut microbiota have the ability to produce metabolites such as short-chain fatty acids (SCFAs), which can improve the function and integrity of the intestinal epithelium and prevent colitis. Maintaining the integrity of the mucous layer structure could guarantee an appropriate intestinal permeability to the host [13].
The spread of antibiotic resistance among pathogens has become a global threat to public health, and it is accelerating much faster than developing new drugs, so severely there is an urgent need to intervene to raise the effectiveness of common antibiotics or find new treatments that work more precisely to kill pathogens and does not target commensal microbes in the gastrointestinal tract [14]. Consequently, finding more effective and safe alternative strategies to prevent or treat pathogens associated with UC is very important. Recently, many compounds have been developed as antibiotic adjuvants to efficiently treat MDR bacteria, such as protein kinases, quinazoline, polypeptides, plant extracts, and endogenous bacteria extracts [15]. In addition, the consumption of nutritional supplements such as probiotic microorganisms, primarily lactic acid bacteria (generally recognized as safe), has become essential in daily diets due to their multiple potential health benefits for humans and animals; besides that, they are associated with many clinical aspects such as attenuating inflammatory bowel disease (IBD), mitigating irritable gastrointestinal syndrome, affecting homeostasis, modulating host immune responses as immunosuppressants or activators, and preventing pathogen infections by motivating the epithelial cells or competing to colonize the intestine [5]. At present, studying the potential health-beneficial effects of probiotics has become a hotspot in the research field of food and health. Various studies reported that probiotics have a high ability to produce countless inhibitory bioactive metabolites, such as glycosyl compounds, gluco-phospholipid, hydrogen peroxide (H2O2), organic acids, steroids, flavonoids, indole, reuterin, indazole, catechol, 2,3-butadione, acetaldehyde, hydroxyl radical, and bacteriocins [16]. These derivative compounds can inhibit a broad spectrum of Gram-negative and Gram-positive pathogenic bacteria, including E. coli, Enterococcus faecalis, and Salmonella [17]. A growing body of literatures has reported that many LAB probiotic strains have potentially beneficial health effects on preventing intestinal infections caused by pathogens, especially may help control MDR-E. coli infection-associated colitis (anti-inflammatory activity), like Lactobacillus gasseri [11], Lacticaseibacillus fermentum [18], Lactobacillus acidophilus [19], Lactobacillus helveticus [20], Lacticaseibacillus rhamnosus [21], and Lactobacillus casei [22, 23]. The mode of action of probiotics may relate to adhesion to gastrointestinal mucosa or the transient and permanent colonization and modulation of the gut microbiome, producing antimicrobial substances, stimulating the host’s immune system response, and protecting the intestinal epithelial barrier [9]. Nevertheless, identifying safe probiotics is still limited and their biological health benefits are strain-specific not at the species level; thus, researchers are constantly screening new probiotic strains with potential desirable functions.
Among the FDA-approved probiotics, Lactiplantibacillus plantarum plays a role in health care through its physiological functions to the host’s body by regulating the secretion of inflammatory cytokines, modulating the intestinal microflora, preventing and curing foodborne diseases, developing the innate immunity, and controlling cancer tumors [24]. However, the reports on the inhibition of MDR pathogenic bacteria infection-associated colitis by probiotics have yet to be addressed extensively very well [17].
Our previous in vitro study has proven that L. plantarum NWAFU-BIO-BS29 possesses potential health benefits to the host due to its tolerance to acid and bile salts and the high adhesion ability with the intestinal receptor analog to avoid bacterial invasion [25]. Otherwise, it can produce metabolites of SCFAs and an antimicrobial substance of bacteriocin named LP-BIO29, which has a good inhibitory effect against a wide-range of foodborne pathogens (Gram + and −) and multidrug-resistant pathogens compared to antibiotics [26]. Moreover, the genomic analysis showed that this strain does not carry virulence factors (VFg) or antibiotic-resistant genes. The purpose of this study is to evaluate the effect of oral administration of L. plantarum NWAFU-BIO-BS29 on preventing the pathogenesis of colitis induced by MDR-E. coli infection and alleviating intestinal inflammation damages by using the BALB/c mice model. Here, we sought to identify the possible protective roles of this probiotic in regulating the expression of colitis-related cytokines, protecting the mucous layer and tight junction proteins from damage, as well as the intestinal epithelial barrier, and re-modulating intestinal microflora. The present study could provide a solid basis for future studies in clarifying the mechanisms of LAB-probiotics as an anti-inflammation treatment against MDR E. coli infection.
Materials and Methods
Bacterial Strains and Growth Conditions
L. plantarum NWAFU-BIO-BS29 was isolated from traditional Chinese fermented milk in our previous study [25], which is generally recognized as safe and could be used as functional food or in food production and preservation. It was stored in De Man, Ragosa, and Sharp broth (Beijing Land Bridge, China) containing 20% glycerol (KESHI Chemical, China) at − 80 °C in the lab of food bioresources at NWAFU. The multidrug-resistant strain of Escherichia coli SX ZFQ 009 EC3 was previously isolated in our lab and found to have antibiotic resistance to ampicillin (AMP)/chloramphenicol (CHL)/cefoperazone (CFP)/kanamycin (KAN)/meropenem (MP)/sulfamethoxazole (SMZ)/sulfamethoxazole-trimethoprim (SXT)/tetracycline (TE) [27]. The E. coli SX ZFQ 009 EC3 and L. plantarum NWAFU-BIO-BS29 were activated before use by cultivation in Luria Bertani (LB, Land Bridge) and MRS broth, respectively, at 37 °C for 12 h and then harvested by using a refrigerated centrifuge of (HC-3016R, Zonkia. China) at 10,000 g for 15 min. The precipitated cell pellets were rinsed twice in cold phosphate-buffered saline (PBS, pH 7.4) and then resuspended to 1 × 108 CFU/mL in PBS. The cells were freshly prepared for in vivo analysis [28].
Determination of the Inhibitory Effect of L. plantarum NWAFU-BIO-BS29 on MDR-E. coli
The agar-well diffusion method (AWDM) was used to evaluate the antibacterial activity of the cell-free supernatant (CFS) of NWAFU-BIO-BS29 against MDR-E. coli SX ZFQ 009 EC3 as described in our previous study [25]. In brief, NWAFU-BIO-BS29 and SX ZFQ 009 EC3 were cultivated overnight in LB broth (Land Bridge, China). The probiotic CFS was collected by centrifugation, and 200 µL was poured into punched holes (diameter, 8 mm) in double-layer plates (semi-solid LB medium with 0.75% agar) containing 106 cfu/mL of MDR-E. coli. The petri dishes were incubated at 37 °C for 24 h and then metering the inhibition zone diameter. To assess the susceptibility of E. coli SX ZFQ 009 EC3 to antibiotics, the tested strain and the standard strain of Escherichia coli ATCC25922 were inoculated separately on LB plates with antibiotics produced from DIYIBIO. China. The inhibition zones were observed after 24 h of incubation [29].
Animal Experiment Design and Treatments
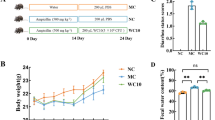
Forty male BALB/c mature mice (6–8 weeks old) were purchased from the Health Science Center of Xi’an Jiaotong University (XJU, China). The mice were housed in a pathogen-free laboratory room with a controlled environment (temperature 23 ± 2 °C and humidity 55 ± 5%) and a 12 h light/dark cycle at Northwest A&F University. All the experiments were performed strictly according to the animal protection guidelines of the Ethics Committee of Northwest A&F University. Moreover, the independent Animal Care and Use Committee in Shaanxi Province, China, issued guidelines that were adhered to and strictly followed. Mice were acclimatized for 2 weeks with free access to a standard diet and water; after that, healthy mice were randomly divided into four groups (n = 10/group) as follows: (1) control group (C) was given 200 µL PBS once a day by oral gavage throughout the whole experiment; (2) model group (M) was given MDR-E. coli suspension by gavage as a challenge; the probiotic intervention groups: designed as (3) pre-treatment group (EP) started gavage before a week from the challenge and continued for another 7 days and (4) post-treatment group (ET) started gavage after the challenge for 7 days; both groups were intragastrically given 200 µL freshly prepared probiotic suspension (5 × 108 CFU/mL) once a day during the schedule period. The mice feed was purchased from Medicine, Professionals for Lab Animal Diets Co. Ltd., Jiangsu, China. Figure 1 A shows the scheme of the experiment. By the end of 4th week, mice were fasted from food and water overnight and then euthanized according to the approved animal protection guidelines [29]. The fecal samples were collected before mice euthanizing and then stored at − 80 °C for SCFAs assessment, while cecum and colonic contents were collected after sacrifice and frozen in liquid nitrogen, subsequently, stored at − 80 °C for gut microbiota analysis [10, 12].
Measurement of Body Weight, Disease Activity Index, and Organ Index After Sacrifice
The mice’s body weights were measured at the beginning of the experiment and continuously every 3 days at 12:00 AM during the challenge period till day 14. Stool consistency and occurrence of blood in the feces were evaluated and recorded every 3 days by a blind observer using a reagent kit from Nanjing Jiancheng Bioengineering Institute (NJBI, China) following the company protocol. The disease activity index (DAI) is a macroscopic appearance index calculated as previously proposed by Tu et al. [13] (Table S1). The DAI scores were assessed according to the severity of losing weight, the stool consistency, and the amount of blood on it by using the following formula:
For the organ index (OI), the liver, kidneys, and spleen were weighed shortly after dissection and placed in liquid nitrogen [29]. OI was calculated using the next formula:
where W0 is the organ weight, and WB is the body weight.
Blood and Tissue Collection
Blood samples were collected from the retro-orbital capillaries after the anesthesia to analyze the inflammation cytokines level. The serum has been separated by centrifugation at 3000 g for 15 min at 4 °C (ZONKIA, high speed refrigerated centrifuge, China) and stored as supernatant at − 80 °C [30]. The tissue weight of the colon, ileum, cecum, dedendum, kidney, spleen, and brain was immediately measured after excising. For histological analysis, a part of the tissue was taken and fixed in paraformaldehyde solution (Servicebio, China); meanwhile, the other part was frizzed and stored in liquid nitrogen for RNA extraction analysis. Finally, the length was measured for colon samples [31].
Histological Analysis
Tissues of the colon, cecum, and kidney samples were tested histologically to see the treatment’s influence and the morphological changes on the cellular infiltration and the damage to the epithelial cells [32]. The method described by Wang et al. [33] has been used to stain and slice the tissues to sections of 5-µm thickness for hematoxylin and eosin test (H&E) in Y&KBIO company, China. First, the tissues were fixed overnight in 10% paraformaldehyde, treated with graded ethanol and xylene, then embedded in paraffin to be sliced into sections using a rotary microtome. A light microscope (Olympus Corporation, Tokyo, Japan) installed with Olympus cellSenns Standard software was used to observe and photograph the stained slices.
RNA Extraction and Quantitative PCR Analysis
Total RNA was extracted individually from tissue samples of the colon, ileum, cecum, dedendum, kidney, spleen, liver, and brain to measure the immunological factors level by using the triazole method protocol as described by Li et al. [10] and Wu et al. [34] with minor modifications. In brief, the TransZol Up reagent (TRANS, China) was used, and we ground the tissues at the tissue lyser of Shanghai Jingxin Industrial Development Co., Ltd, China. The quality of RNAs was evaluated by checking the absorbance of 260/280 nm (DeNOVIX, DS-11 NanoDrop Spectrophotometer, USA), a good quality RNA ranging from 1.8 to 2.2 µg. After that, cDNA synthesize for reverse transcription was performed by following the kit manufacture instructions (Gen Star, Beijing Kangrun Chengye Biotechnology. Co., Ltd. www.gene-star.com): 2 µL total RNA, 1 µL primer, 10 µL 2 X reaction mix, 1 µL StarScript RT Mix; the total reaction volume is 20 µL completed by DEPC-ddh2O. RNA Amplification was performed on the thermal Cycler of BIO-RAD T100™, Singapore, according to the cycle conditions designed by Wang et al. [31].
Determination of the Levels of Immunological Factors in Tissues
The gene expression of pro-inflammatory cytokines, including interleukin (IL-6 and IL-1β), tumor necrosis factor-α (TNF-α), mucins (MUC2 and MUC3), the mRNA levels of heat-stable enterotoxin (Stx2c), toll-like receptor (TLR4), and the tight junction proteins (Occludin and Claudin-1) in the tissues of colon, ileum, cecum, dedendum, kidney, spleen, liver, and brain were measured by using RT-qPCR the QuantStudio 3™ Real-Time PCR Instrument (Thermo Fisher Scientific, Singapore) according to the operating instructions [29]. Quantitative PCR for mRNA protein expression was conducted by mixing 1 µL of the diluted cDNA (1:10), 0.5 µL forward primer, 0. 5 µL rephrase primer, 8 µL water, and 10 µL 2xFast qPCR Master Mixture (SYBR Green) of DiNing (Beijing, China, www.di-ning.com.cn). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as a housekeeping gene as an internal control to normalize the mRNA expression of target genes. The sequences of the primers used for RT-qPCR are listed in Table S2. The mRNA expression levels were measured using the threshold cycle Ct (QuantStudio™ Design & Analysis Software). The relative gene expression was calculated according to the 2−ΔΔCt method [35, 36].
Biochemical Analysis
Myeloperoxidase (MPO) is a biomarker of neutrophil or monocyte/macrophage infiltration in serum and could obliquely reflect the degree of inflammation and neutrophil infiltration in colonic tissue [37]. The MPO activity in serum and colorectal tissue was evaluated using the Nanjing Jiancheng Bioengineering Research Institute kit (China, www.njjcbio.com) [38, 39]. Enzyme-linked immunosorbent assay (ELISA) method was used to determine the inflammation level biochemically by measuring the expression levels of TNF-α, IL-1β, and IL-6 cytokines in mice serum and tissues using a Mouse ELISA kit (Hai Ming Biological Co. Ltd., China). First, ~ 100 mg from the tissues were ground and homogenized with 900 µL PBS pH 7.0 (GCLONE, China), and then, the protein concentration was adjusted by using a bicinchoninic acid protein assay kit (BCA, Shannxi Zhonghui Heaibio Pharmaceutical Technology Co. Ltd., Xi’an, China www.sxzhhc.com). According to the manufacturer’s protocol, the immunological factors were measured at the wavelengths of 450 and 460 nm (UNICO7200 Spectrophotometer, Shanghai, China), respectively [40].
Analysis of Short-Chain Fatty Acids (SCFAs)
The described method by Wang et al. [38] and Wang et al. [35] was used to measure the SCFs concentration (acetic, butyric acid, propionic, valeric, isovaleric, and isobutyric acids) in the feces samples using Shimadzu gas-chromatography (GC-2014C, Kyoto, Japan) equipped with DB-FFAP column (Agilent Technologies, USA). Briefly, nitrogen gas (N2) was used as a carrier and set to a split ratio of 10:1 and a flow rate of 2.0 mL/min; the oven was set at 50 °C for 1 min (initial temperature) and gradually increased at a rate of 15 °C/min, 5 °C/min, and 15 °C/min to 120 °C, 170 °C, and 240 °C for 3 min, respectively.
Analysis of the Expression of 16S rRNA Gene in the Gut Microbiota
The cecum contents (~ 200 mg) of BALB/c mice were collected from each group to extract the DNA. The method of Wang et al. [33] and Wang et al. [12] was used to extract their DNA and then analyze the DNA quality by agarose gel electrophoresis. Frozen genomic DNA samples were sent to Beijing biomarker company for sequencing of 16S rRNA intestinal-bacteria genes. The polymerase chain reaction was performed to amplify the hypervariable V3-V4 regions of 16S rRNA with PCR Master Mix (New England Biolabs), 2 µM of forward (343F) and reverse (798R) primers, and 10 ng genomic DNA template. Afterward, the DNA fragments of purified PCR products were sequenced at Biomarker Company, China. The sequence libraries were generated by using the IlluminaMiSeq platform, and then, the quality of the library was checked by using Qubit2.0 Fluorometer (Thermo Scientific) and an Agilent Bioanalyzer 2100 system [13].
Bioinformatic and Correlation Analysis
All the sequence data was analyzed bioinformatically after being converted to FASTQ format using the BMKCloud platform (http://www.biocloud.net/) of BEIJING BIOMARKER Ltd. (Beijing, China). The operational taxonomic units (OTUs) were generated by clustering the high-quality sequences to find the corresponding species abundance. The following data analyses were performed the same as we described in our previous work [41] based on effective data of paired-end reads to simplify the data structure and show the natural distribution of the samples: (1) alpha diversity of the gut microbiota to analyze the microbial community richness, (2) beta diversity to compare the degree of similarity between species, (3) taxonomic analysis of order species, (4) functional genes prediction, and (5) analysis of significant differences between the community structure among the groups [42].
The correlation between the properties of L. plantarum NWAFU-BIO-BS29 and their roles in ameliorating the infection symptoms was explored using the Pearson correlation coefficient in the R language by using the Corrplot R package.
Statistical Analysis
All the experimental results were expressed as mean (M) ± standard deviation (SD) of replicate experiments. GraphPad Prism (version 8.01) was used for statistical analysis and for drawing the figures. The significant difference between groups was analyzed by ANOVA one-way and Tukey–Kramer for the multiple comparisons test. The significant difference means having P values of < 0.05 (*), < 0.01 (**), and < 0.001 (***).
Results
Inhibitory Effect of L. plantarum NWAFU-BIO-BS29 Against MDR-E. coli In Vitro
The results showed that the free-cell supernatant of NWAFU-BIO-BS29 has a potent antimicrobial activity against E. coli SX ZFQ 009 EC3 with an inhibition zone of 2.8 cm (Figure S1a). The susceptibility assessment exhibited that SX ZFQ 009 EC3 had a high antibiotic resistance compared to the reference strain of Escherichia coli ATCC25922 (Figure S1b).
Body Weight, Disease Activity Index, and Organ Index of Mice
As shown in Fig. 1B, except group C, all the mice groups exhibited significant weight loss (P < 0.05) during the challenge period. Importantly, the post-administration treatment had alleviated the weight loss compared with the infected group (P < 0.05). This result indicated that MDR-E. coli infection had caused severe diarrhea and bleeding, which progressively increased the DAI of mice. Apparently, these damages had been attenuated by the administration of NWAFU-BIO-BS29 (Fig. 2C). Meanwhile, the survival rates for all groups during the experiment showed that some mice were dead in the model group due to the high severity of infection (Fig. 1D). The organ index was calculated for the liver, kidney, and spleen to reflect the health situation of the body organs (Fig. 1E–G).
Histological Analysis to Evaluate the Effects of L. plantarum NWAFU-BIO-BS29 on the BALB/c Mice Infected with MDR-E. coli
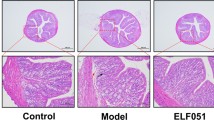
Histopathology analysis of the tissues showed that the mice intestinal villous in the C group had a healthy shape and no pathological damages were observed. Meanwhile, the intestinal cells in the M group (model group) had been damaged, and an incomplete intestinal structure and infiltratory inflammation in the lamina propria were observed (Fig. 2A1, A2, B1, B2, C1, and C2). Conversely, the pathological damages in the administration groups (before and after the challenge) were alleviated in a dose-dependent manner compared to those of the M group. In contrast, H&E staining of kidney sections had shown acute necrosis in the model group, but it was not observed in the negative control and treatment groups (Fig. 2A3, B3, C3, and D3).
Effects of L. plantarum NWAFU-BIO-BS29 on the Intestinal Barrier Integrity and the mRNA Expressions of Toll-Like Receptor (TLR4) in BALB/c Mice Tissues
The results showed that pre- and post-treatments by L. plantarum NWAFU-BIO-BS29 suppressed the markers of MDR-E. coli–induced colitis after 7 days of induction. They shortened colon by 44% (Fig. 3A) and significantly activated the expressions of some epithelial integrity-related proteins in the mouse colon mucous layer like mucins 2 and 3, Claudin-1, and Occludin by 130.00–661.85%, 27.64–57.35%, 75.52–162.51%, and 139.36–177.73%, respectively (Fig. 3B–E), which were decreased in the M group. Furthermore, Fig. 3F shows that the administration of NWAFU-BIO-BS29 in the EP group has downregulated the gene expression of TLR4 by 99.99% in jejunum tissue (P < 0.001) when suffering from challenge compared with the C group. Relative to the pre-treatment group, the challenge significantly increased the protein expression of TLR4 in the ileum and colon by 69.50% and 56.09, respectively (Fig. 3G and H). Moreover, administration of L. plantarum NWAFU-BIO-BS29 decreased expression of heat-stable enterotoxin like Shiga-like toxin (stx2c) compared with the M group (Fig. 3I–K).
Effects of NWAFU-BIO-BS29 on the intestinal barrier integrity and the expressions of TLR4 and stx2c in the mice tissues. Note: A Intestinal length. B MUCIN2 (RE). C MUCIN3 (RE). D Claudin-1 (RE). E Occludin (RE). F TLR4 secretion in jejunum. G TLR4 in the colon. H TLR4 in the ileum. I Stx2c secretion in the brain. J Stx2c in the dedendum. K Stx2c in the kidney. RE is mRNA relative expression to the GAPDH gene. mRNA-RE is mRNA relative expressions
Effects on the Gene Expressions of Immunological Factors
The related signaling pathways that were activated by L. plantarum NWAFU-BIO-BS29 to regulate the pro-inflammatory cytokine expression were studied. The relative gene expressions of tumor necrosis factor-α (TNF-α), interleukin (L-1β), and (IL-6) were measured in the colon, ileum, cecum, jejunum, dedendum, spleen, kidney, liver, and brain tissues of BALB/c mice by RT-qPCR to investigate the inflammation alleviate effects. In detail, all the cytokines had significantly increased in the M group, which was exposed to the challenge when compared with the control group. These findings suggested that the infection by MDR-E. coli led to an occurrence of inflammatory response. Conversely, the administration of L. plantarum NWAFU-BIO-BS29 had significantly prevented the increasing trend of these pro-inflammatory cytokines (P < 0.001) by 87.88–89.93%, 30.73–35.98%, and 19.14–22.32% for IL-6, L-1β, and TNF-α in serum, respectively. In addition, the same cytokines in colon and ileum tissues showed high reducing percentages by 78.96–93.11, 13.67–19.42, and 72.43–93.55, respectively. Obviously, the results of the mRNA expressions of pro-inflammatory genes showed no significant difference between the ET and EP groups (P > 0.05) (Fig. 4A1, A2, A3, B1, B2, B3, C1, C2, D–G, H1, H2, and I).
Influences on the Inflammation Level and Myeloperoxidase (MPO) Activity
The ELISA biochemical analysis of pro-inflammation cytokines levels in mice blood and tissues showed that the infection remarkably induced the production of IL-1β, IL-6, and TNF-α (Fig. 5A–C), which is consistent with the results of mRNA expression in the tissues. On the other side, NWAFU-BIO-BS29 alleviated oxidative stress in the MDR-E. coli–induced colitis mice compared with the control group; the model group showed higher oxidative mediators (MPO) levels 146.5 U/L in serum compared to 63.6 U/L in the ET group and 70.1 U/L in the EP group, and 970.7 U/L in colon tissue compared to 956.2 U/L in the ET group and 896.9 in the PT group (Fig. 5D and E).
Effects of L. plantarum NWAFU-BIO-BS29 Administration on the Level of Short‑Chain Fatty Acid in BALB/c Mouse Feces
SCFAs are usually produced during the fermentation of indigestible carbohydrates inside the body by the gut microbiota through the pathways of methyl malonyl-CoA and acryloyl-CoA, which are strongly associated with immune activation [43]. As shown in Fig. 8, the analysis indicated that the major metabolites were acetic, propionic, valeric, isovaleric, isobutyric, and butyric acids in the mouse feces (Table S3). In this context, the total SCFA concentration was markedly decreased in the infected mice compared with the healthy group, while the administration of L. plantarum NWAFU-BIO-BS29 for seven consecutive days increased it to some degree. The total concentration of the SCFAs reached 901,250 and 1,060,970 µM/mg under EP and ET treatments, respectively, which was higher than that in the model group (727,912 µM/mg) (Fig. 6).
The Gut Microbiota Diversity of BALB/c Mice
The 16S rRNA sequencing showed that NWAFU-BIO-BS29 administration improved the gut microbiota diversity. The sample sizes were confirmed in the rarefaction curve to reflect the species composition (Table S4). While the operational taxonomic units (OTUs) reflected the reliability and quality of corresponding species (Table S5) and the number and distribution of species in samples (Figure S2a). The bioinformatic analyses of effective paired-end reads were shown as follows: (1) The evolutionary branching diagrams of LEfSe and ANOVA analysis showed significant differences of the community structure among the groups (Fig. 7A, B and Fig S2b). (2) The taxonomic analysis cluster presented the species abundance heat map, the species distribution map, and the relative abundance of species communities (Figs. 2C and 2A, B), respectively. (3) Correlation and association analysis showed a correlation > 0.1 and P < 0.05, which was reflected in diagrams of the species at the genus level (Fig. 8C) and node Zi-Pi distribution (Figure S2c). (4) Alpha diversity showed as Boxplot, the dilution and rank-abundance curves, and the Shannon Index indicated that there are differences in the microbial richness between groups (Figures S2d, e, f, and g). (5) Beta diversity presented as the unweighted pair group method with arithmetic mean (UPGMA) histogram combination cluster tree, the principal component analysis (PCA) to obtain the β value, PCoA analysis, and heat map of the phylogenetic species tree, they have confirmed the similarity between species (Fig. 8D, Fig S2h, I, and Fig S3a). (6) The histograms of Orthologous Groups of proteins (COG) showed the genetic information of unknown species and predicted the functions of genes as follows: Fig. 8E is the metabolic pathway for predicting the protein functions, Figure S3b is the COG functions classification chart, Figure S3c is analysis the differences of KEGG metabolic pathways between groups, and Figure S3d a diagram of KEGG metabolic pathways of biogeochemical cycles processes.
Correlation Analysis
A correlation coefficient of Spearman’s was used to evaluate the relationship among the gut microbiota, the disease activity index, the biochemical analysis of inflammation-related parameters (pro-inflammation cytokines and MPO activity), epithelial integrity-related proteins (mucins 2 and 3, Claudin-1, and Occludin), and toll-like receptor (TLR4) and the probiotic role in ameliorating colitis. The matrix in Fig. 9 showed a positive correlation between each other. The results even indicated that the level of pro-inflammation cytokines and MPO activity were positively significantly correlated with the epithelial integrity-related proteins and DAI.
Discussion
The Mechanisms of Probiotics Alleviating the Physical Parameters of Colitis
In recent years, there has been an increasing interest in identifying novel probiotic microorganisms to use as functional foods to improve human and animal health and also to prevent multidrug-resistant pathogen infection [4]. Long-term intestinal inflammation induced by these pathogens could associated with colitis. Probiotics that have beneficial features are still limited, so there is a necessary need to mine new good strains. Meanwhile, the conclusions of the in vitro trials have demonstrated that the underlying mechanisms of the beneficial effects of probiotics mainly consist of (1) improving the intestinal barrier permeability, mucus properties, and epithelial cells [44]; (2) regulating the immune system responses of pro-inflammatory cytokines secretion [45]; and (3) modulating the gut microbiota structural by inhibiting the grows of pathogens due to the capacities of LAB to produce antimicrobial agents and competition on the attachment sites and nutrients, which will prevent adhesion of pathogens [46]. Usually, the adherence ability of LAB is an important feature in exerting their role in the host gastro-tract but might not be the essential characteristic that alleviates the effects of ulcerative colitis [47]. In this regard, till now, the relationship between the probiotic properties (physiological characteristics) and immune regulation functions and their role in ameliorating colitis has yet to be adequately studied. Consistent with our previous findings, L. plantarum NWAFU-BIO-BS29 isolated from traditional fermented milk possessed desirable potential probiotic properties that can be applied in health interventions with no apparent toxicity [41]. It showed abilities of adhesion, tolerance to acid and bile salts, and production of antimicrobial peptide substances (bacteriocins) which are often associated with the inhibition of intestinal pathogens [26]. To explain the protective effects and the potential action modes of NWAFU-BIO-BS29 in alleviating the symptoms of MDR-E. coli–induced colitis, BALB/c mice model was used. Interestingly, the mice that orally garaged probiotic for 14 days (before and after MDR-E. coli infection) showed significant differences in body weights and reduced mortality compared with the M group, while the general appearance of all treated mice was normal with no disfigurements in the body, gait, and behavior; opposite to that, the M group exhibited a tremor, diarrhea, convulsion, and sluggishness. Furthermore, the colon’s appearance has presented that its length in the M group was reduced which indicated a colon dysfunction; the probiotic administration has optimized the colon length (Fig. 3A). This crucial importance fact led us to investigate the effects of NWAFU-BIO-BS29 on MDR-E. coli infection; the DAI score was measured for the mice groups during administration. Results showed that NWAFU-BIO-BS29 partially prevented the increase of the DAI score (Fig. 1C). On the other hand, it improved the degrees of immune organ indices; NWAFU-BIO-BS29 reversed the decrease in kidney index and the increase in the spleen and liver indices of ET and EP groups of BALB/c mice inducted by multidrug-resistant E. coli (Fig. 1E–G).
The Immune and Enzyme Responses of Mice Administrated by NWAFU-BIO-BS29
Inflammatory responses are considered a major symptom of infection. The increasing of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α would aggravate the severity of colitis which is considered to be a pathophysiological mechanism of inflammation due to the infiltration of activated monocytes and macrophages during the infection [42, 48]. As such, these cytokines stimulate the immune system cells to make a response to pathogens [49]. Herein, we investigated the effect of L. plantarum NWAFU-BIO-BS29 administration on alleviating the inflammation response in the BALB/c mice induced by MDR-E. coli infection. The obtained results of mRNA relative expressions and biochemical tests suggested that NWAFU-BIO-BS29 consumption could significantly regulate the expression of some pro-inflammatory cytokines levels including TNF-α, IL-1β, and IL-6 in the colon, cecum, dedendum, jejunum, ileum, liver, brain, kidney, and spleen (Figs. 4 and 5). The analysis of mRNA relative expressions showed that the levels of pro-inflammatory cytokines were all significantly increased in the M group, while the treatments (ET and EP) conspicuously alleviated these adverse changes, except IL-6 in the cecum, ileum, dedendum, and kidney did not alleviate by pre-supplementation with NWAFU-BIO-BS29 (Fig. 4 B1, D, F, and E). Simultaneously, the cytokines levels were reduced in the serum of the (ET and EP) groups compared with the M group. Therefore, these results indicated that treatment with probiotics could significantly ameliorate the symptoms of infection by inhibiting the inflammatory response. These results are consistent with Li et al. [10]; administration by L. rhamnosus JL-1 can downregulate the mRNA expression of TNF-α, IL-1β, and IL-6. Consistent with these results, the biochemical analysis of ELISA to determine pro-inflammatory cytokines levels in the colon, cecum, ileum, brain, and spleen indicated that the administration of this probiotic could lower the pro-inflammation cytokines levels (Fig. 5A–C). Therefore, colonic inflammation induced by MDR-E. coli was successfully established and treatment with NWAFU-BIO-BS29 could partly ameliorate it. The same results were reported by Yun et al. [11]. Additionally, IL-1β levels in brain tissues were measured by using the mRNA expression method and ELISA to see the effect of treatments on alleviating cognitive impairment and depression in the BALB/c mice, where regulating the IL-1β expression in the brain could partially alleviate depression-like behaviors and cognitive dysfunction which induce by nerve-mediated gut–brain signaling [50]; its ET and EP treatments significantly suppressed the overexpression compared with the M group (Fig. 5A); in contrast, mRNA expression indicated just the post-treatment could decrease the IL-1β level (Fig. 4 H2). Likewise, Lactobacillus gasseri NK109 and Lactobacillus mucosae NK41 showed the same features [11, 50].
MPO is a peroxidase enzyme that exists primarily in neutrophil granules that act as a major mediator of pathogen killing by serving as a catalyst in the formation of reactive oxygen intermediates [51]. The MPO results of the M group suggest considerable neutrophil infiltration in blood and the colonic tissues and lowered the presence of immune cells (Fig. 5D and E). Meanwhile, the probiotic administration in the ET and EP groups has corroborated the attenuation of inflammatory mediators. These results agreed with the measurement of cytokines levels and the observations of H&E analysis.
Toll-like receptors (TLRs) are one of the most essential pattern-recognition receptors that play a critical role in innate immune response by recognizing microbial pathogen-associated molecular patterns; they are highly correlated with colitis [10]. Probiotics are highly able to regulate TLRs, and suppressing of TLR4 is considered anti-inflammatory [20]. Correspondingly, we found a high rise in mRNA expression of TLR4 protein in the jejunum, colon, and ileum of the M group compared with the C group. Simultaneously, the ET treatment attenuated the inflammatory response leading to adverse changes, while EP treatment lowered TLR4 in the jejunum (Fig. 3F–H). These results strongly suggested that NWAFU-BIO-BS29 decreased the expressions of pro-inflammatory cytokines partly due to the inhibitory effect of the TLR4 signaling pathway. A previous study also indicated that Lactobacillus amylovorus DSM 16698 T might be associated with inhibiting the TLR4 in the ileum [11].
Probiotic Mechanisms in Promoting Intestinal Barrier Integrity
To clarify the mechanism of inducing colitis in mice by MDR-E. coli infection, basically, the intestinal barrier consists of epithelial cells, mucus, and tight junction proteins; it forms a barrier between the intestinal contents and the external environment. The intestinal barrier disorders might lead to reduced mucus production and increased intestinal epithelial permeability; all these changes will increase the inadequate protection against microbial pathogens invasion [11]. The key component of the internal and external colonic mucous layer is MUC2-3 proteins, which cover the surface of epithelial cells. Injury of the goblet cells suppresses their ability to produce mucins, allowing pathogens’ endotoxins to enter the systemic circulation. Thus, excessive secretion of these genes could provide a protective barrier between the epithelial surfaces and the gut. Our findings exhibited that the infection reduced the gene expression of MUCIN-2 and MUCIN-3 in the colonic, while the ET and EP treatments improved the levels of these proteins (Fig. 3B and C). According to the prior evidence from in vivo experiments, probiotics could help maintain or recover intestinal barrier functions either by direct contact with intestinal epithelium or indirectly through their metabolites effect [18]. However, administration with NWAFU-BIO-BS29 strain could upregulate the mRNA expression of these proteins, indicating that this strain could be maintaining the damage of the epithelial barrier in infected mice. Similar results have been found in the study of Li et al. [14]. On the other hand, the tight junctions (TJ) of the epithelial barrier are built from proteins of ZO-1 and pore-forming proteins such as occludin and claudin, which control the cellular barrier permeability [13]. Consequently, keeping the integrity of the mucous layer structure assures proper intestinal permeability. Similarly, our study indicated that MDR-E. coli infection-associated UC had caused an increase in intestinal permeability and damage to epithelial integrity due to a reduction in the expression of their related protein genes (Occludin and Claudin-1). These adverse effects have been significantly improved after using ET and EP probiotic treatments (Fig. 3D and E). However, the effects on the intestinal barrier integrity showed that treatment with some specific Lactobacillus strains could reverse these alterations due to their high reverse abilities [11]. As such, the intestinal epithelial barrier damage is accompanied with the upregulation of inflammatory factors.
Effects of Probiotic Administration on the Histopathological Changes
To explore the pathological changes in mouse intestines and kidneys, we dissected and inspected them by H&E staining. Histological analysis of cecum and colon integrity showed that the challenge led to disruption of crypt architecture and flattening of villi, mucosal and submucosal inflammation, edema, goblet cell depletion, edema, and epithelial erosion. Meanwhile, the ET and EP groups had standard intestinal mucosa shape and did not present any abnormalities or histopathological changes in tissue structural makeup. Additionally, the intestinal villis of colon tissues were neatly arranged and had a normal structure with a clear and shallow crypt as a result of the absence of goblet cell loss same as in the control group (Fig. 2 A1–D2); also, the kidney tissue sections showed distinct necrosis in the model group compared with tissues from the negative control and treatment groups which were without epithelium necrosis or detachment from lamina propria (Fig. 2 A3–D3). H&E staining confirmed that these damages were obviously ameliorated in the mice treated with NWAFU-BIO-BS29 strain and ameliorated the inflammatory proliferation (Fig. 2 A1–D3). These observations were in line with data of high expression of colitis-related cytokines in the model group. The findings were similar to the study of Yue et al. [40].
The Change in SCFAs Profile
SCFAs are the important indicator of changes in the microflora community structure and have an essential role in regulating epithelial cell differentiation and stimulating immune system response; it could interfere with the production of pro-inflammatory cytokines in particular; butyrate can promote the expression of anti-inflammatory cytokines like (IL-10) [43]. Also, SCFAs ameliorate intestinal mucosal inflammation by regulating the inflammatory pathway-related molecules’ effect on intestinal barrier integrity, inhibiting the growth of some pathogens and promoting the growth of beneficial microorganisms in the host’s intestine [13]. Our results demonstrated that the reduction of SCFAs in feces of the model group was abrogated by administrating with NWAFU-BIO-BS29 strain, specially isobutyric acid and butyric acid, which were increased by 98.04–299.39% and 75.17–289.57%, respectively, and the total concentration reached from 727,912 in the M group to 901,250 and 1,060,970 µM/mg in the EP and ET groups, respectively (Fig. 6). These were similar to the findings by Tu et al. [13]. Therefore, it can be deduced that treatment with this strain had improved SCFAs levels in mouse feces which could promote health benefits to the host and rebalance its gut microbiota; similar results were found in the studies by Cheng et al. [52] and Valvaikar et al. [53].
Modulating Gut Microbiota
Another important feature is the intestinal microbial structure. Gut microbiota have an important role in host health by providing various nutrients, energy balance, and improving SCFAs production that would lead to modulating the immune responses and defense against pathogen infection [54]. Consequently, the imbalance of the gut microbiome composition (dysbiosis) reflects negative shifts in the epithelial cells and mucosal layers, which lead to an increase in the intestinal permeability by collapsing the barrier and also cause metabolic endotoxemia low-grade chronic inflammation through the toll-like receptor pathway [55]. The host’s defense mechanisms are different and complex with another factors, including competition, inhibition, production of antimicrobial substances, as well as anti-adhesion and adhesion to the same receptors [11, 56]. Often, the dietary intervention of some probiotics and their metabolites could modulate the richness of gut microbiome; on the contrary, pathogen infection may cause a loss of gut microbiota diversity and change its composition. In this context, the gut microbiota analysis of the M group in our study indicated that the species distribution histogram showed a decline in the abundance of Lactobacilli but an increase in Oscillospiraceae, Muribaculaceae, Bacteroides, Alistipes, and Lachnospiraceae. As a result, the pre and post-treatments by L. plantarum NWAFU-BIO-BS29 could exert their antiadhesion activity to pathogens and increase the abundance of beneficial bacteria in mice gut like Ligilactobacillus, Bacteriodales bacterium, Lactobacillus, Candidatus Saccharimonas, Enterorhabdus, and Bacilli, besides, Akkermanisia, Clostridia-UCG-014, Odoribacter, Helicobacter in the EP group, with no changes in Desulfovibrio abundance (Fig. 8B). This explained the reason for increasing SCFAs in ET and EP groups which was confirmed by the functional gene predictive analysis (Fig. S3b). Furthermore, alpha diversity indicated that the treatment groups have richness in species due to the decrease of Boxplot, Shannon, rank-abundance curve, and dilution curve indices (Fig. S2d, e, f, and g). The LEfSe analysis of ET and EP groups was conducted to identify the changes that alleviate the inflammation development, which exhibited a significant increase at the genus level of Lactiplantibacillus, Bacillus, and uncultured_rumen_bacterium; the species level of Limosilactobacillus_vaginalis and unclassified_Lactiplantibacillus; and at the species order of Pseudomonadales, Bacillales, Monoglobales, RF39, Rhodospirillales, unclassified_Bacilli, and Adlercreutzia_mucosicola (Fig. 7A and B). Additionally, the data from the unweighted pair group matrix algorithm (UPGMA) tree and the PCA analysis suggested that this probiotic strongly influenced intestinal microflora modification and showed a different clustering pattern at post or pre-challenge treatments. Moreover, EP and ET treatment groups shared 1072 and 1080 OTUs, respectively, with the CN group and only 961 OTUs with the M group (Table S5). These findings agreed with data from other studies by Kim et al. [50], Koo et al. [45], and Yang et al. [57]. The results indicated that NWAFU-BIO-BS29 administration can increase the abundance of beneficial bacteria in the intestinal microbiota community as an emerging approach for therapeutic intervention applications.
Based upon the results of the present study, the oral administration of NWAFU-BIO-BS29 evidently had a good effect on ameliorating MDR-E. coli infection-induced colitis symptoms on BALB/c mice model. The mode of action explanation is strongly related to regulating the immune response and enhancing the intestinal barrier function, moreover, modulating gut microbiota of the host and promoting the production of SCFAs.
Conclusions
One of the emerging approaches in curing multidrug resistance infections is using probiotic administration as a potential therapeutic intervention by changing the gut microbiota structure. This pioneering study has demonstrated that oral administration of L. plantarum NWAFU-BIO-BS29 to BALB/c mice perfectly alleviated or prevented the induced colitis by MDR-E. coli and reversed the dysbiosis of gut microbiota. The probiotic exerted effective immune-response functions through decreasing the pro-inflammatory cytokines mRNA expression of IL-1β, IL-6, and TNF-α in vivo, inhibiting TLR4 signaling pathway and intestinal epithelial barrier damage, as well as modulating gut microbiota and improving the production of SCFAs. It also mitigated the signaling of cognitive impairment and depression-like behaviors by mediating the expression of the IL-1β gut-brain axis cytokine. In addition, its beneficial effects on the mice model had improved the physiological parameters, and no pathological changes were observed; besides that, the H&E staining showed no obvious toxicity or side effects at the effective dose. These findings support the potential health-promoting functions of L. plantarum NWAFU-BIO-BS29 in the host as a therapeutic probiotic. Although treatment with this probiotic is promising, there are many aspects that still need to be studied. This study may prompt to disclose more in future studies which should focus on the changes in gut cytokines of mesenteric lymph nodes that will be informative for explaining the development of intestinal inflammations. Clarifying the systemic mechanism of preventing infection by complementary clinical trials also is needed.
Data Availability
No datasets were generated or analysed during the current study.
References
Dall LB, Lausch KR, Gedebjerg A, Fuursted K, Storgaard M, Larsen CS (2019) Do probiotics prevent colonization with multi-resistant Enterobacteriaceae during travel? A randomized controlled trial. Travel Med Infect Dis 27:81–86
Kumar S, Kumar A (2022) Microbial pathogenesis in inflammatory bowel diseases. Microb Pathog 163:105383
Dubinsky V, Reshef L, Rabinowitz K, Wasserberg N, Dotan I, Gophna U (2022) Escherichia coli strains from patients with inflammatory bowel diseases have disease-specific genomic adaptations. J Crohns Colitis 16(10):1584–1597
Liu GW, Pang B, Li N, Jin H, Li JJ, Wu WQ, Ai CY, Jiang CM, Shi JL (2020) Therapeutic effect of Lactobacillus rhamnosusSHA113 on intestinal infection by multi-drug-resistant Staphylococcus aureus and its underlying mechanisms. Food Funct 11(7):6226–6239
Gilliland A, Chan JJ, De Wolfe TJ, Yang H, Vallance BA (2024) Pathobionts in inflammatory bowel disease: origins, underlying mechanisms, and implications for clinical care. Gastroenterology 166(1):44–58
Bai YF, Wang GH, Qi H, Wang YP, Xu C, Yue L, Hou XL, Yu LY (2020) Immunogenicity of 987P fimbriae of enterotoxigenic Escherichia coli surface-displayed on Lactobacillus casei. Res Vet Sci 128:308–314
Hussain A, Shaik S, Ranjan A, Nandanwar N, Tiwari SK, Majid M, Baddam R, Qureshi IA, Semmler T, Wieler LH et al (2017) Risk of transmission of antimicrobial resistant Escherichia coli from commercial broiler and free-range retail chicken in India. Front Microbiol 8:2120
Atumanya P, Sendagire C, Wabule A, Mukisa J, Ssemogerere L, Kwizera A, Agaba PK (2020) Assessment of the current capacity of intensive care units in Uganda; a descriptive study. J Crit Care 55:95–99
Wang T, Shi C, Wang S, Zhang Y, Wang S, Ismael M, Zhang J, Wang X, Lü X (2022) Protective effects of Companilactobacillus crustorum MN047 against dextran sulfate sodium-induced ulcerative colitis: a fecal microbiota transplantation study. J Agric Food Chem 70(5):1547–1561
Li X, Hu D, Tian Y, Song Y, Hou Y, Sun L, Zhang Y, Man C, Zhang W, Jiang Y (2020) Protective effects of a novel Lactobacillus rhamnosus strain with probiotic characteristics against lipopolysaccharide-induced intestinal inflammation in vitro and in vivo. Food Funct 11(7):5799–5814
Yun SW, Kim JK, Lee KE, Oh YJ, Choi HJ, Han MJ, Kim DH (2020) A Probiotic Lactobacillus gasseri alleviates Escherichia coli-induced cognitive impairment and depression in mice by regulating il-1 beta expression and gut microbiota. Nutrients 12(11):3441
Wang T, Wang S, Dong S, Zhang Y, Ismael M, Wang S, Shi C, Yang J, Wang X, Lü X (2022) Interaction of Companilactobacillus crustorum MN047-derived bacteriocins with gut microbiota. Food Chem 396:133730
Tu A, Wang XC, Chen H, Jia X, Wang T, Yi Y, Liu B, Xin W, Lü X, Shan Y (2021) Ovomucin ameliorates intestinal barrier and intestinal bacteria to attenuate DSS-induced colitis in mice. J Agric Food Chem 69(21):5887–5896
Li N, Pang B, Li JJ, Liu GW, Xu XG, Shao DY, Jiang CM, Yang BW, Shi JL (2020) Mechanisms for Lactobacillus rhamnosus treatment of intestinal infection by drug-resistant Escherichia coli. Food Funct 11(5):4428–4445
Wang T, Zheng J, Dong S, Ismael M, Shan Y, Wang X, Lü X (2022) Lacticaseibacillus rhamnosus LS8 ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated tumorigenesis in mice via regulating gut microbiota and inhibiting inflammation. Probiotics Antimicro Prot 14:947
Aditya A, Peng MF, Young A, Biswas D (2020) Antagonistic mechanism of metabolites produced by Lactobacillus casei on lysis of enterohemorrhagic Escherichia coli. Front Microbiol 11:574422
Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SVS, Rawool DB (2016) Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int J Antimicrob Agents 48(3):265–270
Bhat MI, Kapila S, Kapila R (2020) Lactobacillus fermentum (MTCC-5898) supplementation renders prophylactic action against Escherichia coli impaired intestinal barrier function through tight junction modulation. Lwt-Food Sci Technol 123:109118
Yang KMM, Zhu C, Wang L, Cao STT, Yang XFF, Gao KGG, Jiang ZYY (2021) Early supplementation with Lactobacillus plantarum in liquid diet modulates intestinal innate immunity through toll-like receptor 4-mediated mitogen-activated protein kinase signaling pathways in young piglets challenged with Escherichia coli K88. J Animal Sci 99(6):skab128
Ho SW, El-Nezami H, Shah NP (2020) The protective effects of enriched citrulline fermented milk with Lactobacillus helveticus on the intestinal epithelium integrity against Escherichia coli infection. Sci Rep 10(1):499
Kathayat D, Closs G, Helmy YA, Deblais L, Srivastava V, Rajashekara G (2022) In vitro and in vivo evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 against avian pathogenic Escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiotics & Antimicro Prot 14:1012–1028
Xu CL, Yan SQ, Guo Y, Qiao L, Ma L, Dou X, Zhang BH (2020) Lactobacillus casei ATCC 393 alleviates enterotoxigenic Escherichia coli K88-induced intestinal barrier dysfunction via TLRs/mast cells pathway. Life Sciences 244:117281
Fayyaz I, Zahoor MA, Shahid M, Rasool MH, Nawaz Z (2018) Effect of Lactobacillus casei on serum interleukins following enteropathogenic E. coli infection in experimental rabbits. Pak J Pharmaceut Sci 31(5):2131–2136
Zhou Y, Ni XQ, Duan L, Niu LL, Liu Q, Zeng Y, Wang Q, Wang J, Khalique A, Pan KC et al (2021) Lactobacillus plantarum BSGP201683 improves the intestinal barrier of giant panda microbiota-associated mouse infected by enterotoxigenic Escherichia coli K88. Probiotics Antimicro Prot 13(3):664–676
Ismael M, Gu Y, Cui Y, Wang T, Yue F, Yantin Q, Lü X (2022) Lactic acid bacteria isolated from Chinese traditional fermented milk as novel probiotic strains and their potential therapeutic applications. 3 Biotech 12(12):337
Ismael M, Wang T, Yue F, Cui Y, Yantin Q, Qayyum N, Lü X (2023) A comparison of mining methods to extract novel bacteriocins from Lactiplantibacillus plantarum NWAFU-BIO-BS29. Anal Biochem 661:114938
Guo X, Chen J, Sun H, Luo L, Gu Y, Yi Y, Wang X, Shan Y, Liu B, Zhou Y et al (2020) Mining, heterologous expression, purification and characterization of 14 novel bacteriocins from Lactobacillus rhamnosus LS-8. Int J Biol Macromol 164:2162–2176
Wang T, Wang P, Ge W, Shi C, Xiao G, Wang X, Lü X (2021) Protective effect of a multi-strain probiotics mixture on azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Food Biosci 44:101346
Li N, Pang B, Liu G, Zhao X, Xu X, Jiang C, Yang B, Liu Y, Shi J (2020) Lactobacillus rhamnosus from human breast milk shows therapeutic function against foodborne infection by multi-drug resistant Escherichia coli in mice. Food Funct 11(1):435–447
Wu ZK, Yang KX, Zhang AR, Chang WH, Zheng AJ, Chen ZM, Cai H, Liu GH (2021) Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poultry Science 100(9):101323
Wang T, Zhang L, Wang P, Liu Y, Wang G, Shan Y, Yi Y, Zhou Y, Liu B, Wang X et al (2022) Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur J Nutr 61(1):85–99
Liang WF, Li HT, Zhou HY, Wang M, Zhao X, Sun XH, Li CT, Zhang XM (2021) Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli-infected broiler chickens. Poultry Science 100(4):101007
Wang T, Yan H, Lu Y, Li X, Wang X, Shan Y, Yi Y, Liu B, Zhou Y, Lü X (2020) Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur J Nutr 59(6):2709–2728
Wu T, Shi YT, Zhang YY, Zhang M, Zhang LJ, Ma ZP, Zhao D, Wang L, Yu H, Hou YQ et al (2021) Lactobacillus rhamnosus LB1 alleviates enterotoxigenic Escherichia coli-Induced adverse effects in piglets by improving host immune response and anti-oxidation stress and restoring intestinal integrity. Front Cell Infect Microbiol 11:724401
Wang T, Sun H, Chen J, Luo L, Gu Y, Wang X, Shan Y, Yi Y, Liu B, Zhou Y et al (2021) Anti-adhesion effects of Lactobacillus strains on caco-2 cells against Escherichia coli and their application in ameliorating the symptoms of dextran sulfate sodium-induced colitis in mice. Probiotics Antimicrob Proteins 13(6):1632–1643
Zhu C, Lv YT, Yang J, Bai YS, Ye JL, Wang ZL, Chen Z, Jiang ZY (2020) Proteomic alteration of porcine intestinal epithelial cells after pretreatment with Lactobacillus plantarum followed by infection with enterotoxigenic Escherichia coli F4. Vet Immunol Immunopathol 222:109943
Nieto-Veloza A, Wang Z, Zhong Q, D’Souza D, Krishnan HB, Dia VP (2022) Lunasin protease inhibitor concentrate decreases pro-inflammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis. Food Sci Human Wellness 11(6):1508–1514
Wang T, Wang P, Ge W, Shi C, Xiao G, Wang X, Lü X (2021) The probiotic Companilactobacillus crustorum MN047 alleviates colitis-associated tumorigenesis via modulating the intestinal microenvironment. Food Funct 12(22):11331–11342
Zhao CJ, Hu XY, Bao LJ, Wu KY, Feng LJ, Qiu M, Hao HY, Fu YH, Zhang NS (2021) Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. Plos Pathogens 17(7):e1009774
Yue Y, He ZJ, Zhou YH, Ross RP, Stanton C, Zhao JX, Zhang H, Yang B, Chen W (2020) Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct 11(12):10362–10374
Ismael M, Gu Y, Cui Y, Wang T, Yue F, Qin Y, Lü X (2022) Probiotic of Lactiplantibacillus plantarum NWAFU-BIO-BS29 isolated from Chinese traditional fermented milk and its potential therapeutic applications based on gut microbiota regulation. Foods 11(23):3766
Zhang Q, Zhang LJ, Lyu Y, Shi YT, Zhu LY, Zhang M, Zhao YY, Zhao D, Wang L, Yi D et al (2022) p Dietary supplementation of Lactobacillus zeae regulated the gut microbiome in piglets infected with enterotoxigenic Escherichia coli. Czeh J Anim Sci 67(1):27–38
Li Y, Zhang Y, Wei K, He J, Ding N, Hua J, Zhou T, Niu F, Zhou G, Shi T et al (2021) Review: effect of gut microbiota and its metabolite SCFAs on radiation-induced intestinal injury. Front Cell Infect Microbiol 11:577236
Li YS, San Andres JV, Trenhaile-Grannemann MD, van Sambeek DM, Moore KC, Winkel SM, Fernando SC, Burkey TE, Miller PS (2021) Effects of mannan oligosaccharides and Lactobacillus mucosae on growth performance, immune response, and gut health of weanling pigs challenged with Escherichia coli lipopolysaccharides. J Animal Sci 99(12):skab286
Koo B, Bustamante-Garcia D, Kim JW, Nyachoti CM (2020) Health-promoting effects of Lactobacillus-fermented barley in weaned pigs challenged with Escherichia coli K88 (+). Animal 14(1):39–49
Kaewchomphunuch T, Charoenpichitnunt T, Thongbaiyai V, Ngamwongsatit N, Kaeoket K (2022) Cell-free culture supernatants of Lactobacillus spp. and Pediococcus spp. inhibit growth of pathogenic Escherichia coli isolated from pigs in Thailand. Bmc Vet Res 18(1):60
Lawrence GW, McCarthy N, Walsh CJ, Kunyoshi TM, Lawton EM, O’Connor PM, Begley M, Cotter PD, Guinane CM (2022) Effect of a bacteriocin-producing Streptococcus salivarius on the pathogen Fusobacterium nucleatum in a model of the human distal colon. Gut Microbes 14(1):2100203
Wang G, Tang H, Zhang Y, Xiao X, Xia Y, Ai L (2020) The intervention effects of Lactobacillus casei LC2W on Escherichia coli O157:H7 -induced mouse colitis. Food Sci Human Wellness 9(3):289–294
Xie J, Yu Q, Nie S, Fan S, Xiong T, Xie M (2015) Effects of Lactobacillus plantarum NCU116 on intestine mucosal immunity in immunosuppressed mice. J Agric Food Chem 63(51):10914–10920
Kim JK, Lee KE, Lee SA, Jang HM, Kim DH (2020) Interplay between human gut bacteria Escherichia coli and Lactobacillus mucosae in the occurrence of neuropsychiatric disorders in mice. Front Immunol 11:273
Mitchell JG, Kogure K (2006) Bacterial motility: links to the environment and a driving force for microbial physics. FEMS Microbiol Ecol 55(1):3–16
Cheng S, Li B, Ding Y, Hou B, Hung W, He J, Jiang Y, Zhang Y, Man C (2024) The probiotic fermented milk of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 alleviates constipation via improving gastrointestinal motility and gut microbiota. J Dairy Sci 107(4):1857–1876
Valvaikar S, Vaidya B, Sharma S, Bishnoi M, Kondepudi KK, Sharma SS (2024) Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem Int 174:105691
Liu Y, Wang J, Wu C (2022) Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front Nutr 8:634897
Azad MAK, Sarker M, Li T, Yin J (2018) Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int 2018:9478630
Zheng Y, Liu G, Wang W, Wang Y, Cao Z, Yang H, Li S (2021) Lactobacillus casei Zhang counteracts blood-milk barrier disruption and moderates the inflammatory response in Escherichia coli-induced mastitis. Front Microbiol 12:675492
Yang KM, Zhu C, Wang L, Cao ST, Yang XF, Gao KG, Jiang ZY (2021) Early supplementation with Lactobacillus plantarum in liquid diet modulates intestinal innate immunity through toll-like receptor 4-mediated mitogen-activated protein kinase signaling pathways in young piglets challenged with Escherichia coli K88. J Anim Sci 99(6):skab128
Zhi S, Szelewicki J, Ziebell K, Parsons B, Chui L (2019) General detection of Shiga toxin 2 and subtyping of Shiga toxin 1 and 2 in Escherichia coli using qPCR. J Microbiol Methods 159:51–55
Acknowledgements
The authors would like to thank the working group of food bioresources lab for their valuable contributions during the study and also gratefully acknowledge the financial supports of Agro-Scientific Research in the Public Interest (Grant No. 201503135), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2024A1515012695), and Science and Technology Projects of Guangdong Province (Grant No. 2020B1212060059) for funding this research.
Author information
Authors and Affiliations
Contributions
Mohamedelfatieh Ismael: conceived of the presented idea, carried out the experiment and wrote the manuscript. Nageena Qayyum: contributed to the design and implementation of the research. Yaxin Gu: processed the experimental data, performed the analysis, drafted the manuscript and designed the figures. Li Na: verified the results and analysed the data and prepared figures. Han Haoyue: contributed to the analysis of the results and prepared figures. Muhammad Farooq & Panpan Wang: contributed to the writing of the manuscript and analysis of the results. Qingping Zhong: reviewed the manuscript and supervised the project. Xin Lü: reviewed the manuscript, involved in planning and supervised and funded the project. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0–309-15396–4, and approved by the Animal Ethics Committee of Xi’an Jiaotong University (permission no. SCXK 2018–001).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ismael, M., Qayyum, N., Gu, Y. et al. Functional Effects of Probiotic Lactiplantibacillus plantarum in Alleviation Multidrug-Resistant Escherichia coli–Associated Colitis in BALB/c Mice Model. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10356-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10356-7