Abstract
Lactobacilli strains are considered as a preventive means for treatment of vaginal infections or post-antibiotic treatment to repopulate the vaginal mucosa. This study aimed at establishing the vaginal lactobacillus profile of Algerian women with different vaginal diseases. Afterwards, lactobacilli isolated from swabs were in vitro characterized for their probiotic hallmarks. This prospective study allowed isolation of 44 Lactobacillus strains and 160 potentially pathogens, among which are Escherichia coli (50 isolates), Staphylococcus sp. (38 isolates), Enterococcus sp. (16 isolates), and Candida sp. (56 isolates). All Lactobacilli strains were characterized for their antagonism, adhesion to polystyrene, and resistance to acidity and bile. Consequently, six Lactobacillus strains (Lb. fermentum 5LB4, 5LB10, 5LB12, Lb. plantarum 5LB2, 5LB11, and Lactobacillus sp. 4LB9) were moderately or weakly adherent, and 35 potentially pathogens exhibited weak to strong adhesion to polystyrene. Antagonism was recorded for 36 Lactobacillus strains towards E. coli 6E2, S. aureus 7S3, Enterococcus sp. 5EN8, and Candida albicans C1 used as indicator organisms. Finally, Lb. fermentum 9LB6, 4LB16, and 10LB1 and Lb. plantarum 9LB4 were remarkable for their inhibitory activity, absence of hemolytic potential, and for their resistance to acidity (pH 1.5) and bile (0.5%) harsh conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies aiming at underpinning the impact of human vaginal microbiota (VMB) on the health of women and their descendants are currently of major importance. Lactobacilli are the dominant microorganisms in a healthy human vagina, where they anticipated playing essential roles in protecting women from genital infections. Any alteration in the lactobacillus content can result in an imbalance of the human VMB, leading therefore to a quantitative and a qualitative shift from normally occurring lactobacilli to a mixed microbial content dominated by anaerobic bacteria, among which are Gardnerella vaginalis, Bacteroides, Prevotella, and Mobiluncus species [1]. Lactobacillus species encountered in the VMB of healthy women comprise mainly Lactobacillus crispatus, Lb. jensenii, and Lb. iners [2, 3]. Lactobacilli are tolerated by vaginal epithelial cells and inhibit induction of pro-inflammatory cytokines [4]. The Caucasian, African, and Hispanic women display different Lactobacillus species content. Related to that, Lb. crispatus appeared to be predominant for Caucasian women, and Lb. iners for African and Hispanic women [5, 6]. Lb. crispatus strains produce copious amounts of lactic acid with immunomodulatory, virucidal, and bactericidal activities [7], while the role of Lb. iners remains to be determined [8]. The discrepancies in Lactobacillus species distribution are attributed to cultural, behavioral, and genetic factors [3]. Further factors including lifestyle conditions as dress habits and hygienic practices were noted [9]. The relative abundance of Lactobacillus species in the human VMB is expected to be > 70%, conversely to other mammals where lactobacilli are rarely > 1% [10]. Significant decrease of Lactobacillus amount in VMB could lead to bacterial vaginosis (BV), which is defined also as anaerobic polybacterial dysbiosis. The BV is a hallmark for bacterial and viral infections. The beneficial associated lactobacilli are the key elements for vaginal eubiosis. Lactobacilli produce hydrogen peroxide and mainly lactic acid by using amylase breakdown products of glycogen [11]. The relevance of each of these substances in the vaginal eubiosis has recently been reviewed by Tachedjian et al. [11]. Lactic acid induces autophagy in epithelial cells to degrade intracellular microorganisms and promote homeostasis [4]. Lactobacilli are naturally present in the human VMB or administered as probiotics. Probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host [12]. The safe use of lactobacilli as probiotic agents in the human genitourinary tract dates back to 1915 [13]. Streptococci, Staphylococci, and Enterobacteriaceae are prevalent in women with BV [14]; other (facultative) anaerobic bacteria including Gardnerella vaginalis, Atopobium vaginae, Prevotella spp., and Sneathia spp. are also reported [14, 15]. The burden of BV is remarkable for Sub-Saharan African women, and also their descent living around the world [14]. Currently, available antimicrobial treatments of the vaginal infections can lead to diarrhea, super infections, depression, and even renal failure. Additionally, antimicrobial resistance tends to decrease the effectiveness of this therapy over time, as recently reported [16]. Antibiotics used for BV treatment include clindamycin, metronidazole, and secnidazole [17, 18], but these drugs may negatively impact the vaginal microbiome stability [18], which argues on the need of novel soft therapeutic options. Probiotics may offer favorable microbial balance for the vagina, and as the normal vaginal flora ascends from the rectal mucosa, a convenient form of administration of probiotics could be the oral gastrointestinal route [19]. Presently, the only strains exhibiting clinical effects are Lb. rhamnosus GR-1 and Lb. reuteri. Indeed, when these probiotics are intravaginally administered once weekly or orally administered twice daily, they could reduce recurrences of UTI and restore a normal lactobacillus-dominated vaginal microbiota in patients [16]. As prospect, it is of major importance to explore novel human VMB sources in order to isolate, characterize, and valorize further Lactobacilli strains as probiotics, mainly in the countries where access to antibiotic treatment is limited. The source targeted in this study is the Algerian human VMB, as no studies have been performed on this context. This prospective study was carried out on a limited sample of women and, based on the data obtained, will be completed in the future with a more statistically significant samples recovered from women with different symptoms. This study aimed to study VMB from Algerian women and to investigate the potential beneficial effects of the vaginal ecosystem microbiota in order to select probiotic candidates for human administration.
Materials and Methods
Study Population
The samples were collected by a gynecologist from 10 women (W1-W10) consulting, between January and March 2016, the gynecology service of a private health care unit in Bejaia city (Algeria). Patient history records provided to these patients included questions related to age, reason for consultation, gestational state, infection status, and antecedent of antibiotic therapy. Samples were collected by setting up a sterile speculum without antiseptic cleaning of the exocervix, and a swab was inserted into the endocervix by performing a rotational movement. Then, the swab was introduced into sterile tryptone–salt (TS) solution (Sigma-Aldrich, Steinheim, Germany) prior further analyses.
Lactobacillus and Pathogenic Strains Isolation from Vaginal Swabs
One milliliter of the collected swab was introduced into 5 ml of de Man-Rogosa-Sharpe (MRS) broth (Conda, Spain) (pH 5.4) and incubated for 24 h at 37 °C, allowing enrichment. After this period, MRS plates were inoculated with appropriate inoculum size of the enriched bacterial suspension and incubated at 37 °C for 24–72 h. These swab samples served as well for isolation of pathogenic bacteria. To this end, 1 ml of swab sample was inoculated into 5 ml of the appropriate broth for enrichment (Table 1) and inoculated on the selective agar media listed in Table 1. After a period of incubation at the appropriate temperature, the bacterial isolates were identified using basic taxonomical methods.
Lactobacillus Species Identification by MALDI-TOF Spectrometry
Bacterial isolates grown on MRS medium and anticipated to correspond to Lactobacillus strains were identified by Matrix-Assisted Laser Desorption and Ionisation, Time Of Flight (MALDI-TOF) spectrometry. To this end, pure colonies isolated on MRS agar upon 48 h of incubation were deposited on a ground steel MALDI target. The spots (three spots for each strain) were overlaid with 1 μl of 70% (v/v) formic acid solution (Sigma-Aldrich, Germany), dried at room temperature, and overlaid again with 1 μl of matrix solution (α-cyano-4-hydroxycinnamic acid [HCCA]; Bruker Daltonics) dissolved in 50% (v/v) acetonitrile (Sigma-Aldrich,), 47.5% (v/v) water, and 2.5% (v/v) trifluoroacetic acid (Sigma-Aldrich). The ground steel MALDI target was analyzed by the MALDI-TOF MS spectrometer Autoflex speed TM (Bruker Daltonics, Bremen, Germany) in a linear positive mode. Mass spectra were analyzed in m/z range of 2000 to 20,000 and bacterial test standard “BTS” (Bruker Daltonics) was used for instrument calibration according to the supplier’s recommendations. The determination of m/z ratios of detected ions in each MALDI-MS profile was performed under Flex analysis 3.4 for comparison with database. The following manufacturer-recommended identification scores were used: 2.00–3.00, high-confidence identification; 1.70–1.99, low-confidence identification; 0.00–1.69, no organism identification possible.
Biochemical Identification of the Pathogenic Strains
The pathogens were identified biochemically by using some key tests [20]. Escherichia coli strains were identified based on their biochemical traits on triple-sugar iron (TSI; Conda, Spain) agar (lactose +, gas + and H2S −), Shubert (Conda, Spain) medium (gas + and indole +), and Simmons’s citrate (Himedia, India) agar (citrate −). Identity of Staphylococcus aureus was confirmed using coagulase and DNase tests (coagulase+ and DNase+), and Enterococcus spp. were identified based on their NaCl (6.5% [w/v]) and pH (pH 9.6) tolerance and thermal treatment resistance (63 °C/30 min). Whereas, Candida species, firstly identified microscopically, were further identified by MALDI-TOF spectrometry as described above for lactobacilli.
Aggregation and Cell Surface Hydrophobicity Properties of Lactobacilli and Pathogens
Aggregation assays were performed according to Kos et al. [21]. Briefly, Lactobacillus strains and pathogens (E. coli 6E2, Enterococcus sp. 5EN8, S. aureus 7S3 and Candida albicans C1) were grown for 18 h at 37 °C in MRS or NB broth, respectively. After centrifugation (8000g, 10 min, 20 °C; Hettich Rotina 380R, Germany), the pellets were washed twice with sterile phosphate-buffered saline solution (PBS, 10 mM, pH 7.2) and re-suspended in the same buffer at concentration of about 108 CFU/ml. Cell suspensions were mixed by vortexing. Bacterial auto-aggregation was determined after 2 h of incubation at 37 °C. For this purpose, an aliquot of these bacterial suspensions was carefully removed from the aqueous phase, and the absorbance at 600 nm was read on a spectrophotometer (Specord®, Shimadzu, Germany). The auto-aggregation percentage was calculated using the following formula:
auto − aggregation (%) = 1 − (At/A0) × 100; At represents the absorbance at time t = 2 h and A0 represents the absorbance at t = 0 h.
Co-aggregation with E. coli 6E2, S. aureus 7S3, Enterococcus sp. 5EN8, and C. albicans C1 was studied upon growth of pathogens in the above-described conditions. Equal volumes (2 ml) of lactobacillus and pathogen suspensions were mixed by vortexing for 30 s in glass test tubes. Control assays contained in turns 4 ml of suspension of Lactobacillus or pathogen. The absorbance was read immediately and after 2 h of incubation at 37 °C. The percentage of co-aggregation was calculated using the formula below:
Co − aggregation (%) = (Ax + Ay)/2 − A(x + y)/(Ax + Ay)/2 × 100; A represents the absorbance, x and y represent each of the two strains in the control tubes, and (x + y) represents their mixture.
Cell surface hydrophobicity was determined by the microbial adhesion to hydrocarbons method (MATH) [22] with some modifications. Bacteria from overnight culture were harvested by centrifugation (8000g, 10 min, 20 °C), washed twice with PBS (10 mM, pH 7.2), and re-suspended in the same buffer to about 108 CFU/ml. The absorbance of the cell suspension was measured at 600 nm (A0). One milliliter of xylene was added to 3 ml of cell suspension and mixed by vortexing for 2 min. The suspension was incubated at room temperature to allow phase separation. The aqueous phase was removed and its absorbance was read at 600 nm (A1). The percentage of bacterial adhesion to solvent was determined with the following formula: hydrophobicity (%) = 1 − (A1/A0) × 100, where A1 represents the absorbance of the aqueous phase after two-phase system separation and A0 represents the absorbance of the initial bacterial suspension.
Adhesion of Lactobacillus and Pathogen Strains to Polystyrene Tissue Culture Plates
The semi quantitative method of adhesion to polystyrene culture protocol [23] with some modifications was used in this study. Briefly, 100 μl of each culture in MRS broth for Lactobacillus strains and NB for pathogens was added to the wells of sterile 96-well polystyrene tissue culture plates previously filled with 100 μl of tryptic soy broth “TSB” (Difco, France), and incubated for 24 h at 37 °C. Cultures were decanted and wells were washed twice with sterile TS solution to remove the non-adherent cells. The adherent cells in each well were fixed with 200 μl of 96% ethanol (Sigma-Aldrich, France). Notably, after 15 min, the plates were emptied and left to dry and were strained for 30 min with 0.1% (w/v) crystal violet (Biochem Chemopharma, Quebec, Canada). The stained biofilms were washed twice with 200 μl of TS solution and extracted with 200 μl of 96% ethanol (Sigma-Aldrich). The amount of biofilm was quantified by measuring the OD630 nm using a microplate reader.
Hemolytic Activity
The hemolytic activity of fresh cultures of vaginal Lactobacillus and pathogen strains was evaluated by spotting 10 μl of each culture on a blood agar medium (Columbia agar purchased from Biokar Diagnostics (France) containing 5% [v/v] blood). The plates were incubated for 24 h at 37 °C. After this period, they were examined for the presence of hemolytic activity around the spots.
Inhibition of Pathogens by Vaginal Lactobacilli
The antimicrobial activity of 44 Lactobacillus strains isolated from four different swabs (W4, W5, W9, W10) was tested against E. coli 6E2, S. aureus 7S3, Enterococcus 5EN8, and C. albicans C1 as target strains, selected for their adhesion and hemolytic properties, using the spots-on-lawn test [24]. Briefly, Petri plates were filled with MRS agar and allowed at room temperature for solidification and drying. After which, 5 μl of 18-h-old Lactobacillus cultures at about 108 CFU/ml was deposited as spots on the agar. The plates were then dried for 30 min and incubated at 37 °C/24 h. At the end of this incubation period, the agar was covered with 10 ml of a soft nutrient agar “NA” (8 g agar/l) previously seeded with 1 ml of a fresh culture of the target strain at about 107 CFU/ml, and then re-incubated at 37 °C for 18 h. The antimicrobial activity was revealed by the presence or absence of inhibition zones around the spots. The diameter of these zones was subsequently measured.
Antibiotic Susceptibility
The susceptibility of Lb. fermentum 4LB16, Lb. fermentum 5LB13, Lb. fermentum 5LB14, Lb. fermentum 9LB5, Lb. fermentum 9LB6, Lb. fermentum 10LB1, and Lb. plantarum 9LB4 was tested against ẞ-lactamines, aminoglycosides, tetracyclines, macrolides, glycopeptides, sulfamides, diaminopyrimidine, rifamycines, and aminosides. Bacterial suspensions at about 107 CFU/ml were seeded onto MRS agar plates using the flooding technique. The plates were air-dried for 15 min and then disks impregnated with antibiotics were deposited on the plates. The formation of inhibition zones around the disks was determined after 24 h of incubation at 37 °C. The susceptibility to these antibiotics was determined based on the recommendations of the Antibiogram Committee of the French Microbiology Society [25] for the pathogens and according to the literature for the Lactobacilli strains.
Resistance to Acidity and Bile of the Vaginal Lactobacilli
Bacterial cultures (18 h) containing about 108 CFU/ml of Lb. plantarum 9LB4, Lb. fermentum 4LB16, Lb. fermentum 9LB6, or Lb. fermentum 10LB1 served for assessment of resistance to bile and acidity. To this end, 1 ml of each of the aforementioned bacterial cultures was introduced into 9 ml of MRS broth adjusted at pH 1 or 1.5 with 3 N HCl (Sigma-Aldrich, Germany) and incubated at 37 °C. Aliquots (1 ml) were taken at 0, 1, and 3 h of incubation and plated onto MRS agar to determine the cell viability. Resistance to bile was evaluated as previously described [26] with some modifications. Lactobacillus cultures at about 108 CFU/ ml were centrifuged (5000g, 10 min, 20 °C) and washed twice with PBS (10 mM, pH 7.2). MRS broth containing 0.3, 0.5, or 1% (w/v) porcine bile (Sigma-Aldrich, Germany) was inoculated with the resulting base and incubated for 4 h at 37 °C. Survival rates in the acidic conditions and in the presence of bile were determined by comparing the number of viable cells after incubation (N) to the initial number (0 h, N0) as follows:
Results
Lb. fermentum and C. albicans Were Prevalent Microorganisms in the Vaginal Swabs
Two hundred four (204) microbial isolates were obtained from 10 swab samples, from which 44 isolates were recovered on MRS agar (pH 5.4). Besides the growth of these isolates on MRS (pH 5.4) agar medium, colonies were checked for their cell shape (bacilli), Gram staining (positive Gram), and catalase activity (negative activity). Remarkably, 44 bacterial isolates fulfilled these taxonomical criteria and assumed to belong to Lactobacillus genus. MALDI-TOF identification revealed the dominance of Lb. fermentum (32/44), followed by Lb. plantarum (7/44) and Pediococcus acidilactici (1/44), and four strains (4/44) were not identified using this technology. The 160 remaining bacterial isolates displayed different taxonomical criteria and assumed therefore as E. coli (50 isolates), Enterococcus sp. (16 isolates), Staphylococcus sp. (38 isolates), and Candida sp. (56 yeast isolates). Notably, C. albicans resulted to be the most prevalent species with 80% (45/60), followed by C. tropicalis 13% (7/56), and C. glabrata 7% (4/56). These species were efficiently identified by MALDI-TOF spectrometry.
Lb. fermentum Displayed High Aggregation and Hydrophobicity Properties
Auto-aggregation rates obtained for the 44 selected Lactobacilli strains were ranging from 27.8 to 68.3%, after only 2 h of incubation at 37 °C. The highest levels were registered for Lb. fermentum, with 60.3 to 68.3%. The cell hydrophobicity levels registered for all lactobacillus isolates were ranging from 40.1 to 80.2% and Lb. fermentum displayed the highest surface hydrophobicity (78.2–80.2%). The tested pathogen strains showed as well high auto-aggregation (68–82%) and hydrophobicity (30–56%) levels.
Adhesion of the Lactobacillus and Pathogenic Strains to Polystyrene Tissue Culture Plates
Results depicted on Figs. 1 and 2 reveal the aptitudes of the lactobacillus and pathogen strains to adhere and form biofilms under the tested conditions. These strains were classified in four categories based on the recommendations of Stepanovic et al. [27]. “Ac” is the absorbance of the sterile broth and was used as control. The following interpretations served along this experiment: A ≤ Ac, non-adherent (non-biofilm producer); 2Ac ≥ A > Ac, weakly adherent (weak biofilm producer); 4Ac ≥ A > 2Ac, moderately adherent (moderate biofilm producer); and strongly adherent (strong biofilm producer), A > 4Ac. As consequence, 38 Lactobacilli strains were non-adherents, 4 weakly adherents and 2 moderately adherents. The most adherent strains were Lb. fermentum 5LB12, Lactobacillus sp. 4LB9, Lb. plantarum 5LB11, Lb. fermentum 5LB4, Lb. plantarum 5LB2, and Lb. fermentum 5LB10 (Fig. 1). Regarding the pathogens, the absorbencies recorded for S. aureus and E. coli strains ranged from 0–0.190 to 0.070–0.335, respectively (Fig. 2), and comprised for S. aureus 22 non-adherent isolates, 15 weakly adherent isolates and 1 moderately adherent isolate (S. aureus 2S6). On the other hand, E. coli contained 13 non-adherent isolates, 26 weakly adherent isolates, 11 moderately adherent isolates, and 3 strongly adherent isolates which are E. coli 1E12, E. coli 6E5, and E. coli 6E6. As shown in Fig. 2, for Enterococcus sp., the absorbencies were between 0 and 0.155 and the resulting isolates were classified into 14 non-adherents and 2 weakly adherents (4En1 and 4En2). Similarly, Fig. 2 shows the absorbencies recorded for Candida strains, which are comprised between 0.12 and 0.288, leading to 34 non-adherents, 18 weakly adherents, and 4 moderately adherents (C. tropicalis C56, C. glabrata C26, C. albicans C55, and C. albicans C25). Statistical analysis showed significant differences (P < 0.05) on the adherence ability of the strains of the same genus or species.
Adhesion of the pathogenic strains to polystyrene microplates. A630 nm was used to quantify the adhesion potential. The data are the means of at least three independent experiments. Control corresponds to sterile TSB-YE. The error bars represent the standard deviations. A: E. coli, B: S. aureus, C: Enterococcus sp., and D: Candida sp.
Hemolytic Activity
Among the 44 tested Lactobacillus strains, 11 strains (25%) were deprived of hemolytic activity (Fig. 3). The other 33 strains (75%) displayed large clear halos around the spots indicating a typical ẞ-hemolysis hallmark. Regarding the other strains isolated in the frame of this work, 38 E. coli strains (76%) were devoid of hemolytic activity (Fig. 3). However, 8 strains (16%) showed green halos (α-hemolysis), while the remaining 4 strains (8%) showed clear halos (ẞ-hemolysis). No hemolytic activity was registered for 18 S. aureus strains (47%). However, the remaining 20 strains (53%) revealed a ẞ-hemolysis (Fig. 3). Among the tested Enterococcus strains, 50% were found to be non-hemolytic, 31% were α-hemolytic, and 19% were ẞ-hemolytic. For Candida species, 39 strains (70%) were non-hemolytic, 16 strains (28%) revealed α-hemolysis, and a single strain (2%) was ẞ-hemolytic (C. glabrata C52) (Fig. 3).
Inhibition of Pathogens by Vaginal Lactobacilli
The antimicrobial activity of the 44 recovered Lactobacillus strains was tested against E. coli 6E2, S. aureus 7S3, Enterococcus 5En8, and C. albicans C1, used as indicator organisms based on their adhesion and hemolytic properties. The majority of Lactobacillus strains (84%) showed antimicrobial activity at least against one of the target strains and 70% displayed activity against all the target strains (Fig. 4). Antagonism was observed for 82% of strains against E. coli, 70% against S. aureus, and 72% against Enterococcus sp. Anti-C. albicans C1 was observed for 70% of tested Lactobacillus strains. All the strains with antifungal activity exhibited as well antibacterial activity. The average of the inhibition zone diameters varied from 21 to 42 mm (Fig. 4). The upmost diameter was registered for Lactobacillus 4LB8 against C. albicans C1 and Lb. fermentum 4LB11 against S. aureus 7S3 with 42 mm, respectively. The lowest significant activity against S. aureus 7S3 was observed for Lb. fermentum 5LB1 (21 mm). It is noteworthy that the 2 strains with the upmost antagonism are part of the 16 strains recovered from sample W4, a woman consulting for a fibroma without any microbial infection. All these strains have antibacterial and antifungal activities except for Lb. fermentum 4LB6 which was deprived of an anti-S. aureus activity. Remarkably, all the strains devoid of antagonism were from sample W5 (woman with vaginosis), representing therefore 50% of this group. The strain having the least important activity is part of this group and is the only strain with an antimicrobial power towards the 4 pathogenic strains. All the strains from sample W9 have antimicrobial activity on the 4 tested pathogenic strains except for Lb. fermentum 9LB5 which was not active against S. aureus and Enterococcus strains and Lb. fermentum 9LB8 that lacks antifungal activity. Finally, all the strains having antimicrobial activity on the 4 tested pathogenic strains belong to the sample W10 (healthy woman). These observations may indicate the protective role of the lactobacilli in normal vaginal microbiota and their non-efficiency in the case of its imbalance.
Antibiotic Susceptibility
Resistance to antibiotics was tested for the non-hemolytic and antagonistic Lb. fermentum 4LB16, Lb. fermentum 5LB13, Lb. fermentum 5LB14, Lb. fermentum 9LB5, Lb. fermentum 9LB6, Lb. fermentum 10LB1, and Lb. plantarum 9LB4. The resistance was evaluated by the disk diffusion method for a number of different families of commonly used antibiotics in vaginal infection treatment. All these strains were resistant to streptomycin and vancomycin, except for Lb. fermentum 5LB14 and 10LB1, which exhibited sensitivity to vanvomycin and Lb. fermentum 4LB16 which was sensitive to streptomycin. However, Lb. fermentum 5LB13 was resistant to most antibiotics tested (Table 1). The antibiotic resistance against bactrim, which is a mixture of trimethoprim and sulfamethoxazole, was observed for Lb. fermentum 4LB16, Lb. plantarum 9LB4, Lb. fermentum 5LB13, and Lb. fermentum 9LB5. These data showed the species-dependent trait of antibiotic resistance.
Resistance to Acidity and Bile of Vaginal Lactobacilli
Lb. plantarum 9LB4 and Lb. fermentum 4LB16, 9LB6, and 10LB1 were tested for their resistance to acidity (pH 1.5) and bile (0.3, 0.5, and 1%). The data obtained are depicted on Fig. 5, underlining the resistance trait of all these strains to pH 1.5. Accordingly, Lb. fermentum 10LB1 appeared as the most resistant to pH 1.5 with a survival rate of 10%, then Lb. fermentum 4LB16 which showed a survival rate of 0.16%, whereas Lb. plantarum 9LB4 displayed a survival rate of 0.05%. No survival was registered for Lb. fermentum 9LB6 at this pH value. Notably, at pH 1.0, none of these strains was able to survive. Furthermore, these lactobacilli were resistant to bile (Fig. 6). Indeed, survival rates of 3.1 and 8.5% were registered for Lb. plantarum 9LB4 and Lb. fermentum 9LB6, respectively, in contact of 0.3% bile.
Discussion
The composition and ecology of human VMB have been extensively studied worldwide. The description of this particular ecosystem [10] is important to decipher for a better understanding of the mechanisms by which lactobacilli dominate this niche and avoid possible dysbacteriosis and risk of infections. The human VMB seems to be ethnicity-dependent and different in different geographical location. As abovementioned, lactobacilli are naturally present or administered as probiotics. Apropos of 44 Lactobacillus strains isolated here, Lb. fermentum was the prevalent species (73%), followed by Lb. plantarum (16%) and Pediococcus acidilactici (2%), and four strains (9%) were not identified by MALDI-TOF technology. It should be noted that Lactobacillus species dominating the human VMB of the most reproductive-age women are Lb. crispatus, Lb. iners, Lb. gasseri, and Lb. jensensii [28]. Based on separate studies, Vasquez et al. [19] established differences between lactobacilli recovered from the vagina, and species as Lb. rhamnosus, Lb. pentosus, Lb. fermentum, Lb. plantarum, and Lb. acidophilus considered being dominant in the vagina. Nevertheless, deviating results may be attributed to differences in the handling of samples, vaginal status, or the methods preferred for the isolation but may also reflect differences between populations [19]. The infrequent species as Lb. fermentum and Pediococcus acidiclactici were reported and studies associating their beneficial effects were reported. Kaewnopparat et al. [29] portrayed the effectiveness of Lb. fermentum SK5 against gastrointestinal pathogenic E. coli and vaginal pathogenic Gardnerella vaginalis though production of bacteriocin-like substance (BLIS). Similarly, Sabia et al. [30] underpinned the potency of Lb. fermentum CS57 towards Streptococcus agalactiae and C. albicans through production of BLIS as well. The presence of Pediococcus species in the human VMB is controversial. Indeed, Baldwin et al. [31] detected this species in the preterm premature rupture of membranes, while Park and Lee [32] associated this species to severe pelvic pain. In contrast, Borges et al. [33, 34], Borges, and Teixeira [35] unveiled the potential of this species as probiotic for vaginal application. In this study, we detected E. coli, Staphylococcus sp., Enterococcus sp., and Candida sp. in the swab samples analyzed. The presence of E. coli and Staphylococcus sp. is usually related to aerobic vaginitis [36]. Here, we investigated the in vitro probiotic features of Lactobacillus strains. The auto-aggregation and cell surface hydrophobicity of the 44 vaginal Lactobacilli strains resulted to be strain-dependent. Nevertheless, Lb. fermentum 5LB4, Lb. fermentum 5LB10 and 5LB12, and Lb. plantarum 5LB2 and 5LB11 exhibited high auto-aggregation and cell surface hydrophobicity levels with percentages ranking from 60.3 to 68.3% and 78.2 to 80.2% for Lb. fermentum species, respectively. The aforementioned strains and Lactobacillus sp. 4LB9 were also remarkable for their adhesion onto polystyrene abiotic device. Importantly, four E. coli strains, designed E. coli 1E12, E. coli 6E5, and E. coli 6E6, were marked by their high adhesion levels to polystyrene, which is a sign for their aptitudes to form a biofilm. In any way, the involvement of biofilm in a bacterial infection will complicate the treatment. Biofilm formation by lactobacilli can be considered as determinant element because it can stand as barrier against pathogens in the vaginal mucosa [37]. However, a study using A431 cells showed that only a small proportion of vaginal Lactobacilli strains tested was able to form a biofilm, despite the test conditions mimicking the vaginal environment [38]. For details on biofilms in the vaginal environment, it is recommended to see recent review by Hardy et al. [39]. The other pathogens, Candida species, Staphylococcus sp., and Enterococcus sp., were moderately adherents. To gain more insights on the safety of Lactobacillus strains isolated here, we assessed their hemolytic activities. Therefore, only 11 strains (Lactobacillus 4LB8, Lb. fermentum 5LB1, 4LB11, 9LB5, 9LB8, 4LB16, 5LB13, 5LB14, 9LB6, 10LB1, and Lb. plantarum 9LB4) were devoid of hemolytic activity. The hemolytic activity was not recorded systematically for all pathogens and was exerted in a strain-dependent manner. The antagonism is the main key for impeding dysbacteriosis. Antibacterial, antifungal, and antiviral activities are of major importance in the vaginal ecosystem. This feature is considered as an added value for probiotic design. Most of Lactobacillus strains (70%) were active against a panel of microbes including Gram-negative E. coli 6E2, Gram-positive S. aureus 7S3, and Enterococcus 5En8, and yeast C. albicans C1. Taken individually, E. coli is described as one of the main causes of uncomplicated urinary tract infections (UTI) and responsible for the vaginal infections [40]. Vulvovaginal candidiasis is sustained by Candida yeasts. Deidda et al. [41] portrayed the effectiveness of Lb. plantarum vs. traditional azoles used for treatment of Candida infections. While Enterococcus sp. is a key member of the female genital tract as facultative anaerobe microbe [42], the intriguing presence of S. aureus was recently reported to be prevalent in infertile Iranian women [43]. In this study, we evidenced the highly potent activity of Lactobacillus 4LB8 against C. albicans C1 and Lb. fermentum 4LB11 against S. aureus 7S3. Resistance to antibiotics and bile salt and low pH are as many attributes that need to be studied in depth for probiotic application. Indeed, the resistance to antibiotics can result from their overuse. The propagation of resistance to antibiotics can disqualify for probiotic application. Resistance of Lactobacillus strains is to be considered as key factor in light of vaginal therapy for prophylactic and treatment means. The Lactobacillus strains isolated here were mostly resistant to all antibiotics including vancomycin. Lactobacilli are naturally resistant to several antibiotics but this resistance is in many cases not transferable [44]. The resistance is usually intrinsic; even cases of acquired resistance were reported [45]. Similarly, resistance to bile salts and low pH which are mimicking the GIT environment are key criteria for probiotic design and therefore selection. Thus, the orally consumed probiotics ascend to the vaginal tract after being excreted from the rectum [46]. From our study, raised Lb. fermentum 10LB1 as a strain-defying constraint was caused by acidic (pH 1.5) conditions with amazing survival rate of 10%, but failing in resisting to bile salts. The other strains unveiled Lb. fermentum 9LB6, with resistance percentages of 8.5, 0.61, and 0.036%, followed by 9LB4 3.1, 0.076, and 0.0048%, and 4LB16 with 0.023, 0.0041, and 0.00011%. Taking Lb. fermentum KLD for comparison, this strain was reported to be resistant to GIT with 0.5% of the ingested cells recovered from ileum after 4 h [47]. Reid et al. [48] reported that Lb. rhamnosus GR-1 and Lb. fermentum RC-14, orally administrated twice daily during 14 days for 10 women with recurrent yeast vaginitis, bacterial vaginosis (BV), and urinary tract infections, have positively recovered from the vagina. To sum up, this is the first study dealing with isolation and characterization of human VMB in Algeria. We detected E. coli, Staphylococcus sp., Enterococcus sp., and Candida sp., traditional pathogens responsible for vaginal infections. Regarding the lactobacilli, Lb. fermentum was the prevalent species (73%), followed by Lb. plantarum (16%). Most of lactobacilli isolated here displayed antagonistic activities towards the detected pathogens. These antagonistic lactobacilli fulfilled also in vitro assessments used to design probiotic candidates. This study is anticipated to be pursued mainly through in vivo experiments in order to highlight further probiotic capabilities of these strains.
References
Petricevic L, Domig K, Nierscher F, Sandhofer M, Fidesser M, Kro-ndorfer I, Husslein P, Kneifel W, Kiss H (2014) Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 4(1):5136. https://doi.org/10.1038/srep05136
Al Kassaa I, Hamze M, Hober D, Chihib NE, Drider D (2014) Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb Ecol 67(3):722–734. https://doi.org/10.1007/s00248-014-0384-7
Kamińska D, Gajecka M (2017) Is the role of human female reproductive tract microbiota underestimated? Benefic Microbes 8(3):327–343. https://doi.org/10.3920/BM2015.0174
Witkin SS, Linhares IM (2017) Why do lactobacilli dominate the human vaginal microbiota? BJOG 124(4):606–611. https://doi.org/10.1111/1471-0528.14390
Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ (2007) Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1(2):121–133. https://doi.org/10.1038/ismej.2007.12
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Supplement_1):4680–4687. https://doi.org/10.1073/pnas.1002611107
Lepargneur JP (2016) Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann Biol Clin 74:421–427
Petrova MI, Reid G, Vaneechoutte M, Lebeer S (2017) Lactobacillus iners: friend or foe? Trends Microbiol 25(3):182–191. https://doi.org/10.1016/j.tim.2016.11.007
Le Blanc R-M (2009) Détecter des infections génitales basses chez la femme. Option Biologie 424:19–20
Miller EA, Beasley DE, Dunn RR, Archie EA (2016) Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol 7:1936. https://doi.org/10.3389/fmicb.2016.01936
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA (2017) The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. https://doi.org/10.1016/j.resmic.2017.04.001
FAO/WHO (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutritional paper No. 85
Newman D (1915) The treatment of cystitis by intravesical injections of lactic bacillus cultures. Lancet 186(4798):330–332. https://doi.org/10.1016/S0140-6736(01)53633-8
van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V (2014) The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 9(8):e105998. https://doi.org/10.1371/journal.pone.0105998
Onderdonk AB, Delaney ML, Fichorova N (2016) The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29(2):223–238. https://doi.org/10.1128/CMR.00075-15
Daliri EBM, Lee BH (2015) New perspectives on probiotics in health and disease. Food Sci Human Wellness 4(2):56–65. https://doi.org/10.1016/j.fshw.2015.06.002
Donders GG, Zodzika J, Rezeberga D (2014) Treatment of bacterial vaginosis: what we have and what we miss. Expert Opin Pharmacother 15(5):645–657. https://doi.org/10.1517/14656566.2014.881800
Petrina MAB, Cosentino LA, Rabe LK, Hillier SL (2017) Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe 47:115–119. https://doi.org/10.1016/j.anaerobe.2017.05.005
Vasquez A, Ahrne S, Jeppsson B, Molin G (2005) Oral administration of Lactobacillus and Bifidobacterium strains of intestinal and vaginal origin to healthy human females: re-isolation from faeces and vagina. Microb Ecol Health Dis 17(1):15–20. https://doi.org/10.1080/08910600510031376
Guiraud JP (2003) Microbiologie alimentaire. Dunod, Paris
Kos B, Šušković J, Vuković S, Šimpraga M, Frece J, Matošić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94(6):981–987. https://doi.org/10.1046/j.1365-2672.2003.01915.x
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9(1):29–33. https://doi.org/10.1111/j.1574-6968.1980.tb05599.x
O’Toole G, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30(2):295–304. https://doi.org/10.1046/j.1365-2958.1998.01062.x
Ait Ouali F, Al Kassaa I, Cudennec B, Abdallah M, Bendali F, Sadoun D, Chihib NE, Drider D (2014) Identification of lactobacilli with inhibitory effect on biofilm formation by pathogenic bacteria on stainless steel surfaces. Int J Food Microbiol 191:116–124. https://doi.org/10.1016/j.ijfoodmicro.2014.09.011
Comité de l’Antibiogramme de la Société Française de Microbiologie (CFA-SFM). Recommandations 2015. Paris, France. Société Française de Microbiologie
Bendali F, Durand A, Hébraud M, Sadoun D (2011) Lactobacillus paracasei subsp. paracasei: an Algerian isolate with antibacterial activity against enteric pathogens and probiotic fitness. J Food Nutr Res 50:139–149
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40(2):175–179. https://doi.org/10.1016/S0167-7012(00)00122-6
Nunn KL, Forney LJ (2016) Unraveling the dynamics of the human vaginal microbiome. Yale J Biol Med 89(3):331–337
Kaewnopparat S, Dangmanee N, Kaewnopparat N, Srichana T, Chulasiri M, Settharaksa S (2013) In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe 22:6–13. https://doi.org/10.1016/j.anaerobe.2013.04.009
Sabia C, Anacarso I, Bergonzini A, Gargiulo R, Sarti M, Condò C, Messi P, de Niederhausern S, Iseppi R, Bondi M (2014) Detection and partial characterization of a bacteriocin-like substance produced by Lactobacillus fermentum CS57 isolated from human vaginal secretions. Anaerobe 26:41–45. https://doi.org/10.1016/j.anaerobe.2014.01.004
Baldwin EA, Walther-Antonio M, MacLean AM, Gohl DM, Beckman KB, Chen J, White B, Creedon DJ, Chia N (2015) Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. Peer J 3:e1398. https://doi.org/10.7717/peerj.1398
Park TC, Lee HJ (2013) Pregnancy coexisting with uterus didelphys with a blind hemivagina complicated by pyocolpos due to Pediococcus infection: a case report and review of the published reports. J Obstet Gynaecol Res 39(7):1276–1279. https://doi.org/10.1111/jog.12049
Borges S, Barbosa J, Silva J, Teixeira P (2013) Evaluation of characteristics of Pediococcus spp. to be used as a vaginal probiotics. J Appl Microbiol 115(2):527–538. https://doi.org/10.1111/jam.12232
Borges S, Costa P, Silva J, Teixeira P (2013) Effects of processing and storage on Pediococcus pentosaceus SB83 in vaginal formulations: lyophilized powder and tablets. Biomed Res Int 680767
Borges S, Teixeira P (2014) Pediococcus pentosaceus SB83 as a potential probiotic incorporated in a liquid system for vaginal delivery. Benefic Microbes 5(4):421–426. https://doi.org/10.3920/BM2013.0084
Vicariotto F, Mogna L, Del Piano M (2014) Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study. J Clin Gastroenterol 48:106–112
Martin R, Soberon N, Vaneechoutte M, Florez A, Vazquez F, Suarez J (2008) Characterizaton of indigenous vaginal lactobacilli from healthy women as probiotic candidates. Int Microbiol 11(4):261–266. https://doi.org/10.2436/20.1501.01.70
Strus M, Kucharska A, Kukla G, Wloch M, Maresz K, Heczko P (2005) The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect Dis Obstet Gynecol 13(2):69–75. https://doi.org/10.1080/10647440400028136
Hardy L, Cerca N, Jespers V, Vaneechoutte M, Crucitti T (2017) Bacterial biofilms in the vagina. Res Microbiol. https://doi.org/10.1016/j.resmic.2017.02.001
Leccese Terraf MC, Juarez Tomás MS, Rault L, Le Loir Y, Even S, Nader-Macías ME (2017) In vitro effect of vaginal lactobacilli on the growth and adhesion abilities of uropathogenic Escherichia coli. Arch Microbiol 199(5):767–774. https://doi.org/10.1007/s00203-016-1336-z
Deidda F, Amoruso A, Nicola S, Graziano T, Pane M, Allesina S, Raiteri E, Del Piano M, Mogna L (2016) The in vitro effectiveness of Lactobacillus fermentum against different Candida species compared with broadly used azoles. J Clin Gastroenterol 50:S171–S174. https://doi.org/10.1097/MCG.0000000000000686
Gibbs RS (1987) Microbiology of the female genital tract. Am J Obstet Gynecol 156(2):491–495. https://doi.org/10.1016/0002-9378(87)90318-8
Akhi MT, Esmailkhani A, Sadeghi J, Niknafs B, Farzadi L, Akhi A, Nasab EN (2017) The frequency of Staphylococcus aureus isolated from endocervix of infertile women in northwest Iran. Int J Fertil Steril 11(1):28–32
Rabia A, Chah N (2011) Antibiotic resistance of probiotics organisms and safety of probiotic dairy products. Int Food Res J 18:837–853
Delgado S, Flórez AB, Mayo B (2005) Antibiotic susceptibility of Lactobacillus and Bifidobacterium species from the human gastrointestinal tract. Curr Microbiol 50(4):202–207. https://doi.org/10.1007/s00284-004-4431-3
Lee YK (2014) What could probiotic do for us? Food Sci Human Wellness 3(2):47–50. https://doi.org/10.1016/j.fshw.2014.06.001
Flourié B, Nancey S (2007) Propriétés fonctionnelles des probiotiques. Cahier de Nutrition Diététique 42:38–44. https://doi.org/10.1016/S0007-9960(07)91320-6
Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 30(1):49–52. https://doi.org/10.1111/j.1574-695X.2001.tb01549.x
Funding
Experiments performed at Charles Violette Institute (Lille University) were funded by CPER/FEDER Alibiotech program (2016-2020). Those performed in Algeria were funded by the Algerian Ministry of High Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ouarabi, L., Chait, Y.A., Seddik, H.A. et al. Newly Isolated Lactobacilli strains from Algerian Human Vaginal Microbiota: Lactobacillus fermentum Strains Relevant Probiotic’s Candidates. Probiotics & Antimicro. Prot. 11, 43–54 (2019). https://doi.org/10.1007/s12602-017-9360-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9360-0



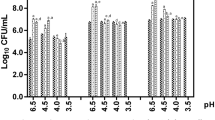



 ), E. coli (■), S. aureus (
), E. coli (■), S. aureus ( ), Enterococcus sp. (
), Enterococcus sp. ( ), and Candida sp. (
), and Candida sp. ( )
)

