Abstract
Whole-cell immobilisation technology involving ℽ-aminobutyric acid GABA biosynthesis using lactic acid bacteria (LAB) has been extensively studied owing to its numerous benefits over free-living bacteria, including enhanced productivity, improved cell viability, ability to prevent cell lysis and protect cells against bacteriophages and other stressful conditions. Therefore, a novel LAB biocatalyst was developed using various fruit and fruit waste, immobilising a potential probiotic strain, Lactiplantibacillus plantarum B7, via an adsorption method to improve GABA and cell viability. Apple and watermelon rind have been known to be the ideal natural supports for L. plantarum B7 owing to higher GABA and lactic acid production and improved cell viability among the other natural supports tested and selected to be used in repeated batch fermentation (RBF) to improve GABA production and cell viability. In general, immobilisation of L. plantarum B7 on natural support has better GABA and lactic acid production with improved cell viability via RBF compared to free cells. Watermelon rind-supported cells and apple-supported cells could produce nine and eight successful GABA cycles, respectively, within RBF, whereas free cells could only produce up to four cycles. When using watermelon rind-supported cells and apple-supported cells in RBF, the GABA titer may be raised by up to 6.7 (218.480 ± 0.280 g/L) and 6 (195.439 ± 0.042 g/L) times, respectively, in comparison to GABA synthesis by free cells in single batch fermentation (32.65 ± 0.029 g/L). Additionally, natural support immobilised L. plantarum B7 could retain half of its cell viability even after the 12th cycle of RBF, while no cell was observed in control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA) is a four-carbon free amino acid that possesses numerous health benefits, such as improving the secretion of growth hormone, regulating plasma levels, preventing chronic-related diseases and maintaining a healthy cardiovascular system [1]. Moreover, GABA plays a role in preventing diabetes by promoting insulin release [2]. In diabetic patients, GABA levels decrease, leading to nerve damage. A medication using a GABA analogue, known as gabapentin, can help treat diabetic neuropathy [3]. In the meantime, Ohmori et al. [4] reported that GABA decreased blood glucose levels in rats. Besides treating diabetes, GABA may slow or prevent the spread of cancer cells in the colon, liver and mammary glands via apoptosis [3]. Gao et al.’s [5] study proves the production of cadherin and GABA type B could be helpful in treating ovarian cancer. In stage 1 and 2 breast cancer patients, higher GABA levels were associated with longer survival in a clinical trial [6]. GABA also helps prevent and cure neurological disorders. When GABA levels are low, it can lead to conditions like epilepsy, seizures, Huntington’s disease and Parkinsonism [7]. According to Okada et al. [8], it was effective to treat various neurological disorders with a daily oral intake of rice germ containing 26.4 mg GABA. Yoga sessions can also boost GABA levels and potentially help with certain autonomic illnesses [9]. Microbial GABA production has been extensively studied as the parameters affecting GABA production are much easier to manipulate than those in plants, which have low levels of GABA. Meanwhile, GABA from animals cannot breach the blood-brain barrier, which prevents its beneficial effect on humans [10, 11]. Owing to its straightforward process, low cost of substrate, high transformation ratio and increasing demands for naturally-made GABA, microbial fermentation using naturally GABA-producing microorganisms becomes the most preferred method for producing GABA [12, 13]. In various naturally GABA-synthesising microorganisms, lactic acid bacteria (LAB) are the most preferred by many researchers due to their wide availability in nature, distinct physiological properties, probiotic effects and, most importantly, their safe (GRAS) status [14]. Among various LAB species, L. plantarum is also one of the species extensively studied for its GABA production and its potential probiotic properties [12, 13]. Besides improved GABA production through optimisation of GABA-producing fermentation parameters, the immobilisation technique could be further used to improve GABA to meet market demand [15, 16].
The immobilisation concept and methods for effective biocatalysts-viable cells (microbial, plant, or animal) and enzymes have added a new facet to the rapidly expanding field of biotechnology [17]. Numerous studies have been conducted to investigate the production of GABA using whole-cell and/or glutamate decarboxylase (GAD) enzyme immobilisation techniques. Glutamate decarboxylase (GAD) is the key enzyme that catalyses the conversion of glutamate into GABA and is usually found within various LAB species such as Lactobacillus, Lactococcus, Pediococcus and Streptococcus [18]. Several studies have investigated the use of immobilised GAD enzymes to catalyse glutamate into GABA. For example, Lee and Jeon [19] reported that nickel chelate sepharose immobilised GAD achieved a maximum conversion of 97.8% of GABA from 50 mM L-glutamate in a continuous flow system. However, the usage of metal affinity resin (a synthetic polymer) immobilised GAD in food and pharmaceutical industries, especially nickel-chelating resin, has raised safety concerns due to the toxicity of heavy metals [20]. Besides, the purification of GAD is also costly, in addition to its activity that can be quickly reduced.
Hence, whole-cell immobilisation within natural polymers has drawn much attention to improving GABA productivity due to safety concerns, cost-effectiveness and higher productivity. Apart from that, immobilised whole-cell systems have vast benefits over free-living bacteria, such as higher productivity, greater cell survival compared to free suspension, improved bacterial balance, improved plasmid stability, prevention of cell lysis, protection against bacteriophages and stressful conditions like shear damage, and improved by-product secretion [21]. Immobilised cell technology has been successfully used in GABA-producing fermentation processes involving lactic acid bacteria, as reported by several researchers. For instance, immobilised whole cells of L. brevis CGMCC 1306 in a fixed bed reactor yielded 5.67 g/L of GABA after 10 h of fermentation [22]. According to Kook and Cho [23], isomalto-oligosaccharides added to alginate bead-immobilised L. brevis GABA 057 strains in fed-batch mode converted 534 mM of MSG into 223 mM GABA in 48 h. The production of GABA and the stability of bacterial cells were both improved by the addition of isomalto-oligosaccharides to alginate beads [16]. Moreover, the immobilisation of engineered high-GABA-producing strain L. brevis GadADC14 with gellan gum gel beads achieved maximum GABA production of 87.56 g/L after 10 consecutive fermentation cycles under pH 4.4 at 40 °C [24].
Although cell entrapment within natural polymer support has numerous advantages, its application is restricted by limited diffusion, mechanical strength and a lack of open space to facilitate active cell growth, resulting in the release of cells into the growth medium and, eventually, their loss. In addition, scaling up is challenged by the operational cost [25, 26]. Therefore, alternative matrices are required to support cells, such as natural support derived from plant sources, which is composed of non-digestible cellulose components and serves as the base for cell attachment [25]. There have been several studies conducted using fruits as the natural support to immobilise the cell, such as apple and quince immobilised L. casei to improve lactic acid and ethanol production [27] apple immobilised yeast to improve wine-making at low and room temperature [28], guava immobilised yeast in wine-making [29], as well as cheese production using apple and pear immobilised L. casei [30].
Additionally, agro-waste, another type of natural support, could be used to immobilise cells owing to its high availability in nature and cheaper carrier source. Increasing agricultural production annually increases agro-waste worldwide. Nearly 998 MMT of agro-waste are produced per year globally, with 1.2 MMT from Malaysia [31]. Among those, 0.20 MMT of agro-waste was generated by the cultivation of tropical fruits [32]. As fruit is processed, the skin, rind, core and base are often removed, making up around 80% of the whole fruit known to be agro-waste [33]. As a result, effective waste management is essential for managing agriculture sustainably. Hence, the immobilisation of cells on agro-wastes may provide cheaper carriers than natural and synthetic polymers while also helping in effective agricultural waste management. The rinds of durian, jackfruit and mangosteen used as carriers to support L. acidophilus and L. bulgaricus during the fermentation of soy milk demonstrated faster growth, higher lactic acid and acetic acid production, as well as a greater reduction in carbon sources [33]. Lye et al. [34] discovered that soymilk fermented with agro-wastes (cempedak, durian and mangosteen) immobilised lactobacilli exhibited improved bioactive properties. Watermelon rind-supported yeast improves the fermentation rate, vitality and viability of yeast cells in wine-making applications [35].
Therefore, this study hypothesised that fruit waste (agro-waste) and fruit could be used as carriers for a potential probiotic strain, Lactiplantibacillus plantarum B7 to improve GABA production and its viability by reusing the cell via repeated batch fermentation (RBF).
Experimental Section
Samples
Initially, 84 LAB isolates have been isolated from various fermented food sources, including Budu (fermented anchovy sauce), Cincaluk (fermented shrimp), Tempeh (fermented soybean), Tapai cassava (fermented cassava), Tapai pulut (fermented glutinous rice), Kombucha tea, Mulberry tea and Kimchi (fermented vegetables); however, only 37 LAB isolates have shown GABA-producing capability. Among them, Lactiplantibacillus plantarum B7 isolated from Budu was identified as the highest GABA-producing strain (data not shown). Therefore, L. plantarum B7 (GenBank accession no. OL818343), a potential probiotic strain, was selected to be used in this study and stored at Microbial Culture Collection Unit (UNiCC), UPM (UPMC 1491) for further use. The strain was activated via subculture twice at 37 °C for 24 h, using MRS broth (Oxoid LTD, Basingstoke, Hampshire, GB), which was autoclaved at 121 °C for 15 min. All the fruit samples (list all the samples) were purchased from a local market at Serdang, Selangor.
Growth, GABA and Lactic Acid Production Profile of L. plantarum B7
The growth, GABA and lactic acid production profile of L. plantarum B7 were assessed for 96 h in MRS modified medium (5.2% (w/v) of MRS medium added with 1% (w/v) of glucose, 2.5% (w/v) of yeast extract, 2 ppm of each of tween 80, calcium carbonate and potassium iodide) at optimum GABA fermentation condition (35.6 °C, pH 5.66, 335.61 mM of initial MSG concentration, 0.723 mM PLP concentration and 63.66 h of incubation time) (unpublished data). Based on the procedure outlined by Kook and Cho [23], fermentation involving the synthesis of GABA using free-cell L. plantarum B7 was carried out. About 1% (v/v) of active culture was transferred into MRS medium containing 1% (w/v) of MSG (Sigma-Aldrich, MO, US) and incubated for 24 h as an inoculum. After that, 10% (v/v) of the inoculum (~9 log CFU/mL) was added into MRS modified medium (fermentative media), followed by shaking for 96 h at 35.6 °C in an incubator (Lab Companion shaker IS-971R, MN, US). Every 6 h during the first 24 h, sampling was done. Then, every 24 up to 96 h later, sampling was conducted with an additional sampling point at 63.66 h, which is the maximum GABA production time. Viable cell count, pH and both the GABA and lactic acid concentration were evaluated. The conventional plate count technique was used to determine the number of viable cells, which were expressed as log colony forming units per millilitre (log CFU/mL). The sample was serially diluted ten times using sterile 0.85% (w/v) NaCl solution (R&M Chemicals, Essex, GB). A 100 µL aliquot of the sample was diluted, plated on MRS agar (Oxoid LTD, Basingstoke, Hampshire, GB) and then incubated anaerobically at 37 °C for 48 h. A colony counter was employed to manually count the bacterial colonies (Stuart, GB). The pH was measured using Eutech pH 700 Meter (Thermo Fisher Scientific Inc, Massachusetts, US). Lactic acid was quantified using the spectrophotometric method, as described by Borshchevskaya et al. [36], while GABA was determined using a colourimetric procedure, as described by Dikshit and Tallapragada [37] with minor modifications.
Cell Immobilisation
The cell immobilisation method was carried out based on the method described by Kourkoutas et al. [27] with minor modifications. Two different types of natural support were used to immobilise a potential probiotic strain, L. plantarum B7, known as fruit and fruit waste. The fruits included apple (Malus domestica ‘Gala’), pear (Pyrus bretschneideri ‘Chinese white pear’), pomegranate (Punica granatum), strawberry (Fragaria ananassa ‘Senga Sengana’) and guava (Psidium guajava ‘Tropic white’); meanwhile, fruit waste such as watermelon rind (Citrullus lanatus ‘Glamour’), mangosteen rind (Garcinia mangostana ‘Manggis’), pomegranate peel (Punica granatum), banana peel (Musa acuminata ‘Cavendish’) and orange peel (Citrus sinensis ‘Navel’) were used to immobilise. All the fruits and fruit waste selected in this study were chosen for the reason that these fruits and fruit waste have been previously used as natural carriers for immobilising various strains to improve numerous targeted productions by many researchers [27,28,29, 33, 35, 52]. All the fruit and fruit waste were cut into small pieces (1 cm3) and sterilised at 121 °C for 15 min. About 1% (v/v) of active culture (~9 log CFU/mL) and the ratio of 1 to 2 of natural support to the total volume of the medium were introduced into MRS broth containing 1% (w/v) of MSG (Sigma-Aldrich, MO, US) and incubated at 37 °C with 125 rpm for 12 to 15 h in an incubator (Lab Companion shaker IS-971R, MN, US). For instance, Fig. 1 displays apple and watermelon rind pieces immobilised with L. plantarum B7. Upon complete immobilisation, the biocatalysts were washed twice (0.5 mL) with MRS broth containing 1% (w/v) of MSG, and the fermented liquid was decanted. The biocatalysts were then used for GABA fermentation.
GABA Fermentation Using Various Biocatalysts
Fermentation involving GABA production using immobilised L. plantarum B7 proceeded according to the method described by [27] with minor modifications according to [38]. The fermentation was conducted at optimum GABA-fermentation conditions using optimised fermentative media. The 10% (w/v) of inoculum (whole-cell immobilised biocatalysts) was transferred into the optimum GABA-fermentation medium. The fermentations were conducted for 63.66 h at 35.6 °C with 125 rpm. After complete incubation, the supernatant was extracted using a centrifuge (Microfuge 16 Centrifuge, Krefeld, DE) at 9000 × g for 15 min under 4 °C. The extracted supernatants were stored at −20 °C for further analysis. Fermentation using each fruit and fruit waste without L. plantarum B7 cell served as a negative control.
Successive Fermentation Batches of GABA
Based on the analysis, each of the two types of natural support was chosen to be used in RBF based on their best capability to immobilise a potential probiotic strain, L. plantarum B7 and produce GABA. The number of successive GABA fermentation batches was determined based on the GABA level of each batch required to be at least 50% of the initial GABA concentration (1st cycle) [39]. About 10% (w/v) of chosen whole-cell immobilised biocatalysts were transferred into the optimum GABA-producing fermentation medium and incubated at optimised GABA fermentation parameters. At the end of each fermentation batch, the liquid was decanted, and the support was washed twice with MRS broth containing 1% MSG before being transferred into the new fresh optimum GABA-producing medium. The decanted liquids were centrifuged (Microfuge 16 Centrifuge, Krefeld, DE) at 9000 × g for 15 min under 4 °C for the supernatants and stored at −20 °C for further analysis. RBF was completely stopped once the GABA concentration was produced below the successive level. A 10% (v/v) of free cells with an initial viable cell concentration of ~9 log CFU/mL were transferred into the optimum GABA-producing fermentation medium and incubated at optimised GABA fermentation parameters as a control. At the end of each fermentation batch, around 90% of the media was decanted, and the remaining 10% of the previous media was transferred into a new fresh optimum GABA-producing medium. Then the decanted media were centrifuged to collect the supernatant and stored for further analysis.
Biocatalyst and Free Cell Viable Count
The viable cell count on the biocatalysts was identified once after completing the immobilisation (total number of cells immobilised) and at the end of fermentation (number of cells retained) according to the procedure reported by Aguirre-Guzmán et al. [40] with minor modifications. About 1 g of immobilised samples was blended with 9 mL of sterile 0.85% (w/v) of NaCl solution (R&M Chemicals, Essex, GB) for 2 min using Panasonic blender MX-895. About 1 mL of the blended samples was serially diluted from 101 – 109 times, whereas 0.1 mL of each diluted culture was equally dispersed on MRS agar plates and incubated anaerobically for 48 h at 37 °C. Using a colony counter, the bacterial colonies were manually counted. Meanwhile, free cell viable count for each sample was detected using fermentation medium at the end of each cycle of RBF. About 1 mL of fermentation medium of each sample subjected to be diluted and spread plate on MRS agar plates and incubated anaerobically for 48 h at 37 °C.
Analyses
The final pH of the sample was measured using the Eutech pH 700 Meter (Thermo Fisher Scientific Inc., Massachusetts, USA). The lactic acid concentration was determined using a spectrophotometric method, as described by Borshchevskaya et al. [36]. A 25-µL-centrifuged sample was added to 1 mL of iron(III) chloride mixture absorbance and measured at 390 nm under a UV-Vis spectrophotometer (Uviline 9400 spectrophotometer, Alès, FR) at 25 ± 5 °C within 15 min after preparing the solution. The absorbance was measured against a reference solution of 25 µL of fermentation medium without cell (negative control) with 1 mL of iron(III) chloride. A lactic acid standard curve ranging from 0.02 to 0.2 mg/µL was then developed. GABA concentration was quantified using the colourimetric method, as described by Dikshit and Tallapragada [37] with minor modifications. A TLC plate (Merck, Darmstadt, DE) was aliquoted with 2 µL of sample and developed using n-butanol-acetic acid-H2O [5:3:2 (v/v/v)] (Merck, Darmstadt, DE) before being sprayed with 1% (w/v-ethanol) of ninhydrin solution. The developed TLC plate was dried in an oven at 60 °C for 30 min before the GABA spots were scratched out of the paper and placed in 3 mL of borate buffer (pH 7) and 0.5 mL of 0.8% (w/v of acetone) ninhydrin reagent. The solution was incubated for 20 min at 70 °C, while absorbance was read at 570 nm under a UV-Vis spectrophotometer (Uviline 9400 spectrophotometer, Alès, FR). A GABA standard curve was developed using a GABA standard solution (10 mg/mL).
Scanning Electron Microscopy
Based on the analysis, each of the two types of natural support biocatalyst was chosen to monitor the immobilisation of L. plantarum B7 on the biocatalysts once after completing the immobilisation (initial) and at the end of RBF (final) using scanning electron microscopy. The immobilised biocatalysts were washed with MRS broth containing 1% MSG and dried overnight at 30 °C. After drying, the samples were subjected to coating with gold in a BAL-TEC SCD 005 Sputter coater for 2 min before being examined in a JEOL JSM IT-100 scanning electron microscope. The samples were viewed at 5000× magnifications.
Statistical Analysis
GraphPad Prism 9.0 was used for the analysis of experimental data (San Diego, CA, USA). The statistical differences between the means of the data were determined using a one-way analysis of variance ANOVA, with a significance level set at p < 0.05. Tukey’s multiple range tests were used to compare multiple means. All experiments were carried out in triplicate, while the mean values and standard error of each sample were expressed as mean ± SEM.
Results and Discussion
The experiments were conducted according to the objectives of this study, which started from the construction of the L. plantarum B7 growth curve together with lactic acid and GABA production profile in an optimised MRS medium at 35.6 °C for 96 h, followed by the screening of the ideal natural support biocatalyst based on the lactic acid. GABA analysis and number of cells retained, and finally, RBF was conducted using the ideal natural support to improve further GABA production under optimum GABA production conditions. Along with the determination of GABA, measuring the amount of lactic acid produced by L. plantarum B7 is significant in this study, especially in the section on RBF, since during RBF, it is impractical to count the strain’s cells at each batch of fermentation, as they are immobilised in natural support that must be transferred into the following batch. Therefore, the cell count of immobilised L. plantarum B7 in each batch was estimated by determining the lactic acid concentration, as it is the primary metabolite and is often considered a growth-associated product of lactic acid bacteria [41]. It is also used to relate pH changes and GABA production in each batch.
L. plantarum B7 Growth, GABA and Lactic Acid Production Profile
In this study, the growth profile, together with pH changes, GABA and lactic acid production by L. plantarum B7 in an optimised MRS medium at 35.6 °C, was examined every 6 h for a 24-h period, followed by a 24-h interval for sampling until 96 h (Fig. 2). In terms of the cell viability count, the cells were counted to be at 7.640 ± 0.001 log CFU/mL at 0 h, representing the concentration of cells obtained from the 24-h incubated inoculum culture. Despite the fact that no lag phase was observed during the 6-h sampling period, it can be assumed that the lag phase occurred within this period. As reported by Ding and Li [42], L. plantarum Z7 entered an exponential phase just after 3 h of fermentation in MRS medium, indicating a very short lag phase. During the first 12 h of the fermentation, L. plantarum B7 grew and rapidly multiplied, reaching a log CFU/mL of 11.403 ± 0.003. Multiple rounds of DNA synthesis, together with transcription and translation, were involved during exponential bacterial growth and replication to produce the essential macromolecules [43]. The stationary phase of L. plantarum B7 was then observed at the 18th hour of fermentation and remained plateaued until the end of fermentation at 96 h.
Apart from cell viability, the concentration of lactic acid and changes in pH usually correlate with the growth of lactic acid bacteria, since its metabolism could synthesise various organic acids throughout the growth period, such as lactic acid, acetic acid, succinic acid and 3-hydroxypropionic acid. Hence, the production of various organic acids will affect the pH of the medium during the fermentation [44]. Based on the result, the production of lactic acid follows the trend observed for cell growth, as most of the lactic acid is usually produced at the log phase of cell growth; therefore, it is known to be a growth-associated production [45]. During the exponential phase between 0 h until the 18th hour, lactic acid was produced at a very high rate, going from 0 to 39.947 ± 0.128 g/L. This is anticipated as lactic acid is the major metabolism product of lactic acid bacteria such as L. plantarum B7 [46]. Due to the high accumulation of lactic acid, the pH of the medium changed. During the same period, the pH values significantly dropped from pH 5.66 to 4.34. Meanwhile, lactic acid reached its maximum concentration at 63.66 h (42.056 ± 0.061 g/L). There are several reasons for the reduction in lactic acid concentration after 63.66 h. Nutrient depletion or insufficient substrate during the fermentation may slow down the growth of LAB and reduce the production of lactic acid. Besides that, the accumulation of the metabolic products of LAB, mainly organic acids including lactic acid, acetic acid and others may reduce the pH of the culture to become more acidic, which creates an unfavourable environmental condition for the growth of LAB and eventually reduces the production of lactic acid [47].
For the GABA yield, the L. plantarum B7 cells produced GABA at a fairly slow rate during the first 18 h of the fermentation, as opposed to lactic acid and log CFU/mL. At the 18th hour, it only managed to produce GABA at 4.236 ± 0.092 g/L, which was not much of an increase from the GABA at the 12th hour (1.927 ± 0.112 g/L) and the 6th hour (0.528 ± 0.094 g/L). However, after entering the stationary phase at the 18th hour, GABA started to be produced at an exponential rate, going from 12.992 ± 0.033 g/L at 24 h to reaching the maximum GABA of 32.65 ± 0.029 g/L at 63.66 h. This result can be supported by Yogeswara et al. [48], who reported that L. plantarum FNCC 260 was able to produce the highest GABA concentration after 60 h of fermentation. Moreover, several literatures also reported that the highest GABA production by lactic acid bacteria can be seen in the range between 48 and 72 h of fermentation [49, 50]. After that, the GABA production by L. plantarum B7 began to decline until the end of fermentation at the 96th hour. This might be due to substrate (MSG) depletion, which prevents L. plantarum B7 from producing further GABA. Besides that, increasing the concentration of GABA increases the pH, which eventually activates the enzymes (γ-aminobutyric acid aminotransferase (GABA-AT) and succinic semialdehyde dehydrogenase (SSADH)) that are responsible for GABA degradation [10, 12, 18, 51]. The produced GABA will be degraded by GABA-AT into succinic semialdehyde, followed by irreversible oxidation of it catalysed by SSADH, producing succinate, which then enters the TCA cycle and eventually transforms into the precursor (oxaloacetate) that undergoes gluconeogenesis. Based on the results obtained, it can be concluded that the optimum fermentation time for the production of GABA by L. plantarum B7 was 63.66 h. Hence, for the subsequent experiments, all sampling procedures were taken at 63.66 h of fermentation.
Screening of Ideal Natural Support for L. plantarum B7
The screening of the ideal natural support was conducted using two types of natural support, namely fruits (apple, pear, pomegranate, strawberry and guava) and fruit waste (watermelon and mangosteen rind, pomegranate, banana and orange peel) based on the GABA production, lactic acid concentration and the number of cell retention after fermentation. The lactic acid, GABA production and final pH of the medium were assessed after 63.66 h of fermentation using various types of fruit-immobilised L. plantarum B7 (Fig. 3). The results demonstrated that, among the tested fruit support biocatalysts, apple-immobilised L. plantarum B7 recorded the highest GABA (31.967 ± 0.008 g/L), while guava-immobilised L. plantarum B7 produced the highest lactic acid (40.832 ± 0.813 g/L), with no significant difference (p > 0.05) compared to the rest of tested fruit pieces support biocatalysts. From the initial pH value of the liquid medium that was set at 5.66 ± 0.01 at the start of the fermentation, the lowest final pH (pH 4.29 ± 0.01) was observed in the sample containing guava support L. plantarum B7 and apple support L. plantarum B7. In order to evaluate the potential of the fruit pieces to support L. plantarum B7, the number of cells immobilised on each fruit was assessed after the immobilisation process. As tabulated in Table 1, the number of cells immobilised on various fruits ranged from 8.170 ± 0.051 to 8.340 ± 0.032 log CFU/g. The highest amount of cells was immobilised on apple pieces (8.340 ± 0.032 log CFU/g) with no significant difference (p > 0.05) compared to the other fruits tested. To investigate the strength of natural support in retaining the cells after the fermentation process, cell retention was calculated and shown in a percentage. At the end of fermentation, apple biocatalyst significantly showed the highest percentage (86.662 ± 1.162%) of cells retained than all the other tested fruit biocatalysts except for pear pieces (Fig. 3).
The effects of Lactiplantibacillus plantarum B7 immobilised on various fruit samples on lactic acid, GABA concentration and final pH of the fermentation medium after incubating for 63.66 h at 35.6 °C with 125 rpm. Each data represents the mean ± SEM of three replicates. Different lowercase letters on the top of the bar indicate statistically significant differences (p < 0.05) of the results among various biocatalysts. Negative control represents no GABA and lactic acid production
Apart from fruit biocatalyst, the effects of various fruit wastes immobilised L. plantarum B7 on the production of lactic acid, GABA and final pH of the fermentation medium were also assessed (Fig. 4). It was discovered that the watermelon rind support cell has the highest GABA concentration (31.772 ± 0.022 g/L) with a significant difference (p < 0.05) among the rest of the fruit waste biocatalysts tested. Meanwhile, the highest lactic acid produced by banana peel supported L. plantarum B7 with no significant difference (p > 0.05) compared to orange peel and watermelon rind biocatalysts. Watermelon rind immobilised L. plantarum B7 has the lowest final pH (pH 4.34 ± 0.01) with no significant difference (p > 0.05) among the rest. Based on Table 2, the number of cells immobilised on various fruit wastes ranged from 8.006 ± 0.018 to 8.724 ± 0.008 log CFU/g. Among the tested fruit waste biocatalysts, watermelon rind biocatalyst has the highest number of cells immobilised (8.724 ± 0.008 log CFU/g) and the percentage of cells retained (90.158 ± 0.071%). After 63.66 h of incubation, none of the natural supports without cells (negative control) showed any significant changes in the fermentative medium. Negative control samples showed no lactic acid or GABA content, with no live cells that could be counted. Hence, this finding demonstrates that the production of lactic acid and GABA was completely dependent on various natural supports immobilised L. plantarum B7.
The effects of Lactiplantibacillus plantarum B7 immobilised on various fruit waste samples on lactic acid, GABA concentration and final pH of the fermentation medium after incubating for 63.66 h at 35.6 °C with 125 rpm. Each data represents the mean ± SEM of three replicates. Different lowercase letters on the top of the bar indicate statistically significant differences (p < 0.05) of the results among various biocatalysts. Negative control represents no GABA and lactic acid production
Among the screened fruits, the apple was found to be the best fruit support for a potential probiotic strain, L. plantarum B7, to synthesise GABA and lactic acid, as it can immobilise a greater number of cells compared to the other tested fruits. Besides, a higher number of cells were retained in the apple after the fermentation was completed. Apple has been frequently used by several researchers in their studies as a natural whole-cell supporter owing to its immobilisation capabilities and increased productivity. For instance, apples were used by Kourkoutas et al. [27] to immobilise Lacticaseibacillus casei to improve the lactic acid production in fermented milk. Additionallyf, employing the apple-immobilised Saccharomyces cerevisiae strain AXAZ-1, Plessas et al. [52] revealed superior alcoholic fermentation under low temperatures along with improved sensory characteristics of wine. Moreover, apple-immobilised Lactobacillus delbrueckii subsp. bulgaricus has been found to have a higher survival rate during storage under freeze-drying and thermal drying processes and was able to maintain successive lactic acid production even after an immediate reactivation in whey [53]. Apart from the fruits evaluated in this study, other fruits, such as mango, sapota, quince, banana and pineapple, also have the ability to immobilise whole cells [27, 54].
Based on the result, watermelon rind was identified as the best fruit waste supporter for a potential probiotic strain, L. plantarum B7, with a high amount of GABA and lactic acid due to the higher number of cells immobilised compared to other fruit waste. Unlike other tested fruit wastes, watermelon rind has been found to be able to retain more cells even after fermentation. Previously, watermelon rinds have been used as an immobiliser for Saccharomyces cerevisiae 101 in the wine-making process [35]. It was then discovered that the use of immobilised S. cerevisiae 101 had a better fermentation rate, vitality and viability of the strain in the process of wine-making. Additionally, the flavour, aroma, taste and overall quality of the wine have also been enhanced. Plessas et al. [52] also investigated an orange peel as a potential immobiliser for S. cerevisiae in an alcoholic fermentation at various temperatures. They claimed that employing S. cerevisiae immobilised on orange peel resulted in better ethanol productivity with less fermentation time needed. Furthermore, Teh et al. [33] ground up durian, mangosteen and cempedak rinds (agro-wastes) into powder to increase the total surface area, which promoted a greater number of cells to immobilise before being used to support Lactobacillus acidophilus FTDC 1331, FTDC 2631, FTDC 2333, FTDC 1733 and Lactobacillus bulgaricus FTCC 0411 in soymilk fermentations. It was then discovered that cells immobilised on the agro-waste powder had increased substrate utilisation and cell growth, which enhanced the production of lactic and acetic acids in the fermented soymilk.
In comparison, no significant difference (p > 0.05) was observed between the concentrations of lactic acid and GABA produced by L. plantarum B7 immobilised on apple pieces and watermelon rinds. However, a significant difference was noticed in the number of cells immobilised on watermelon rinds and apple pieces. Although there were fewer cells immobilised on the apples compared to watermelon rinds, there was no significant difference in the production of lactic acid or GABA, which is most likely a result of the sugar being utilised by L. plantarum B7 immobilised on apple fruit that contains more fermentable carbohydrates than the watermelon rinds, where the cells consumed the sugar to enhance their growth and metabolic production. Based on a study conducted by Vitola et al. [55], it was discovered that different fruits (pineapple, guava and kiwi) resulted in different amounts of Lacticaseibacillus casei CSL3 cells immobilised to the fruits, albeit the same bacterial concentration used for each fruit. They deduced that this may be due to the undigested carbohydrate content present on the fruits, explaining the different log CFU/g and cell retention for each fruit piece analysed. Cells can attach to fruits because of the non-digestible carbohydrates that constitute the fruit matrix [25]. Furthermore, the apple’s fermentable carbohydrate utilisation by the immobilised cells was proved by the lower percentage of cells retained on apple pieces (86.662 ± 1.162%) than the watermelon rinds (90.158 ± 0.071%) after the fermentation, since more cells were falling off from the apple biocatalyst than from the watermelon rind biocatalyst as a result of the loss of mechanical support between the cells and the natural support digested by the cells during fermentation.
RBF Using Natural Support Immobilised L. plantarum B7
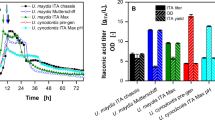
The ability to reuse cells for multiple fermentation cycles to increase productivity at a reduced cost and eliminate the time of preparing a new inoculum for each batch of fermentation is among the advantages of employing immobilised cells over free cells [39]. To further increase the GABA titre via RBF, the ideal natural support from fruit (apple pieces) and fruit waste (watermelon rind) biocatalysts containing a potential probiotic strain was employed. Based on Figs. 5 and 6, there was a decreasing trend in GABA and lactic acid concentrations by immobilised cells and free cells. Compared to free cells, the immobilised L. plantarum B7 has a better production of lactic acid and GABA due to the fact that GABA and lactic acid production by free cells decrease more rapidly throughout the RBF than that observed for the immobilised cells. Additionally, the production of GABA and lactic acid by free cells entirely stopped after the seventh and ninth cycles of RBF, respectively. Nonetheless, immobilised cells were still able to synthesise GABA and lactic acid up to the twelfth cycle of RBF. The final pH of the medium was measured at the end of each fermentation cycle to support the results. According to Fig. 7, samples containing free cells showed a rapid increase in pH until the 9th batch and remained stable after that throughout RBF compared to samples containing immobilised cells, in which the pH (in the range of 4.34 ± 0.01 to 4.89 ± 0.01) was slowly increased. The decrease in the synthesis of organic acids, particularly lactic acid, by L. plantarum B7 increased the pH due to the loss of cells during RBF. It can be observed that nearly half of the initial concentration of cells immobilised on the apple pieces and watermelon rinds were lost after the 12th cycle of RBF (Table 3). However, for the sample containing free cells, there was absolutely no cell count at the end of RBF. The rapid reduction in lactic acid and GABA concentrations in samples containing free cells was due to the rapid reduction in cell count until the 9th batch and no cell count at all thereafter throughout the RBF (Fig. 8). However, no significant changes were noticed in the free cell count within immobilised samples throughout RBF. The stationary final pH of the sample containing free cells at the final three batches of RBF indicated that no fermentation occurred due to no viable cell count present, which kept the initial pH unchanged even after the incubation. Additionally, viewing the biocatalysts under a scanning electron microscope (SEM) proved the immobilisation of L. plantarum B7 on the surface of apple and watermelon rind pieces (Fig. 9). As observed from the SEM images, the cells seemed to be naturally attached to the surface of both watermelon rinds and apple pieces. The mechanism involved in the attachment may be classified as passive immobilisation, which is defined as the use of the natural tendency of microorganisms to attach or adsorb to surfaces-natural or synthetic and grow on them [56]. This result is supported by Teh et al.’s [33] statement, which stated that the majority of passive immobilisations can be found in natural supports such as fruits, plants and organic substances. Therefore, it is reasonable to assume that the cells were naturally adsorbing to the natural support surfaces. Besides, according to Junter and Jouenne [57], the immobilisation of cells in an ideal environment will cause the cells to adsorb to the surface and eventually colonise the support, which results in the development of a biofilm. In essence, a biofilm develops when cells are attached to one another and adhere to a surface [58], leading to the conclusion that L. plantarum B7 cells naturally adsorb to fruits and fruit waste pieces, and the development of biofilm may have also facilitated the attachment. Zur et al.’s [59] statement that bacteria can grow planktonically or create a biofilm that adheres to the surface they adsorb onto lends further weight to this conclusion. Moreover, the SEM results qualitatively demonstrated the reduction of cell attachment on the immobiliser at the end of RBF.
GABA production at various cycles by whole-cell immobilised Lactiplantibacillus plantarum B7 on watermelon rind, apple and free cell as a control. Successful GABA production cycle is noticed above black line (free cell), green line (watermelon rind biocatalyst) and red line (apple biocatalyst). Each data represents the mean ± SEM of three replicates. Different lowercase letters on the top of the bar indicate statistically significant differences (p < 0.05) of the results among various cycles
Lactic acid concentration at various cycles by whole-cell immobilised Lactiplantibacillus plantarum B7 on watermelon rind, apple and free cell as a control. Successful lactic acid production cycle is noticed above black line (free cell), green line (watermelon rind biocatalyst) and red line (apple biocatalyst). Each data represents the mean ± SEM of three replicates. Different lowercase letters on the top of the bar indicate statistically significant differences (p < 0.05) of the results among various cycles
Scanning electron microscopic (SEM) image of whole-cell immobilised Lactiplantibacillus plantarum B7 on watermelon rind before the fermentation (a) and after the 12th cycle of fermentation (b); whole-cell immobilised Lactiplantibacillus plantarum B7 on apple pieces before the fermentation (c) and after the 12th cycle of fermentation (d)
During transfers of cells from one cycle to another, free cells in suspension will be quickly diluted and lost throughout the RBF until no cells can be observed. Meanwhile, during the RBF period, immobilised cells firmly attached to the carrier prevented it from being lost or leaked. Apart from the second cycle, every RBF cycle exhibits a considerable loss of GABA and lactic acid titers due to cell leakage, which results in a small number of cells found within the fermentation medium at the end of fermentation (Fig. 8). In general, when cell leakage occurs, the metabolic products of immobilised cells often decrease [60]. A significant increase in both GABA and lactic acid was seen in the second cycle of RBF, which might be due to the incomplete washing of the biocatalyst before being transferred into the subsequent cycle of RBF, allowing the excess free cells from the previous cycle to stick on the biocatalysts, transferred together with the immobilised cells into the subsequent fermentation medium and eventually grew and affected the analysis [61]. Apart from that, the increment in both GABA and lactic acid concentrations observed in the 2nd cycle of RBF might be a result of the growth and metabolic production of leaked cells from the biocatalyst, which acted as free cells in the fermentation medium [62]. According to Yuvadetkun and Boonmee [38], a similar outcome was obtained when a small number of free cells of Candida shehatae were found in the fermentation medium containing coconut bract immobilised C. shehatae during ethanol production from rice straw hydrolysate. Additionally, during the ethanol production process by Saccharomyces cerevisiae cells immobilised on orange peel, Plessas et al. [52] reported a threefold higher cell concentration within the immobiliser compared to the leaking free cell concentration. The composition of the cellular wall and immobiliser, cell maturation, pH and ionic content of the medium are some of the main causes of cell leakage [63].
As shown in Fig. 8, the sample containing apple biocatalyst had a higher concentration of free cells than the sample containing watermelon rind biocatalyst due to the fact that apples generally have more carbohydrates than watermelon rinds [64, 65], which allows the apple immobilised cells to digest it and eventually falls off the biocatalyst from the loss of support and becomes a free cell. According to Geethanjali and Subash [66], the decline in GABA and lactic acid concentrations may be the result of cell loss during the washing of the biocatalyst before being transferred into the subsequent fermentative medium. Likewise, Wang et al. [62] observed a rapid decrease in β-cyclodextrin (β-CD) produced by free cells, increasing the fermentation cycle compared to a slow decrease in β-CD produced by Bacillus circulans ATCC immobilised on a palm curtain. In the meantime, a decreasing trend of sorbitol production by sodium-alginate immobilised Lactiplantibacillus plantarum (BAA-793) was observed by increasing the repeated cycle of solid-state fermentation [39].
The production of GABA (Fig. 5) and lactic acid (Fig. 6) by free cells was higher during the first cycle of RBF compared to the immobilised samples since the initial concentration of free cells was noticeably higher than the initial immobilised cell count (Table 3). This is unavoidable as it is difficult to precisely control the desired amount of initial cell concentration to be immobilised onto the carrier. Additionally, compared to the immobilised L. plantarum B7, the free cell produced more GABA and lactic acid due to its larger surface area in contact with the nutritive contents and MSG within the fermentative medium. A similar result has been found by Wang et al. [62], where free Bacillus circulans ATCC 21783 synthesised higher β-CD compared to the immobilised cells. Moreover, Yuvadetkun and Boonmee [38] reported that free cell Candida shehatae ATCC 22984 produced more ethanol than the immobilised cells. In contrast, okara-immobilised Lactiplantibacillus plantarum 70810 produced higher levels of lactic acid and acetic acid during soymilk fermentation than the free cells as okara-immobilised L. plantarum 70810 has faster growth and metabolic production rates compared to free cells due to okara’s prebiotic values [67].
Figure 10 shows the images of watermelon rind and apple pieces captured before and after the 12th cycle of RBF. It could be observed that the size and shape of the apple biocatalyst were significantly changed at the end of RBF (Fig. 10). Since the structure of apple pieces was softer and flimsier compared to the watermelon rinds, the apple pieces were prone to be broken into tiny little pieces when transferring the fruit pieces from one batch to another, causing some cell losses during the process. This may also be due to cells consuming the digestible carbohydrates in the apple and leaving non-fermentable cellulose residue. From the lower composition of digestible carbohydrates in watermelon rind than in apples, no significant changes for watermelon rind biocatalysts were observed after finishing the RBF. Additionally, compared to apples, watermelon rind exhibited higher levels of cellulose (fibre), which allowed the watermelon rind biocatalyst to maintain its original size and volume following fermentation [68, 69]. Several studies have previously reported that the volume of natural support biocatalysts decreased throughout the fermentation [27,28,29, 35]. Besides, a significant decrease in the size of the apple pieces at the end of RBF in this study might also be due to the leaching of some organic constituents into the fermentation medium, which might eventually be consumed again by the cell, such as glucose, fructose and sucrose. However, it is expected that fewer organic constituents will be leached from watermelon rind as compared to apple pieces due to no significant decrease in its size at the end of RBF. Apart from that, both apple and watermelon rind pieces might also leach various types of organic constituents such as vitamins, antioxidants, organic acids and phenolic compounds [70, 71]. This leaching process might be helpful as it may also contribute to the flavour and distinctive aroma of the finished product. The leaching process of the guava organic constituents in the wine has been previously discussed by Reddy et al. [29].
In general, it could be deduced that the successful GABA production cycle for free cells was up to the fourth cycle, apple biocatalyst up to the eighth cycle and watermelon rind up to the ninth cycle of RBF at 35.6 °C for 63.66 h (Fig. 5). Meanwhile, free cells, apple biocatalysts and watermelon rind biocatalysts produced successful lactic acid concentration until the fifth, eighth and ninth cycles of RBF, respectively (Fig. 6). To be deemed as a successful production cycle, the production of GABA and lactic acid from each cycle must be at least 50% of its initial production. It has been assumed that a cycle’'s production of less than 50% of its initial production was unworthy of continuing the next RBF cycle. Zuriana and Sakinah [39] employed a similar approach to identify whether the sorbitol-producing cycle by L. plantarum (BAA-793) within RBF can be deemed successful. The RBF was stopped at the 5th cycle when the sorbitol production was 7.619 g/L (approximately < 50% of the initial sorbitol production) and considered unworthy to continue. The findings demonstrated that immobilised L. plantarum B7 outperformed the free cells in terms of GABA and lactic acid production under RBF mode (Table 4). Earlier, Kourkoutas et al. [27] reported a total of 15 successive fermentation batches of whey-producing lactic acid carried out using apple and quince-immobilised Lacticaseibacillus casei, while only six batches were obtained using free cells. Reddy et al. [29, 35] reported that utilising watermelon rind and guava immobilised yeast cells produced 12 successive wine-making fermentation batches (3 batches for each temperature of 15, 20, 25 and 30 °C). Meanwhile, around seven successive cycles of β-CD production have been reported using palm curtain immobilised Bacillus circulans ATCC 21783 with more than 80% of remaining enzyme activity [62].
According to Table 4, watermelon rind biocatalyst produced the highest amounts of total successful GABA (218.480 ± 0.280 g/L) and lactic acid (286.719 ± 0.207 g/L) content, followed by apple biocatalyst and finally free cell. When compared to GABA and lactic acid produced by free cells in single batch fermentation (first cycle of RBF), GABA and lactic acid from the free cell, RBF rose 3.0 and 4.2 times, respectively (Figs. 5 and 6). However, compared to the production of GABA by the free cell L. plantarum B7 from the first cycle of RBF, GABA was enhanced to 6.7 (218.480 ± 0.280 g/L) and 6.0 (195.439 ± 0.042 g/L) times for watermelon rind and apple biocatalysts, respectively, when employed in the RBF. Meanwhile, the lactic acid production by free cells in the first cycle of RBF could be raised by 6.8 (286.719 ± 0.207 g/L) and 6.0 (253.889 ± 0.140 g/L) times when using watermelon rind and apple-supported cells via RBF.
Studies using cellulosic materials as probiotic carriers are new to ensuring the viability of the probiotic strain in the gastrointestinal tract. This is due to the fact that there are no cellulosic compounds that can be digested within the human digestive system that enable the cellulose-immobilised probiotics to reach the colon safely without significant loss [27]. Probiotic strains found in the large intestine serve to sustain human health by developing a balanced gut flora [72]. As a result, fruits and fruit wastes with high cellulose content are known as the best carrier option for probiotics that might be used in the functional foods and beverages industries to produce highly nutritional and valuable products containing GABA and other bioactive compounds. Apart from enhancing the nutritional quality of food by adding probiotics, the shelf life of the food could also be improved. Additionally, the food’s flavour and aroma could be also enhanced [27]. Besides, using natural carriers such as fruits and fruit waste also improves the nutritional value of the final product as it contains nutritious and bioactive components. Apples, for instance, are an excellent source of various bioactive substances, particularly phenolic compounds, including phenolic acids, flavonols, flavan-3-ols, dihydrochalcones and anthocyanins [73]. It also contains much ascorbic acid [74]. In the meantime, pectin, a common polysaccharide used as an additive in the food industry [75], and citrulline, a non-protein amino acid with antioxidant and vasodilatation activity [76], are both naturally present in the watermelon rind. Additionally, compared to its flesh, watermelon rind has a higher concentration of phenolic compounds [76]. Therefore, fruits and fruit wastes have a better prospect as natural carriers for the immobilisation of probiotics to produce specific components or products, especially in the functional food and beverage industries, which have vast health benefits for humans.
There are two distinct approaches to employing whole-cell immobilisation in various applications: column-packed whole-cell biocatalysts and dispersed whole-cell biocatalysts. Column-packed whole-cell biocatalyst involves the packing of whole-cell immobilised matrices within a column, offering a controlled and stable environment for reactions and facilitating separation. It is particularly well-suited for continuous processes but can face challenges related to mass transfer limitations and the potential for clogging or fouling of the column [77]. In contrast, dispersed whole-cell biocatalyst disperses a solid or fine powder-immobilised whole-cell within a reaction mixture, providing a high surface area for reactions and the potential for faster reaction rates with higher productivity [77]. Also, dispersed biocatalyst systems are often easier to scale up for industrial processes compared to column-packed biocatalysts. Moreover, the bioreactor system for dispersed biocatalysts (stirred tank reactor) is much cheaper compared to column-packed biocatalysts (packed bed reactor) [78]. Therefore, a dispersed biocatalyst approach was used in this study to evaluate GABA production via an RBF system.
Besides, upscaling the production of GABA using the method described in this study can yield various economic implications. On a positive note, this approach optimises resource utilisation by utilising fresh fruit and fruit waste, potentially reducing waste and raw material costs [35, 54]. Lower production costs may also be achieved through the accessibility and affordability of fruit and fruit waste [27, 28, 54], enabling a diversified product line and offering different fruit flavours and potential health benefits to cater to a broader consumer base. Additionally, adopting sustainable practices by repurposing fruit waste can enhance a company’s brand image among environmentally conscious consumers. It may provide opportunities for market differentiation, allowing for premium pricing. In addition, producing GABA via a repeated batch fermentation approach can enhance resource efficiency by allowing multiple fermentation cycles with the same immobilised cells, potentially reducing production costs [60]. Nevertheless, challenges include the significant initial investment in equipment and facilities, potential increases in operating costs, batch-to-batch variability in product yield, scaling complexities, market demand fluctuations and the need for a consistent supply of high-quality raw materials, especially when dealing with seasonal and regional variations. Thus, a thorough business plan and a feasibility analysis are essential prerequisites before embarking on such an economically impactful endeavour.
Conclusion
In summary, a potential probiotic strain, Lactiplantibacillus plantarum B7 immobilised on natural supports has improved the biosynthesis of GABA, lactic acid and its survivability via RBF compared to free cells. Apple pieces and watermelon rind, respectively, were found to be the ideal natural supports for GABA and lactic acid synthesis by L. plantarum B7, with greater cell viability among the examined fruits and fruit wastes. Additionally, both GABA and lactic acid with good yield were produced up to the 9th cycle using watermelon rind-supported cells and up to the 8th cycle using apple-supported cells in the RBF. When compared to GABA production by free cells in single batch fermentation (first cycle of RBF), GABA concentration could be increased up to 6.7 (218.480 ± 0.280 g/L) and 6.0 (195.439 ± 0.042 g/L) times utilising watermelon rind supported cells and apple pieces supported cells, respectively. Moreover, lactic acid titre by free cells in a single batch fermentation could be increased by 6.8 (286.719 ± 0.207 g/L) and 6.0 (253.889 ± 0.140 g/L) times using watermelon rind immobilised cells and apple immobilised cells, respectively, via RBF. Besides, when immobilised on natural support, L. plantarum B7 was able to retain ~50% of its cell viability even after the 12th cycle of RBF, in contrast to free cells that were only up to the 4th (GABA) and 5th (lactic acid) cycles.
Data Availability
All the data underlying the results are available as part of the article, and no additional source data are required.
References
Moore JF, DuVivier R, Johanningsmeier SD (2021) Formation of γ-aminobutyric acid (GABA) during the natural lactic acid fermentation of cucumber. J Food Compos Anal 96
Keshani S, Daud WRW, Nourouzi MM, Namvar F, Ghasemi M (2015) Spray drying: an overview on wall deposition, process and modeling. J Food Eng 146:152–162
Huang CY, Kuo WW, Wang HF, Lin CJ, Lin YM, Chen JL, Kuo CH, Chen PK, Lin JY (2014) GABA tea ameliorates cerebral cortex apoptosis and autophagy in streptozotocin-induced diabetic rats. J Funct Foods 6:534–544
Ohmori T, Tahara M, Ohshima T (2018) Mechanism of gamma-aminobutyric acid (GABA) production by a lactic acid bacterium in yogurt-sake. Process Biochem 74:21–27
Gao J, Lin S, Gao Y, Zou X, Zhu J, Chen M, Wan H, Zhu H (2019) Pinocembrin inhibits the proliferation and migration and promotes the apoptosis of ovarian cancer cells through down-regulating the mRNA levels of N-cadherin and GABAB receptor. Biomed Pharmacother 120:109505
Brzozowska A, Burdan F, Duma D, Solski J, Mazurkiewicz M (2017) Gamma-amino butyric acid (GABA) level as an overall survival risk factor in breast cancer. Annals Agri Environ Med 24:3
Ochoa-de la Paz LD, Gulias-Cañizo R, Ruíz-Leyja ED, Sánchez-Castillo H, Parodí J (2021) The role of GABA neurotransmitter in the human central nervous system, physiology, and pathophysiology. Rev Mex Neurociencia 22:67–76
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J Jpn Soc Food Sci Tech 47:596–603
Van Thu T, Foo HL, Loh TC, Bejo MH (2011) Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. African J Biotech 10:1359–1363
Sahab NRM, Subroto E, Balia RL, Utama GL (2020) γ-Aminobutyric acid found in fermented foods and beverages: current trends. Heliyon 6
Diana M, Quílez J, Rafecas M (2014) Gamma-aminobutyric acid as a bioactive compound in foods: a review. J Funct Foods 10:407–420. https://doi.org/10.1016/j.jff.2014.07.004
Gomaa EZ (2015) Enhancement of γ-amminobutyric acid production by co-culturing of two lactobacilli strains. Asian J Biotechnol 7:108–118. https://doi.org/10.3923/ajbkr.2015.108.118
Pannerchelvan S, Rios-Solis L, Wong FWF, Zaidan UH, Wasoh H, Mohamed MS, Tan JS, Mohamad R, Halim M (2023) Strategies for improvement of gamma-aminobutyric acid (GABA) biosynthesis via lactic acid bacteria (LAB) fermentation. Food Funct 14:3929–3948. https://doi.org/10.1039/D2FO03936B
Ming LC, Halim M, Rahim RA, Wan HY, Ariff AB (2016) Strategies in fed-batch cultivation on the production performance of Lactobacillus salivarius I 24 viable cells. Food Sci Biotechnol 25:1393–1398
Diez-Gutiérrez L, San Vicente L, Luis LJ, del Villarán M, C, Chávarri M, (2020) Gamma-aminobutyric acid and probiotics: multiple health benefits and their future in the global functional food and nutraceuticals market. J Funct Foods 64:103669. https://doi.org/10.1016/j.jff.2019.103669
Hsueh YH, Yang JH, Ou SF, Chen ST, Kuo JM, Wu CH (2021) Mass production of γ-aminobutyric acid by semi-continuous fermentation. Food Sci Technol 140:110640
Lou WY, Fernández-Lucas J, Ge J, Wu C (2021) Editorial: enzyme or whole cell immobilization for efficient biocatalysis: focusing on novel supporting platforms and immobilization techniques. Front Bioeng Biotechnol 9:620292
Cui Y, Miao K, Niyaphorn S, Qu X (2020) Production of gamma-aminobutyric acid from lactic acid bacteria: a systematic review. Int J Mol Sci 21:1–21. https://doi.org/10.3390/ijms21030995
Lee JY, Jeon SJ (2014) Characterization and immobilization on nickel-chelated sepharose of a glutamate decarboxylase A from Lactobacillus brevis BH2 and its application for production of GABA. Biosci Biotechnol Biochem 78:1656–1661
Lin Q, Li D, Qin H (2017) Molecular cloning, expression, and immobilization of glutamate decarboxylase from Lactobacillus fermentum YS2. Electron J Biotechnol 27:8–13. https://doi.org/10.1016/j.ejbt.2017.03.002
Aeron G, Shiwangi M (2017) Immobilization and microencapsulation. J Adv Res Biotechnol 2:1–4
Xu N, Wei L, Liu J (2017) Biotechnological advances and perspectives of gamma-aminobutyric acid production. World J Microbiol Biotechnol 33. https://doi.org/10.1007/s11274-017-2234-5
Kook MC, Cho SC (2013) Production of GABA (gamma amino butyric acid) by lactic acid bacteria. Korean J Food Sci Anim Resour 33:377–389. https://doi.org/10.5851/kosfa.2013.33.3.377
Lyu CJ, Liu L, Huang J, Zhao WR, Hu S, Mei LH, Yao SJ (2019) Biosynthesis of γ-aminobutyrate by engineered Lactobacillus brevis cells immobilized in gellan gum gel beads. J Biosci Bioeng 128:123–128. https://doi.org/10.1016/j.jbiosc.2019.01.010
Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y (2013) Immobilization technologies in probiotic food production. J Nutr Metab
Iqbal M, Saeed A (2005) Novel method for cell immobilization and its application for production of organic acid. Lett Appl Microbiol 40:178–182. https://doi.org/10.1111/j.1472-765X.2004.01646.x
Kourkoutas Y, Xolias V, Kallis M, Bezirtzoglou E, Kanellaki M (2005) Lactobacillus casei cell immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Process Biochem 40:411–416. https://doi.org/10.1016/j.procbio.2004.01.029
Kourkoutas Y, Komaitis M, Koutinas AA, Kanellaki M (2001) Wine production using yeast immobilized on apple pieces at low and room temperatures. J Agric Food Chem 49:1417–1425. https://doi.org/10.1021/jf000942n
Reddy LVA, Reddy YHK, Reddy OVS (2006) Wine production by guava piece immobilized yeast from Indian cultivar grapes and its volatile composition. Biotechnology 5:449–454
Kourkoutas Y, Bosnea L, Taboukos S, Baras C, Lambrou D, Kanellaki M (2006) Probiotic cheese production using Lactobacillus casei cells immobilized on fruit pieces. J Dairy Sci 89:1439–1451
Fadzil NF, Othman SA (2021) The growing biorefinery of agricultural wastes: a short review. J Sustain Nat Resour 2:46–51. https://doi.org/10.30880/jsunr.2021.02.02.006
Liong MT, Rosma A, Azhar ME, Afiza TS, Wan Nadiah WA (2008) Vitamin-B contents of yeast extracts from yeasts grown on agrowastes. In: Proceedings of the international conference on environmental research technology. pp 197–201
Teh SS, Bhat R, Ahmad R, Wan-Abdullah WN, Liong MT (2010) Growth characteristics of agrowaste-immobilised lactobacilli in soymilk during refrigerated storage. Int J Food Sci Technol 45:2089–2095. https://doi.org/10.1111/j.1365-2621.2010.02376.x
Lye HS, The SS, Lim TJ, Bhat R, Ahmad R, Wan-Abdullah WN, Liong MT (2012) Bioactive property of soymilk fermented by agrowastes-immobilized lactobacilli. Br Food J 114:1339–1353
Reddy LV, Kumar YH, Anjaneya LP, Sarathi OV (2008) Wine production by novel yeast biocatalyst prepared by immobilization on watermelon (Citrullus vulgaris) rind pieces and characterization of volatile compounds. Process Biochem 43:748–752. https://doi.org/10.1016/j.procbio.2008.02.020
Borshchevskaya LN, Gordeeva TL, Kalinina AN, Sineokii SP (2016) Spectrophotometric determination of lactic acid. J Anal Chem 71:755–758. https://doi.org/10.1134/S1061934816080037
Dikshit R, Tallapragada P (2015) Screening and optimization of γ-aminobutyric acid production from Monascus sanguineus under solid-state fermentation. Front Life Sci 8:172–181. https://doi.org/10.1080/21553769.2015.1028654
Yuvadetkun P, Boonmee M (2018) Comparison between free cells and immobilized cells of Candida shehatae in ethanol production from rice straw hydrolysate using repeated batch cultivation. Renew Energy 115:634–640. https://doi.org/10.1016/j.renene.2017.08.033
Zuriana SA, Sakinah AMM (2017) Production of sorbitol by repeated batch fermentation using immobilized of. Food Res 1:176–182
Aguirre-Guzmán G, Ricque-Marie D, Cruz-Suárez LE (2002) Survival of agglomerated Saccharomyces cerevisiae in pelleted shrimp feeds. Aquaculture 208:125–135
Abdullah A, Winaningsih I, Hadiyarto A (2021) Lactic acid fermentation from durian seeds (Durio zibethinus Murr.) using Lactobacillus plantarum. In: IOP conference series: materials science and engineering, vol 1053. p 012032. https://doi.org/10.1088/1757-899x/1053/1/012032
Ding T, Li Y (2021) Beneficial effect and mechanism of walnut oligopeptide on Lactobacillus plantarum Z7. Food Sci Nutr 9:672–681
Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JC (2012) Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol 194:686–701
Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, Bai X, Xie J, Wang Y, Geng W (2021) Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol 9:612285
Fu W, Mathews AP (1999) Lactic acid production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem Eng J 3:163–170
Bintsis T (2018) Lactic acid bacteria: their applications in foods. J Bacteriol Mycol Open Access 6
Papadimitriou K, Alegría Á, Bron PA, De Angelis M, Gobbetti M, Kleerebezem M, Lemos JA, Linares DM, Ross P, Stanton C, Turroni F (2006) Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 80:837–890
Yogeswara IBA, Maneerat S, Haltrich D (2020) Glutamate decarboxylase from lactic acid bacteria—a key enzyme in Gaba synthesis. Microorganisms 8:1–24. https://doi.org/10.3390/microorganisms8121923
Rayavarapu B, Tallapragada P, Usha MS (2021) Optimization and comparison of ℽ-aminobutyric acid (GABA) production by LAB in soymilk using RSM and ANN models. Beni-Suef Univ J Basic Appl Sci 10:14
Di Cagno R, Mazzacane F, Rizzello CG, Angelis MDE, Giuliani G, Meloni M, Servi BD, Gobbetti M (2010) Synthesis of γ- aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl Microbiol Biotechnol 86:731–741
Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H (2002) GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 213:1–47
Plessas S, Bekatorou A, Koutinas AA, Soupioni M, Banat IM, Marchant R (2007) Use of Saccharomyces cerevisiae cells immobilized on orange peel as biocatalyst for alcoholic fermentation. Bioresour Technol 98:860–865. https://doi.org/10.1016/j.biortech.2006.03.014
Dimitrellou D, Kandylis P, Kourkoutas Y (2019) Assessment of freeze-dried immobilized Lactobacillus casei as probiotic adjunct culture in yogurts. Foods 8:374
Patel P, Patel V, Subhash R (2015) Milk fermentation efficacy of immobilized L.paracasei cells on selected fresh fruit pieces. J Pure Appl Sci 22&23:52–58
Vitola HRS, dos Santos Cruxen CE, da Silva FT, Thiel PR, de Lima MJ, da Silva WP, Fiorentini ÂM (2020) Lactobacillus casei CSL3: evaluation of supports for cell immobilization, viability during storage in Petit Suisse cheese and passage through gastrointestinal transit in vitro. LWT 127:109381
Martins CSS, Miranda CM, Maria LCGF, Tedde SS (2013) Immobilization of microbial cells: a promising tool for treatment of toxic pollutants in industrial wastewater. African J Biotechnol 12:4412–4418. https://doi.org/10.5897/ajb12.2677
Junter GA, Jouenne T (2017) Immobilized viable cell biocatalysts: a paradoxical development. Ref Modul Life Sci
Lopez D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2:1–12
Zur J, Wojcieszyńska D, Guzik U (2016) Metabolic responses of bacterial cells to immobilization. Molecules 21. https://doi.org/10.3390/molecules21070958
Villalba M, Verdasco-Martín CM, dos Santos JC, Fernandez-Lafuente R, Otero C (2016) Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzym Technol 90:35–44
Sriputorn B, Laopaiboon L, Laopaiboon P (2023) Repeated-batch ethanol fermentation from sweet sorghum stem juice under a very high gravity condition using a stirred tank bioreactor coupled with a column bioreactor by immobilized Saccharomyces cerevisiae. Fermentation 9. https://doi.org/10.3390/fermentation9020159
Wang J, Hu Y, Qiu C, Fan H, Yue Y, Jiao A, Xu X, Jin Z (2018) Immobilized cells of Bacillus circulans ATCC 21783 on palm curtain for fermentation in 5 L fermentation tanks. Molecules 23:2888. https://doi.org/10.3390/molecules23112888
Genisheva Z, Teixeira JA, Oliveira JM (2014) Immobilized cell systems for batch and continuous winemaking. Trends Food Sci Technol 40:33–47. https://doi.org/10.1016/j.tifs.2014.07.009
Abdualrahm MAY, Olayinka BU, Etejere EO (2018) Comparative study between local and imported apple (Malus domestica) fruits and their uses in juice production. Sci Int 3:1060–1066. https://doi.org/10.17311/sciintl.2015.69.72
Olayinka BU, Etejere EO (2018) Proximate and chemical compositions of watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai cv red and cucumber (Cucumis sativus L. cv Pipino). Int Food Res J 25:1060–1066
Geethanjali S, Subash A (2013) Optimization and immobilization of purified Labeo rohita visceral protease by entrapment method. Enzyme Res 1–7
Xiudong X, Ying W, Xiaoli L, Ying L, Jianzhong Z (2016) Soymilk residue (okara) as a natural immobilization carrier for Lactobacillus plantarum cells enhances soymilk fermentation, glucosidic isoflavone bioconversion, and cell survival under simulated gastric and intestinal conditions. PeerJ 4:e2701. https://doi.org/10.7717/peerj.2701
Al-Sayed HMA, Ahmed AR (2013) Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann Agric Sci 58:83–95. https://doi.org/10.1016/j.aoas.2013.01.012
Aziz M, Anwar M, Uddin Z, Amanat H, Ayub H, Jadoon S (2013) Nutrition comparison between genus of apple (Malus sylvetris and malus domestica) to show which cultivars is best for the Province of Balochistan. J Asian Sci Res 3:417–424
Wu J, Gao H, Zhao L, Liao X, Chen F, Wang Z, Hu X (2007) Chemical compositional characterization of some apple cultivars. Food Chem 103:88–93
El-Behairy UA, Tork EM, El-Gammal MH (2022) Analytical study of the components of watermelon rind and evaluation of its use as a substitute for flour. J Agric Environ Sci 22:119–125
Markowiak P, Ślizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9. https://doi.org/10.3390/nu9091021
Pereira JAM, Berenguer CV, Andrade CFP, Câmara JS (2022) Unveiling the bioactive potential of fresh fruit and vegetable waste in human health from a consumer perspective. Appl Sci 12:2747. https://doi.org/10.3390/app12052747
Almeida DP, Gião MS, Pintado M, Gomes MH (2017) Bioactive phytochemicals in apple cultivars from the Portuguese protected geographical indication “Maçã de Alcobaça:” basis for market segmentation. Int J Food Prop 20:2206–2214
Marinelli V, Lucera A, Incoronato AL, Morcavallo L, Del Nobile MA, Conte A (2021) Strategies for fortified sustainable food: the case of watermelon-based candy. J Food Sci Technol 58:894–901
Tarazona-Díaz MP, Viegas J, Moldao-Martins M, Aguayo E (2011) Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J Sci Food Agric 91:805–812
Sikyta B (1995) Techniques in applied microbiology. Prog Ind Microbiol 31:373–411
Basso A, Serban S (2019) Industrial applications of immobilized enzymes—a review. Mol Catal 479:110607
Funding
This research was funded by the Universiti Putra Malaysia under Geran Putra GP-IPS (UPM-IPS/2023/9742200). The financial assistance of the Graduate Research Fellowship (GRF) was provided by Universiti Putra Malaysia.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sangkaran Pannerchelvan and Murni Halim; Methodology and Resources: Sangkaran Pannerchelvan, Faris Nulhaqim Muhamad, Fadzlie Wong Faizal Wong, Helmi Wasoh, Mohd Shamzi Mohamed, Rosfarizan Mohamad and Murni Halim; Formal analysis and investigation: Sangkaran Pannerchelvan and Faris Nulhaqim Muhamad; Writing—original draft preparation: Sangkaran Pannerchelvan and Murni Halim; Writing—review and editing: Fadzlie Wong Faizal Wong, Helmi Wasoh, Mohd Shamzi Mohamed and Rosfarizan Mohamad; Funding acquisition: Murni Halim; Supervision: Murni Halim. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pannerchelvan, S., Muhamad, F.N., Wasoh, H. et al. Improvement of ɣ-Aminobutyric Acid Production and Cell Viability of Lactiplantibacillus plantarum B7 via Whole-Cell Immobilisation in Repeated Batch Fermentation System. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10200-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10200-4