Abstract
The aims of this study were to evaluate the effects of Lactococcus lactis subsp. lactis on the growth, intestinal microbiota, digestive enzyme activity, and disease resistance of Litopenaeus vannamei. Diets containing four different concentrations of L. lactis (0 [basal diet], 106, 107, and 108 CFU g−1) were fed to white shrimps L. vannamei (average weight 5.89 ± 0.36 g) for 8 weeks. At the end of the feeding trial, shrimps were immersed in Caspian Seawater (10.8 ppt) contaminated with 106 CFU ml−1 pathogenic V. anguillarum for 2 h. Results revealed that growth rate, survival, and body protein level were increased with dietary supplementation of L. lactis. The activities of digestive enzymes (cellulose, lipase, amylase, and protease) were significantly higher in the groups fed with diets containing 107 or 108 CFU g−1 L. lactis than those in the control. The Lactobacillus and Bacillus counts were higher (P < 0.05) in the intestine of shrimps fed with L. lactis-supplemented diets. In addition, higher level of L. lactis supplementation decreased the Vibrio counts. Moreover, L. vannamei fed diet supplemented with 108 CFU g−1 of L. lactis exhibited significantly the highest hematocyte count and post-challenge survival rate (79.2 %). Collectively, these results suggest that dietary supplementation of L. lactis subsp. lactis at 108 CFU g−1 can promote growth performance, digestive enzyme activity, and disease resistance of L. vannamei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White shrimps (Litopenamei vannamei) are one of the most important farmed crustacean species in the world. The production of white shrimps has increased from 154,515 metric ton (mt) in 2000, representing 13.6 % of total production of shrimps and prawns (1,137,048 mt), to 3,314,447 mt in 2013, representing 74.4 % of total shrimp and prawn production (4,454,602 mt) [1]. Asian countries share about 80 % (2,668,180 mt) of global L. vannamei production in 2015 [1]. However, problems of adverse environmental conditions and disease outbreaks have seriously affected the shrimp production worldwide [2]. Bacterial pathogens pose most serious threat to L. vannamei culture. Vibrio spp. have been reported as one of the major causes of bacterial infections in farmed shrimps. Vibrio infection causes massive colonization of the appendages, mid gut, foregut, hepatopancreas, and a terminal septicemia [3]. Though antibiotics and chemotherapeutics provide a promising approach to combat disease problems in aquaculture, their excessive use may result in an increase in drug-resistant pathogens, environmental hazards, and food safety concerns [4]. Therefore, it is imperative that natural or eco-friendly therapeutics approaches be developed to ensure sustainability of aquaculture.
Recently, application of beneficial bacteria as probiotics in an aquaculture practice is gaining momentum. Probiotics have widely been used to boost the immunity of farmed shrimps against various bacterial pathogens [5, 6]. Probiotics can increase the nutritional status of the host species by producing digestive enzymes, and in turn, promote their growth and survival rates [7, 8]. Among probiotics, various bacilli and lactic acid bacteria (LAB) (e.g., genera Lactobacillus, Lactococcus, and Pediococcus) are considered as an alternative to antibiotics or chemotherapeutics in shrimp aquaculture to induce immunity against pathogenic bacteria [8, 9]. Probiotics have the potential to counter diseases caused by various species of vibrio such as V. anguillarum, V. vulnificus, V. alginolyticus, and V. harveyi [6, 9]. Many probiotics including LAB [9–11] have been reported to inhibit vibrio infection in shrimp aquaculture. Further, LAB enhanced the growth and survival of Penaeus indicus challenged intra-muscularly with V. alginolyticus [10] and L. vannamei challenged with V. harveyi [3]. However, the antagonistic effect of LAB against Vibrio spp. is highly dependent on the ability of the probiotic to survive and dominate in the gut environment of shrimps [6].
The members of the genera Lactococcus and Lactobacillus are most commonly known for the “generally regarded as safe” (GRAS) status [12]. Lactococcus lactis improved the feed utilization and modulated the immune functions in wild kuruma shrimps (Marsupenaeus japonicus) [13]. Application of Lactobacillus plantarum increased the immune responses and survivability of Macrobrachium rosenbergii and L. vannamei against Aeromonas hydrophila [14] and Vibrio harveyi [6] infections, respectively. Information regarding the possible application of L. lactis as a probiotic for L. vannamei is scanty. Therefore, the present study was aimed to evaluate the effects of dietary supplementation of L. lactis subsp. lactis on the growth performance, intestinal microbiota, digestive enzyme activities, and disease resistance of L. vannamei.
Materials and Methods
Animals and Culture Facility
A total of 720 juvenile white shrimps (L. vannamei) (average weight 5.89 ± 0.36 g) were obtained from a research center of Bandar Abbas province, Iran. Their health status was verified by bacteriological, viral, and parasitological studies [15]. Shrimps were acclimatized in fiberglass tanks (1000 L capacity) filled with Caspian Sea water for 2 weeks. Tanks were provided with spongy filters (flow rate of 300 L h−1). Water temperature, dissolved oxygen, electrical conductivity, and salinity were 28.3 ± 1.8 °C, 6.84 ± 0.52 mg L−1, 5846.3 ± 314.6 MM cm−1, 10.8 ± 0.42 ppt, respectively, and pH was 8.2 ± 0.23 throughout the study period. The photoperiod was set at 14-h light (using natural lighting) and 10-h dark cycles. During the acclimation period, shrimps were fed with basal diet (Table 1) thrice a day.
Potential Probiotics
The potential probiotics used in the study were previously isolated from the intestine of healthy white shrimps, which was prepared as a lyophilized stock. Standard biochemical identification protocols [16] and comparative 16S rRNA analysis revealed the isolate as Lactococcus lactis subsp. lactis. Further, the isolate exhibited in vitro inhibitory activities against Vibrio anguillarum and V. harveyi [17]. The isolate was cultured in MRS (De Man Rogosa & Sharp broth) medium (pH = 6.7) and incubated at 30 °C for 48 h. After incubation, cells were harvested by centrifugation (3000×g for 15 min) and washed thrice with phosphate buffered saline (PBS; pH 7.3).
Test Diets
Hygienic environment was strictly maintained during the whole process of diet preparation. A basal diet containing 48.76 % crude protein, 9.16 % crude lipid, 8.7 % ash, 9.2 % moisture, and a gross energy of 4.52 kcal g−1 was prepared (Table 1). The basal diet was divided into four portions, and L. lactis was incorporated into diets at 0 (control), 106, 107, and 108 CFU g−1, respectively [17]. The diets were made into pellets, air dried, and stored at 4 °C until use. Prior to the preparation of the experimental diets, trials were conducted to determine the incorporation success and viability of the potential probiotics into the diet.
Experimental Design
After the acclimatization period, shrimps were randomly divided into four groups across 12 tanks (3 tanks/group; 1000 L water/tank). Each group consists of 180 shrimps (60 shrimps × 3 tanks). Shrimps were fed with basal diet or one of the three experimental diets (thrice a day) for 8 weeks, at 7 and 6 % of body weight during the first month and second month, respectively. The amount of feed consumed was determined by daily recovery of excess feed, which was then adjusted every 15 days by batch weighing after 24 h of starvation.
Proximate Composition
At the end of the feeding trial, shrimps were starved for 24 h, after which they were netted, counted, and the average weight (g) and length (cm) were measured. Five shrimps from each tank were frozen alive for final body composition analyses. Initial body analyses were performed on a pooled sample of larvae, which was weighed and frozen before the study. Proximate analyses of whole body water, protein, lipid, and ash were performed according to standard AOAC [18] methods.
Growth Performance
At the end of the feeding trial, 30 shrimps from each tank (i.e., 30 × 3 = 90 shrimps per group) were randomly collected for growth performance analysis. Growth performances and survival rate of shrimps were calculated using the following formula:
-
Percent weight gain (PWG) = (Wf − Wi/Wi) × 100
-
Specific growth rate (SGR) = 100 (ln Wf − ln Wi)/t,
-
Feed conversion ratio (FCR) = dry feed intake (g)/live weight gain (g).
-
Survival rate (%) = 100 (final number of shrimps/initial number of shrimps).
-
Where, Wi and Wf are the initial and final weight (g), and “t” is the time of experiment days.
Digestive Enzyme Activity
At the end, 10 shrimps from each tank (i.e., 10 × 3 = 30 shrimps per group) were starved for 24 h and subsequently used for digestive enzyme analyses. The whole intestines were removed and rinsed with distilled water [19]. The intestinal samples were homogenized in PBS (pH 7.5), centrifuged (12,000×g for 15 min at 4 °C), and supernatants were stored at −80 °C for enzymatic analysis [19]. Among the enzymatic parameters, protease [20], amylase [21], cellulase [5], and lipase [5] activities were measured.
Intestinal Microbiota
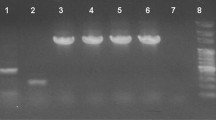
Intestinal samples from nine shrimps per group were aseptically pooled to conduct the quantitative analysis of intestinal microbiota, namely Vibrio spp., Lactobacillus spp., Micrococcus spp., and Bacillus spp. Selective agar method was applied as described by earlier researchers [15, 22, 23]. Also, molecular identifications were conducted by sequencing 16S rRNA analysis as described elsewhere [24]. For 16S rRNA gene sequencing, chromosomal DNA was extracted and purified using the phenol–chloroform extraction method [24]. PCR amplification was carried out with universal bacterial primers as described previously: forward, 5′-GGTTACCTTGTTACGACTT-3′ and reverse, 5′-AGAGTTTGATCCTGGCTCAG-3′. The thermal PCR steps were 1 cycle 95 °C for 2 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing temperature at 50 °C for 30 s, and extension at 72 °C for 1 min. This was followed by a final extension of 10 min at 72 °C [25]. The PCR products were separated by agarose gel electrophoresis, purified with gel extraction kit (Qiagen), and subjected to nucleotide sequence analysis by the dideoxy chain termination method. Sequencing process was performed in CinnaGen Company, Tehran, Iran. The 16S rRNA gene sequence of four isolates and the representatives of the other species from the GenBank database were analyzed using the MEGA 6 software. Similarity between the sequences of the tested isolates and sequences available at GenBank was defined with the use of the MEGA 6 software.
Total Hematocyte Counts (THC)
At the end of the feeding trial, 10 shrimps from each tank (i.e., 10 × 3 = 30 shrimps per group) were selected randomly. Hemolymph (0.2 ml) was drawn from the ventral sinus of shrimps using 1-ml sterile syringe containing anticoagulant solution (30 mM trisodium citrate, 0.34 M sodium chloride, and 10 mM EDTA, at a pH of 7.5) at 1:1 (v/v) ratio and then mixed. A drop of hemolymph (20 μl) was placed on a hemocytometer, and the THC was determined using light microscope (Nikon, Japan) with ×400 magnification [26]. THC count was expressed as log cells ml−1 hemolymph.
Challenge Test
Pathogenic V. anguillarum (ATCC12486) was grown in tryptic soy broth (Merck, Germany) supplemented with 20 g L−1 NaCl for 48 h at 30 °C. At the end of the feeding trial, 45 shrimps (15 × 3 = 45 shrimps) from each group were challenged with Vibrio anguillarum. For this purpose, shrimps from each group were immersed in sterile Caspian seawater (10.8 ppt salinity) contaminated with 106 CFU ml−1 of V. anguillarum for 2 h [6]. The challenged shrimps were kept under observation for 14 days and fed a basal diet. The mortality was recorded daily up to 14 days.
Statistical Analysis
All experimental data were subjected to statistical analysis using the SPSS software, version no. 18 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to determine whether significant differences existed between the treatments at a 95 % confidence limit. Duncan’s multiple range tests were used to compare means when F values from the ANOVA were significant (P < 0.05). All data were presented as means ± standard error of the mean (SEM) of three replicates.
Results
The effect of L. lactis subsp. lactis supplementation on the growth performances of L. vannamei is shown in Table 2. The growth parameters such as PWG and SGR were increased significantly in L. lactis supplementation groups (Table 2), with the highest (P < 0.05) PWG (121.96 ± 10.2), and SGR (1.42 ± 0.14) were recorded in 108 CFU g−1 of L. lactis supplementation group. FCR value decreased gradually (P < 0.05) in L. lactis supplementation groups, and the lowest FCR value (0.76 ± 0.11) was recorded in 108 CFU g−1 of L. lactis fed group. During the experimental period, the highest survivability (93.3 ± 1.1 %) was observed in 107 and 108 CFU g−1 supplementation groups, followed by 107 CFU g−1 fed group (88.6 ± 2.2 %).
The result of the shrimp’s body composition analysis is presented in Table 3. Supplementation of L. lactis at 108 CFU g−1 significantly increased the protein as well as lipid content in shrimps. Body protein content increased gradually with increasing level of L. lactis supplementation (Table 3). However, moisture content was almost similar in all the groups during the experimental period.
The result of the digestive enzyme activity analysis is shown in Table 4. The enzyme protease, amylase, cellulose, and lipase activities were significantly higher in 107 and 108 CFU g−1 of L. lactis supplementation groups. The highest enzymatic activities were recorded in 108 CFU g−1 supplementation group. The supplementation of L. lactis at 106 CFU g−1 had no significant effect on enzymatic activities.
L. lactis supplementation significantly affected the bacterial counts in the intestinal tract of L. vannamei (Table 5). Lactobacillus and Bacillus counts were increased (P < 0.05) with higher concentration of L. lactis. However, Vibrio counts decreased significantly in experimental groups fed with 107–108 CFU g−1 of L. lactis, compared to the control group (Table 5). However, supplementation of L. lactis had no significant effect on the intestinal Micrococcus count (Table 5).
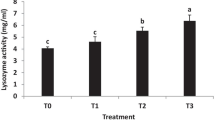
The supplementation of L. lactis at 107 CFU g−1 as well as 108 CFU g−1 significantly increased the THC in juvenile L. vannamei (data not shown). Supplementation of 108 CFU g−1 of L. lactis resulted in the highest (P < 0.05) THC.
Dietary supplementation of L. lactis for 8 weeks enhanced the resistance of L. vannamei against V. anguillarum challenge (Fig. 1). Cumulative percent mortality was lowest in 108 CFU g−1 of L. lactis supplementation group. The highest (P < 0.05) post-challenge survival rate (79.2) was recorded in 108 CFU g−1 of L. lactis supplementation group, followed by 107 CFU g−1 of L. lactis (73 %) and 106 CFU g−1 of L. lactis (62 %) supplementation.
Discussion
The results of the present study showed that supplementation of L. lactis subsp. lactis significantly improved growth performances and survivability of white shrimps. Positive effects of different LAB on the growth performances of shrimps had been reported earlier. Balcazar et al. [27] found that L. vannamei larvae fed with B. subtilis UTM 126-supplemented diets exhibited enhanced the growth performance and survivability. Dietary addition of probiotic Bacillus coagulans increased the final weight gain of L. vannamei [22]. Higher growth performances have also been reported in Fenneropenaeus indicus [19], P. vannamei [28], and L. vannamei [6] fed diets supplemented with LAB probiotics. Further, the significantly lowest FCR was recorded in 108 CFU g−1 of L. lactis supplementation group, which is consistent with the earlier reports [6, 28]. Moreover, lower FCR (P < 0.05) in the experimental groups suggested that shrimps utilized dietary nutrients more efficiently when supplemented with L. lactis.
The higher growth performance and lower FCR of white shrimps fed with L. lactis-supplemented diets may be related to the ability of this bacteria to stimulate digestive enzymes, which increase feed digestibility and in turn, the energetic benefits improved the growth rate. The significant increase in the specific activities of protease, amylase, and lipase of white shrimp treated with 107or 108 CFU g−1 L. lactis may enhance digestion and increase absorption of diet, which in turn contributes to better growth performance [22]. Supplementation of potential probiotic LAB increased the specific activities of digestive enzymes in P. vannamei [22, 28], Litopenaeus stylirostris [29], and F. indicus [19]. It has been suggested that the enhancement of digestive enzyme activities in farmed fishes and shrimps fed with LAB probiotic might be attributed to the improved gut maturation [30], prevention of intestinal disorders, and pre-digestion of anti-nutrient factors found in the feed ingredients [31, 32].
The increase in Lactobacillus spp. and Bacillus spp. counts and decrease in Vibrio spp. counts in L. vannamei fed with L. lactis-supplemented diets may be an indication of the positive role of this potential probiotic in improving the growth and feed efficiency of L. vannamei. It has been reported that supplementation of probiotics can improve intestinal microbial balance [14, 33, 34]. Probiotic bacteria maintain or re-establish a favorable relationship between beneficial and pathogenic microorganisms in digestive tract of the host. Intestinal microbiota plays an important role in immune responses and resistance to infectious diseases due to its ability to produce antibacterial materials which prevent pathogenic bacterial infection [22, 35, 36]. Gullian & Rodríguez [37] reported that Vibrio spp. which normally colonize the hepatopancreas of white shrimps were dominated by Bacillus spp. when it was added to the rearing water. Dietary supplementation of live or freeze dried Bacillus to tiger shrimp P. monodon significantly lowered the concentrations of vibrios in the culture water as well as in the hepatopancreas and intestine of tiger shrimps [34].
Intestinal microbiota also plays an important role in the nutrition of several aquatic animals [31, 32, 34]. Some intestinal microbiota can produce extracellular enzymes (e.g., proteases and lipases) which aid in food digestion processes in penaeid shrimp (Penaeus chinensis) [22]. This microbiota may also serve as a supplementary source of food such as vitamins, essential amino acids, and fatty acids [38]. It has been reported that the haematocyte plays an important role in the invertebrate immune responses [6]. Therefore, the higher post-challenge survival and increases in THC with higher level of L. lactis in the present study may reflect the improved capability of white shrimps to act against foreign materials. Similarly, supplementation of autochthonous L. plantarum increased the THC after V. harveyi injection and increased LAB populations in the digestive tract in L. vannamei [6]. This suggests that LAB may possess inhibitory activities against pathogenic bacteria [3, 13, 35]. Moreover, adding L. plantarum in the diet of white shrimps induced immune modulation and enhanced the immune responses like phenoloxidase activity, superoxide dismutase activity, pathogen clearance efficiency, and prophenoloxidase and peroxinectin (PE) mRNA transcription, and thus increased the resistance of shrimps against V. alginolyticus infection [11].
L. vannamei fed diets supplemented with L. lactis exhibited higher post-challenge survival against V. anguillarum challenge. Dietary administration of L. lactis at 108 CFU g−1 resulted in the highest post-challenge survival (79.2 %) of L. vannamei. Supplementation of potential probiotics increased the resistance of shrimps against pathogen infection [2, 3, 5, 6, 10, 27, 29, 39]. The elevated intestinal microbial balance, digestive enzyme activities, and THC in L. vannamei fed with 108 CFU g−1 of L. lactis-supplemented diet might be associated with the improved resistance of L. vannamei against V. anguillarum and resulted in higher post-challenge survival rate. Earlier, Vieira et al. [3] found that larval white shrimps fed with two LAB strains isolated from juvenile white shrimps showed inhibitory activities against V. harveyi and thus exhibited higher post-challenge survival rate.
In conclusion, the present study provides the evidence that dietary supplementation of L. lactis subsp. lactis at 108 CFU g−1 for 8 weeks can modulate the growth performance, digestive enzyme activities, and beneficial intestinal microbiota of L. vannamei. Clearly, the dietary administration of L. lactis at 108 CFU g−1 has significantly improved the resistance of white shrimps against V. anguillarum challenge. Therefore, we recommend that supplementation of L. lactis at 108 CFU g−1 can improve the growth performance and disease resistance of white shrimps. However, future studies should be focused on immune mechanism, stress responses, and resistance against other shrimp pathogens for exploring the feasibility of its commercial application in shrimp aquaculture.
References
FAO (2015) The state of world fisheries and aquaculture (SOFIA) 2015. FAO Fisheries and Aquaculture Department, Rome
Wu CC, Chang YP, Wang JJ, Liu CH, Wong SL, Jiang CM, Hsieh SL (2015) Dietary administration of Gynura bicolor (Roxb. Willd.) DC water extract enhances immune response and survival rate against Vibrio alginolyticus and white spot syndrome virus in white shrimp Litopeneaus vannamei. Fish Shellfish Immunol 42:25–33
Vieira FN, Pedrotti FS, Neto CCB, Mouriño JLP, Beltrame E, Martins ML (2007) Lactic-acid bacteria increase the survival of marine shrimp, Litopenaeus vannamei, after infection with Vibrio harveyi. Brazilian J Oceanography 55:251–255
Giri SS, Sen SS, Chi C, Kim HJ, Yun S, Park SC, Sukumaran V (2015) Effect of guava leaves on the growth performance and cytokine gene expression of Labeo rohita and its susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol 46:217–224
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 33:683–689
Kongnum K, Hongpattarakere T (2012) Effect of Lactobacillus plantarum isolated from digestive tract of wild shrimp on growth and survival of white shrimp (Litopenaeus vannamei) challenged with Vibrio harveyi. Fish Shellfish Immunol 32:170–177
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34: 660–666
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14
Ninawe AS, Selvin J (2009) Probiotics in shrimp aquaculture: avenues and challenges. Crit Rev Microbiol 35:43–66
Ajitha S, Sridhar M, Sridhar N, Singh ISB, Varghese V (2004) Probiotic effects of lactic acid bacteria against Vibrio alginolyticus in Penaeus (Fennero penaeus) Indicus (H. Milne Edwards). Asian Fisheries Sc 17:71–80
Chiu CH, Guu YK, Lui CH, Pan TM, Cheng W (2007) Immune response and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol 23:364–377
Soccol CR, de Souza Vandenberghe LP, Spier MR, Medeiros ABP, Yamaguishi CT, De Dea LJ, Pandey A, Thomaz-Soccol V (2010) The potential of probiotics: a review. Food Technol Biotechnol 48:413–434
Maeda M, Shibata A, Biswas G, Korenaga H, Kono T, Itami T, Sakai M (2014) Isolation of lactic acid bacteria from kuruma shrimp (Marsupenaeus japonicus) intestine and assessment of immunomodulatory role of a selected strain as probiotic. Mar Biotechnol 16:181–192
Dash G, Raman RP, Pani Prasad K, Makesh M, Pradeep MA, Sen S (2014) Evaluation of Lactobacillus plantarum as feed supplement on host associated microflora, growth, feed efficiency, carcass biochemical composition and immune response of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquaculture 432:225–236
Li K, Zheng T, Tian Y, Xi F, Yuan J, Zhang G, Hong H (2007) Beneficial effects of Bacillus licheniformis on the intestinal microflora and immunity of the white shrimp, Litopenaeus vannamei. Biotechnol Lett 29:525–530
Austin B, Austin DA (2007) Bacterial fish pathogens, diseases of farmed and wild fish. Springer Praxis Publishing, Chichester
Heo WS, Kim YR, Kim EY, Bai SC, Kong IS (2013) Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus). Aquaculture 376–379:20–24
AOAC (2005) Official Methods of Analysis of the Association of Official Analytical Chemists. Arlington, Virginia
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi A-R, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Shyne Anand PS, Kohli MPS, Kumar S, Sundaray JK, Dam Roy S, Venkateshwarlu G, Sinha A, Pailan GH (2014) Effect of dietary supplementation of biofloc on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 418–419:108–115
Wang X, Li H, Zhang X, Li Y, Ji W, Xu H (2000) Microbial flora in the digestive tract of adult penaeid shrimp (Penaeus chinensis). J Ocean Univ China 30:493–498
Wang Y, FU L, LIN J (2012) Probiotic (Bacillus coagulans) cells in the diet benefit the white shrimp Litopenaeus vannamei. J Shellfish Res 31:855–860
Merrifield DL, Burnard D, Bradley G, Davies SJ, Baker RTM (2009) Microbial community diversity associated with the intestinal mucosa of farmed rainbow trout (Oncoryhnchus mykiss Walbaum). Aquaculture Res 40:1064–1072
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Dees PM, Ghiorse WC (2001) Microbial diversity in hot synthetic compost as revealed by PCR-amplified rRNA sequences from cultivated isolates and extracted DNA. FEMS Microbiol Ecol 35:207–216
Sapcharoen P, Pengpipat S (2013) Effects of the probiotic Bacilus subtilis (BP11and BS11) on the growth and survival of Pacific white shrimp, Litopenaeus vannamei. Aquaculture Nutr 19:946–954
Balcazar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebrate Pathol 96:147–150
Wang YB (2007) Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture 269:259–264
Castex M, Chim L, Pham D, Lemaire P, Wabete N, Nicolas JL (2008) Probiotic P. acidilactici application in shrimp Litopenaeus stylirostris culture subject to vibriosis in New Caledonia. Aquaculture 275:182–193
Tovar D, Zambonino J, Cahu C, Gatesoupe FJ, Vazquez-Juarez R, Lesel R (2002) Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae. Aquaculture 204:113–123
Thompson FL, Abreu PC, Cavalli R (1999) The use of microorganisms as food source for Penaeus paulensis larvae. Aquaculture 174:139–153
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotics bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671
Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen TF (1999) Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl Environ Microbiol 65:969–973
Boonthai T, Vuthiphandchai V, Nimrat S (2011) Probiotic bacteria effects on growth and bacterial composition of black tiger shrimp (Penaeus monodon). Aquaculture Nutr 17:634–644
Sugita H, Matsuo N, Shibuya K, Deguchi Y (1996) Production of antibacterial substances by intestinal bacteria isolated from coastal crab and fish species. J Marine Biotechnol 4:220–223
Ringø E, Myklebustd R, Mayhewe TM, Olsen RE (2007) Bacterial translocation and pathogenesis in the digestive tract of larvae and fry. Aquaculture 268:251–264
Gullian M, Rodríguez J (2002) Immunostimulant qualities of probiotic bacteria. Global Aquaculture Advocates 5:52–54
Sakata T (1990) Microflora in the digestive tract of fish and shellfish. In: Lesel R (ed) Microbiology in Poecilotherms. Elsevier, Amsterdam, pp. 171–176
Vaseeharan B, Ramasamy P (2003) Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. J Appl Microbiol 36:83–87
Acknowledgments
This study was funded by the Iranian Fisheries Research Organization (Tehran, Iran) and Sari Agricultural Sciences and Natural Resources University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Adel, M., El-Sayed, AF.M., Yeganeh, S. et al. Effect of Potential Probiotic Lactococcus lactis Subsp. lactis on Growth Performance, Intestinal Microbiota, Digestive Enzyme Activities, and Disease Resistance of Litopenaeus vannamei . Probiotics & Antimicro. Prot. 9, 150–156 (2017). https://doi.org/10.1007/s12602-016-9235-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9235-9