Abstract
Plant resistance to phytophagy is one of the pillars of integrated pest management. Five wild rice accessions viz. IRGC99577, IRGC104646, IRGC105270, IRGC105275, CR100204 along with susceptible (TN1) and resistant (Ptb33) controls were studied for levels of antibiosis resistance and their defense response to brown planthopper (BPH), Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) feeding. Parameters involved in antibiosis resistance viz., honeydew excretion, nymph emergence, nymph survival and development duration and the potential role of antioxidant enzymes viz. superoxide dismutase (EC 1.15.1.1), peroxidase (EC 1.11.1.7), catalase (EC 1.11.1.6), ascorbate peroxidase (EC 1.11.1.11) and H2O2 content in imparting antibiosis against BPH was studied during wet (Kharif) crop seasons of 2017 and 2018. Increased level of antioxidant enzymes were recorded after BPH infestation both in leaf blade and sheath in IRGC99577. Higher induced level of peroxidase was observed in Ptb33 and IRGC99577. Maximum percent increase in H2O2 content after insect infestation was observed in IRGC104646 and CR100204 accessions. The correlation between nymph emergence and superoxide dismutase, peroxidase and H2O2 was significantly negative (r = −0.99, −0.89, −0.93, respectively), while, it was significantly positive with catalase and ascorbate peroxidase (r = 0.99 and 0.98, respectively). Enhanced activities of enzymes may impart resistance in selected accessions against BPH as indicated by correlation and regression analysis. IRGC99577 has come out a potential source of resistance against BPH, which could be used in breeding programmes to develop BPH resistant varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown planthopper (BPH), Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), is a serious sap-sucking and destructive insect pest of rice (Oryza sativa L.). It feeds on phloem sap by its piercing and sucking mouthparts and in case of relevant infestations, plants get completely wilted which may result in ‘hopper burn’ conditions. Furthermore, BPH also transmits several rice viral diseases such as grassy stunt virus and ragged stunt virus, thus causing heavy losses to rice crop (Bottrell and Schoenly 2012). BPH outbreaks on high yielding varieties (HYVs) are mainly considered due to habitat modification rather than to genetic vulnerability of the HYVs. Currently, conventional insecticides can manage this insect pest, but can also detrimentally affect the delicate balance between BPH and natural enemies in rice ecosystem (Yang et al. 2017). Host plant resistance has been recognized as one of the most economic, effective and environment-friendly approach for the management of this pest. Several wild Oryza species showed high level of resistance against BPH (Jena et al. 2015). However, there has been no detailed study on the biochemical resistance as well as the antibiosis resistance in the wild rice accessions which will ultimately affect the BPH behavioural and fitness response to plants.
Plants have selected a wide range of defense mechanisms against pathogens as well as insect pests. BPH feeding results in physiological, morphological and chemical changes in the accumulation of defensive compounds in rice plant (Ashrith et al. 2017). A rapid and transitory production of reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide and hydroxyl radicals, caused by biotic stress, is a characteristic phenomenon in plants (Torres 2010). Overproduction of ROS may result in cell and tissue damage in plants. Superoxide dismutase (SOD) acts as first line of defense against elevated levels of ROS and protect the plant tissues from oxidative damage by scavenging ROS (Mittler et al. 2004). Similarly, peroxidase (POD), ascorbate peroxidase (APX) and catalase (CAT) enzymes play a significant role in defense against insect feeding (He et al. 2010). The induced levels of these enzymes in plants are regarded as a resistance mechanism of the plants in response to phytophagous insects. Such a defense response is an important factor of host plant resistance against insect pests and the success of defense response by plants depends on the quick recognition of and immediate action against insect attack (War et al. 2013).
Currently, information about the induced defense mechanisms to BPH, especially the role of ROS and antioxidative enzymes, is limited. Therefore, the present investigations were undertaken to evaluate the biochemical changes in wild rice accessions after BPH infestation. The association of enzymatic response of plants and feeding and growth of BPH was also studied. These studies will facilitate the use of the accessions for the development of rice varieties with durable resistance against BPH.
Material and methods
All the studies related to antibiosis resistance against BPH and level of antioxidant enzymes at constitutive and induced level were carried out in Rice Entomology laboratory screen house (7.80 × 3.30 × 3.40 m) at Rice Research Farm (30°54′ latitude and 75°48′ longitude, 247 m above mean sea level) and Biochemistry laboratories of the Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana during wet seasons (Kharif) of 2017 and 2018.
Plant material
Five wild rice accessions viz. IRGC99577, IRGC105270, IRGC105275, IRGC104646, CR100204 along with a susceptible control, TN1 and resistant control, Ptb33 were grown separately in earthen pots (20 cm diameter). These pots were placed in water trough in completely randomized design (CRD) under screen house at 28 ± 2 °C and 75 ± 5% RH conditions. Experiments related to antibiosis studies were replicated five times and one potted plant for each accession represented one replication. Separate set of experiments were laid out to study antioxidant enzymes in the leaf blade and leaf sheath of plants under constitutive and induced conditions and such studies were replicated thrice.
BPH colony
BPH colony was maintained in rectangular cages (0.68 × 0.50 × 0.50 m) on food plants (TN1) in screen house conditions as above. Field collected BPH adults were released on potted TN1 plants and were reared continuously for several generations at temperature of 28 ± 2 °C, 75 ± 5% relative humidity and 14:10 h light: dark photoperiod.
Antibiosis mechanism of resistance
Antibiosis response to feeding
The measurement of honeydew excretion is widely used method to assess feeding activity and consequently a reliable measure of resistance to insect feeding in the host plant (Han et al. 2018). In our study, sachet method (Pathak et al. 1982) was used to assess the honeydew excretion by BPH. The parafilm sachets (5 × 5 cm) were made and weighed before attaching them to the leaf sheath of each individual plant of each accession. Five one day old female BPH adults (previously starved for 2 h) were released in these sachets and allowed to feed on plants for 24 h. After feeding, the sachets were weighed again with honeydew.
Nymph emergence
On 30 day old potted plants, two pair of newly emerged male and female BPH adults was released (Khan and Saxena 1986). The insects were confined on plants by using plastic cups (5 cm dia and 6 cm height). BPH female lays eggs in a straight line along the mid-region inside the leaf sheath. Five days after release, adults were removed from the plants and emerging nymphs were counted daily. The total number of nymphs emerged on each test accession was considered as nymph emergence for each accession.
Nymph survival and development duration
In a separate experiment, fifty newly hatched BPH nymphs from eggs were released on 30 day old potted plants of each accession. To prevent the escape of released nymphs and emerging adults, plants were caged with cylindrical mylar cages and secured with cotton swab from upper end. The emerging adults were counted daily until all nymphs reached adult stage and total number of adults was considered as nymph survival. For development duration, nymphs were observed daily and the number of days taken to reach the adult stage was recorded as nymph development duration.
Biochemical constituents
The biochemical constituents were examined from leaf blade and leaf sheath portions of the tested accessions under constitutive (before BPH infestation) and induced (after BPH infestation) conditions. For induced response, twenty five 2nd-3rd instar nymphs were allowed to feed on single plant of each accession for 72 h.
Extraction and assay of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX).
For the extraction of SOD and POD, freshly collected samples of leaf blade and leaf sheath (100 mg) at constitutive and induced levels were homogenized in 2 ml of 0.1 M potassium phosphate buffer (pH 7.5) containing 1% PVP (Polyvinyl pyrrolidone), 1 mM EDTA (Ethylene diamine tetra acetic acid) and 10 mM β-mercaptoethanol using pre-chilled pestle and mortar. Similarly, for CAT and APX, tissue (100 mg) was homogenized in 2 ml of 0.05 M sodium phosphate buffer (pH 7.5) having 1% PVP. The extract was filtered through muslin and centrifuged at 10,000 g at 4 °C for 10 min and supernatant was used for the assay of enzymes as described below.
SOD activity was estimated as described by Marklund and Marklund (1974). Briefly, 1.5 ml Tris HCl buffer (pH 8.2), 0.5 ml of 6 mM EDTA, 1 ml of 6 mM pyrogallol and 0.1 ml of enzyme extract was added. Change in absorbance was recorded at 420 nm wavelength for 3 min at 30 s interval. A unit of enzyme activity was defined as the amount of enzyme causing 50% inhibition of auto-oxidation of pyrogallol as compared to blank.
POD activity was estimated according to the method of Shannon et al. (1966). In a cuvette, 3 ml of 0.05 M guaiacol prepared in 0.1 M potassium phosphate buffer (pH 6.5) and 0.05 ml of enzyme extract was added and the absorbance was set to zero. Then 0.1 ml of 0.8 M H2O2 was added and change in absorbance was recorded at 470 nm wavelength for 3 min at 30 s interval. The reaction mixture without H2O2 was used as blank. The unit of specific activity of enzyme was defined as the change in absorbance at 470 nm wavelength min−1 mg−1 protein.
CAT activity was assayed following method of Chance and Maehly (1955). 1.95 ml of 50 mM sodium phosphate buffer (pH 7.5) and 0.05 ml of enzyme extract was added in a cuvette. To start the reaction, 1 ml of H2O2 was added and utilization of H2O2 was observed for 3 min at interval of 30 s at 240 nm wavelength. The reaction mixture without H2O2 was used as blank. The specific activity of catalase was expressed as μmoles of H2O2 decomposed min−1 mg−1 protein. Protein concentration was measured as per method given by Bradford (1976).
APX activity was assayed according to the method described by Nakano and Asada (1987). In cuvette, 1 ml of 50 mM sodium phosphate buffer (pH 7.5), 0.8 ml of 0.5 mM ascorbic acid, 0.2 ml of enzyme extract and 1 ml of H2O2 was added and the enzyme activity was recorded at 30 s interval for 3 min at 290 nm. Specific activity of APX was expressed as nmoles of monodehydroascorbic acid formed min−1 mg−1 protein.
H2O2 content
H2O2 content at constitutive and induced level was estimated following the method of Sinha (1971). The tissue (0.5 g) was homogenised in 3 ml of ice cold 10 mM potassium phosphate buffer (pH 7.0). The homogenate was centrifuged at 10,000 g for 20 min and the collected supernatant was diluted to volume of 2 ml with 10 mM potassium phosphate buffer (7.0). To this, 2 ml of 5% K2Cr2O7 and glacial acetic acid (1:3 v/v) was added and the reaction mixture was centrifuged at 5000 g for 5 min. The absorbance was recorded at 570 nm. H2O2 content was expressed as μmoles g−1 of fresh tissue.
Statistical analysis
The data on antibiosis parameters were subjected to one-way analysis of variance while for biochemical constituents; data were subjected to two-way analysis of variance for the effects of test accessions and BPH infestation using IBM SPSS statistics. The means were compared by Tukey’s test (P = 0.05) and all the results are expressed as mean ± SE. The correlations between antibiosis parameters and biochemical constituents were carried out using Pearson’s correlation analysis. Regression models were developed by keeping antibiosis resistance parameters as dependent variable and biochemical constituents as independent variables through linear regression analysis.
Results
Antibiosis mechanism of resistance
Antibiosis response to feeding
Honeydew weight was minimum on IRGC99577 followed by Ptb33, IRGC105270, IRGC104646, IRGC105275, CR100204 while, it was maximum on TN1. There was a significant difference among the tested accessions (F6,28 = 3884.64, P ≤ 0.001) (Table 1). BPH feeding rate was significantly less on resistant accession IRGC99577 and on resistant control, Ptb33.
Nymph emergence
Lowest number of nymphs emerged from IRGC99577 followed by resistant control, Ptb33 and IRGC105270 while, the emergence was higher from CR100204 and susceptible control, TN1 (Table 1). There was significant difference in the emergence of nymphs among accessions (F6,28 = 43,176.63, P ≤ 0.001) (Table 1).
Nymph survival and development duration
Nymph survival ranged from 49 to 89% (F6,28 = 22.76, P ≤ 0.001) and nymph development duration ranged from 10.9 days to 22.5 days amongst accessions (F6,28 = 139.10, P ≤ 0.001) (Table 1). Accessions ranked from minimum to maximum nymph survival as IRGC99577, Ptb33, IRGC105270, IRGC104646, IRGC105275, CR100204 and TN1. Nymph development duration was 2.06 times longer on IRGC99577 than on TN1.
Biochemical constituents
Superoxide dismutase (SOD) (EC 1.15.1.1)
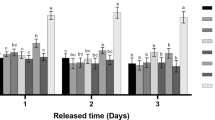
There was about 12% increase in SOD activity in leaf blade and 2% increase in leaf sheath of IRGC99577, after BPH infestation (Fig. 1). Activity of SOD differed significantly among accessions at constitutive and induced level in both leaf blade and leaf sheath (Table 2).
Catalase (CAT) (EC 1.11.1.6)
Activity of CAT differed significantly at constitutive and induced level in both leaf blade and leaf sheath of accessions (Table 2). In leaf blade, CAT activity showed an increase of 17% in TN1 whereas, in all other accessions activity decreased after infestation. In IRGC99577, CAT activity declined by 11.86% in leaf blade and by 15.43% in leaf sheath after BPH infestation (Fig. 2).
Peroxidase (POD) (EC 1.11.1.7)
Maximum induction of POD activity after BPH infestation was found in Ptb33 followed by IRGC99577 and its activity differed significantly among all the accessions (Table 3). The activity of POD was higher by 60% in Ptb33, 57% in IRGC99577 and 41% in IRGC105270 than TN1, after infestation (Fig. 3).
Ascorbate peroxidase (APX) (EC 1.11.1.11)
Highest APX activity was observed in TN1 than other accessions and after BPH infestation, an increase in activity was recorded in all the tested accessions (Table 3). In leaf blades of Ptb33, three times (33%) increase in APX activity was observed than in TN1 (11%) after BPH infestation, while two times higher APX activity (42%) was observed in Ptb33 leaf sheath as compared to TN1 (19%) (Fig. 4).
Hydrogen peroxide (H2O2)
H2O2 content was comparatively lower in susceptible control (TN1) as compared to other accessions (Table 4). After BPH infestation, maximum increase in H2O2 content was observed in leaf blade of IRGC104646 (42.45%) and leaf sheath of CR100204 (42.86%) (Fig. 5).
Correlations between antibiosis resistance parameters and biochemical constituents.
All the parameters studied in antibiosis resistance such as honeydew excretion, nymph emergence, nymph survival and nymph development duration were also correlated with biochemical constituents (Table 5). Honeydew excretion has a significant negative correlation with SOD (r = −0.95, P = 0.001), POD (r = −0.82, P = 0.023) and H2O2 (r = −0.89, P = 0.007) activity. Similarly, nymph emergence and nymph survival had significantly negative correlation with SOD, POD and H2O2 while, positive with CAT activity (Table 5). Time taken to complete development by nymphs was positively correlated with activities of SOD, POD and H2O2 content indicating that more activity of these enzymes resulted in prolonged development duration of BPH nymphs.
Linear regression analysis between antibiosis resistance parameters and biochemical constituents.
Furthermore, regression analysis was done to develop regression models on antibiosis resistance parameters and to know the dependence of antibiosis resistance on biochemical constituents (Table 6). All the regression equations have R2 value ≥0.92 indicating that all the equations are significant. When significance of independent variables was calculated, it was found that CAT contributed more in honeydew excretion and nymph emergence while, SOD contributed more in nymph survival and nymph development duration. It can be concluded that, all antioxidant enzymes in combination contribute towards antibiosis resistance against BPH rather than a single factor.
Discussion
BPH resistance has been documented in many wild accessions of rice belonging to Oryza nivara, O. australiensis, O. latifolia and O. minuta species (Madurangi et al. 2011). Many genes responsible for resistance have already been identified but constant search for new genes is also very important. Therefore, wild accessions of rice were studied to evaluate antibiosis resistance and biochemical constituents in these lines, which might play a crucial role in imparting resistance against BPH. Antibiosis resistance to feeding was measured by quantifying the honeydew excretion of BPH on different accessions (Han et al. 2018). In our study, less honeydew excretion by BPH was noticed on IRGC99577 followed by Ptb33. BPH ingests more plant sap on susceptible hosts and to prevent dilution of digestive enzymes, excess water is passed directly from foregut to hindgut by filter chamber in the form of honeydew. While feeding, BPH drain-out mainly excess sugars and amino acids from the plants and this is the indicator of resistance or susceptibility of the host plant (Udayasree and Rajanikanth 2018). Since BPH feeds less on resistant plants, low amount of honeydew is excreted (Madurangi et al. (2011); Balakrishna and Satyanarayana (2013); Gangaraju et al. (2017); Udayasree and Rajanikanth (2018)). The quality and quantity of sap sucked by BPH affects its growth and development rate and this will indicate level of antibiosis in plants against the BPH (Heinrichs et al. 1985). Reduced growth results in reduced oviposition, egg survival, nymph survival and overall insect vigour. In our study, comparatively lower nymph emergence and less egg hatching was observed on IRGC99577, IRGC105270 and IRGC104646 accessions. This might be due to the chemical environment surrounding the developing eggs which affected the hatching by reducing the permeability of eggs (Alagar and Suresh 2007). Thamarai and Soundararajan (2017) also recorded significantly lower nymph survival and more development duration of BPH on resistant genotypes. This might be the result of inadequate nutrients, absence of essential nutrients or reduced ingestion of adequate nutrition which will be responsible for prolongation of immature duration and lower survival in insects (Bhogadhi and Bentur (2015); Jena et al. (2015); Reddy et al. (2016)). It is a general concept that resistant lines prolonged the development duration and reduced the survival rate of insect pests.
The absence or non-availability of adequate nutrients to the insect pest could be due to the biochemical constituents (enzymatic or non-enzymatic) of the plants. Under stress conditions, reactive oxygen species (ROS) such as superoxide radicals, hydrogen peroxide and singlet oxygen, are produced in the plants which can damage cellular components of plants. Superoxide dismutases (SOD) react with superoxide radicals to produce hydrogen peroxide thus preventing the oxidative stress (War et al. 2012). This enzyme is unique as its activity determines the concentration of H2O2 and O2, thus is likely to be central in the defense mechanism. In our study, after BPH infestation, there was an increase in SOD activity in all accessions. Simova-Stoilova et al. (2009) also reported increased activity of SOD in stressed plants thus showing the anti-oxidative defense as an indispensable part of basic metabolism, enabling the plants to cope with stress conditions. Similarly, Kaur et al. (2014) recorded an increase in SOD activity in the test genotypes of pigeon pea in response to Helicoverpa armigera (Hübner) feeding. A 1.1 fold increase in SOD activity was recorded in flag leaves of aphid infested wheat as compared to uninfested wheat (Kaur et al. 2017). SOD transforms O2− to H2O2 while catalase (CAT) and peroxidase (POD) rapidly converts H2O2 into water, but they allow low steady levels of H2O2 to persist so as to maintain signalling pathways (Sofo et al. 2015). In our study, CAT activity tends to decrease in resistant accessions but increased in TN1 after BPH infestation. Increased SOD activity should have led to simultaneous increase in CAT activity. However, suppression in CAT activity after BPH infestation might be due to high levels of superoxide radicals as superoxide radical can gain access to the hemes of CAT due to its small size and converts CAT into ferro-oxy state which is inactive state (Kono and Fridovich 1982). Decreased catalase activity was documented in black gram leaves after whitefly, Bemisia tabaci (Gennadius) infestation (Taggar et al. 2012). Also, the decline in CAT activity due to herbivore damage has been reported by Khattab (2007); Kaur et al. (2014).
CAT is an antioxidative enzyme and its inhibition leads to elevated levels of H2O2. However, tight regulation of H2O2 is also required to avoid cytotoxic effects on host cells (Grant and Loake 2000). The decreased CAT activity is compensated by increase in ascorbate-peroxidase (APX) activity as both CAT and APX are major enzymatic scavengers of H2O2 (Caverzan et al. 2012). So, higher APX activity at induced levels observed in present studies might be due to increased levels of H2O2 as a result of BPH infestation. Increased APX activity in groundnut after Aphis craccivora (Koch.) infestation has been reported by War et al. (2013). More APX activity may result in decreased level of hydrogen peroxide in plant tissues. A well-established role of hydrogen peroxide as a signal molecule during the hypersensitive response was reported by Grant and Loake (2000). Decrease in CAT activity in resistant accessions and increase in CAT activity in TN1, in this study, can also be correlated to changes in hydrogen peroxide content. CAT catalyzes the dismutation of H2O2 into water and O2. Thus, catalase activity and hydrogen peroxide content are negatively correlated to each other. The H2O2 production has, e.g., been shown to be induced by Helicoverpa zea (Boddie) feeding on Glycine max (L.) Merr. (Bi and Felton 1995), by Heterodera glycines (plant parasitic nematode) feeding on Arabidopsis thaliana (Waetzig et al. 1999) and Spodoptera littoralis (Boisduval) feeding on Phaseolus lunatus L. (Maffei and Bossi 2006). Resistant genotypes tend to induce more H2O2 after insect infestation than susceptible ones (War et al. 2013), this could be highly advantageous, since the timing of induction of defensive response is an important factor for defending the plants against subsequent insect and pathogen invasion (Torres 2010). In addition, H2O2 also acts as a signalling molecule due to its high stability and freely diffusible property (Maffei et al. 2007) and stimulates the cascade of events that trigger physiological and molecular plant responses such as activation of defense-related genes which further cause the generation of anti-oxidative enzymes and toxic secondary metabolites. In our study, H2O2 was found to be positively correlated with nymph development duration of BPH while negatively with amount of honeydew excreted, number of nymphs emerged and nymph survival. Similar results were also reported by Yang et al. (2017) regarding positive correlation of H2O2 with BPH infestation.
Cellular damage caused by piercing behaviour of BPH trigger the activity of POD enzyme in host plant. This enzyme catalyzes the production of semiquinone free radicals and subsequent formation of quinones after (Barbehenn et al. 2010). It also mediates the oxidation of hydroxylcinnamyl alcohols into free radical intermediates, oxidation of phenols, cross-linking of monomers and polysaccharides, lignification and suberization (Chen et al. 2009) which further reduce the digestibility of proteins by insects. In our study, POD activity was enhanced after BPH infestation in all the accessions, but the expression rhythms of activities varied in all of them. Observed difference in POD activity in different accessions could be due to difference in resistance levels against BPH. A 9-fold increase in POD activity was recorded after infestation by Russian wheat aphid in resistant cultivar but only 3-fold increase was observed in susceptible cultivar (Ni et al. 2001). Yang et al. (2017) also observed enhanced POD activity in BPH infested rice seedlings as compared to uninfested seedlings. POD also catalyzes the conversion of H2O2 into water and oxygen as CAT enzyme. It is known that, in BPH infestation plant hormones such as salicylic acid (SA) and jasmonic acid (JA) get activated in the rice plants and SA, in particular, plays a major role in resistance of rice against BPH (Li et al. 2017). SA mediated response is closely associated to the cell-redox state, which indicates that anti-oxidant enzymes are involved in resistance against BPH (Zhao et al. 2016). This could be the reason that SOD and CAT are the key anti-oxidant enzymes imparting antibiosis resistance in wild accessions against BPH as observed in regression models developed in this study.
Conclusions
Five wild accessions of rice were evaluated for antibiosis resistance parameters and biochemical constituents to understand the mechanism of resistance against brown planthopper (BPH). The study has indicated that BPH infestation has altered the biochemical constituents of O. punctata (IRGC99577), O. australiensis (IRGC105270) and O. nivara (IRGC104646). An increase in activity of antioxidant enzymes such as SOD and POD was observed. Even though, the CAT activity declined after herbivory, its higher constitutive level in resistant accessions along with other enzymes may have determined the resistant status of these accessions against BPH. Induction of H2O2 after BPH infestation indicates that it is initial molecule involved in defense response leading to production of further defensive components. Negative correlation between BPH development and enzyme activities suggested their role in imparting resistance against BPH. The study has also suggested that resistance is not the result of a single factor but combination of different parameters which together governs the exhibition of resistance. Such information may prove to be beneficial in developing enzyme markers to identify resistant accessions in future. In our studies, IRGC99577 has come out a potential source of resistance against BPH, which could be used in breeding programmes to develop BPH resistant varieties.
References
Alagar, M., & Suresh, S. (2007). Antibiosis mechanism of resistance to brown planthopper, Nilaparvata lugens (Stal.) on selected rice genotypes. Annals of Plant Protection Sciences, 15, 283–286.
Ashrith, K. N., Sreenivas, A. G., Guruprasad, G. S., Hanchinal, S. G., & Krishnamurthy, D. (2017). Biochemical basis of resistance in rice against brown and whitebacked planthopper. International Journal of Current Microbiology and Applied Sciences, 6, 1699–1706.
Balakrishna, P., & Satyanarayana, P. V. (2013). Genetics of brown planthopper (Nilaparvata lugens Stal.) resistance in elite donors of rice (Oryza sativa L.). The Bioscan, 4, 1413–1416.
Barbehenn, R., Dukatz, C., Holt, C., Reese, A., Martiskainen, O., Salminen, J. P., Yip, L., Tran, L., & Constable, C. P. (2010). Feeding on poplar leaves by caterpillars potentiates foliar peroxidase action in their guts and increase plant resistance. Oecologia, 164, 993–1004.
Bhogadhi, S. C., & Bentur, J. S. (2015). Screening of rice genotypes for resistance to brown planthopper biotype 4 and detection of BPH resistance genes. International Journal of Life Sciences Biotechnology and Pharma Research, 4, 90–95.
Bi, J. L., & Felton, G. W. (1995). Foliar oxidative stress and insect herbivory—Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. Journal of Chemical Ecology, 21, 1511–1530.
Bottrell, D. G., & Schoenly, K. G. (2012). Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high yielding rice production in tropical Asia. Journal of Asia-Pacific Entomology, 15, 122–140.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Caverzan, A., Passaia, G., Rosa, S. B., Ribeiro, C. W., Lazzarotto, F., & Pinheiro, M. M. (2012). Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genetics and Molecular Biology, 35, 1011–1019.
Chance, B., & Maehly, A. C. (1955). Effects of saline stress and calcium on lipid composition in bean roots. Phytochemistry, 32, 1131–1136.
Chen, Y., Ni, X., & Buntin, G. D. (2009). Physiological, nutritional and biochemical bases of corn resistance to foliage-feeding fall armyworm. Journal of Chemical Ecology, 35, 297–306.
Gangaraju, P., Shivashankar, T., & Lohithaswa, H. C. (2017). Genetic basis of resistance to brown planthopper (Nilaparvata lugens Stal) in local landraces of rice. International Journal of Current Microbiology and Applied Sciences, 6, 3388–3393.
Grant, J. J., & Loake, G. L. (2000). Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiology, 124, 21–29.
Han, Y., Chao, W., Yang, L., Zhang, D., & Xiao, Y. (2018). Resistance to Nilaparvata lugens in rice lines introgressed with the resistance genes Bph14 and Bph15 and related resistance types. PLoS One, 13(6), e0198630. https://doi.org/10.1371/journal.pone.0198630.
He, J., Chen, F., Chen, S., Lv, G., Deng, Y., Fang, W., Liu, Z., Guan, Z., & He, C. (2010). Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. Journal of Plant Physiology, 168, 687–693.
Heinrichs, E. A., Medrano, F. G., & Rapusas, H. R. (1985) Genetic Evaluation for Insect Resistance in Rice. Pp 356. International Rice Research Institute, Los Banos, Philippines.
Jena, M., Panda, R. S., Sahu, R. K., Mukherjee, A. K., & Dhua, U. (2015). Evaluation of rice genotypes for rice brown planthopper resistance through phenotypic reaction and genotypic analysis. Crop Protection, 78, 119–126.
Kaur, R., Gupta, A. K., & Taggar, G. K. (2014). Role of catalase, H2O2 and phenolics in resistance of pigeonpea towards Helicoverpa armigera (Hubner). Acta Physiologiae Plantarum, 36, 1513–1527.
Kaur, H., Salh, P. K., & Singh, B. (2017). Role of defense enzymes and phenolics in resistance of wheat crop (Triticum aestivum L.) towards aphid complex. Journal of Plant Interactions, 12, 304–311.
Khan, Z. R., & Saxena, R. C. (1986). Behavioural and physiological responses of Sogatella furcifera (Horvath) (Delphacidae: Hemiptera) to selected resistant and susceptible rice cultivars. Journal of Economic Entomology, 78, 1280–1286.
Khattab, H. (2007). The defense mechanism of cabbage plant against phloem-sucking aphid (Brevicoryne brassicae L.). Australian Journal of Basic and Applied Sciences, 1, 56–62.
Kono, Y., & Fridovich, I. (1982). Superoxide radical inhibits catalase. The Journal of Biological Chemistry, 257, 5751–5754.
Li, C., Luo, C., Zhou, Z., Wang, R., Ling, F., Xiao, L., Lin, Y., & Chen, H. (2017). Gene expression and plant hormone levels in two contrasting rice genotypes responding to brown planthopper infestation. BMC Plant Biology, 17, 57.
Madurangi, S. A. P., Samarasinghe, W. L. G., Senanayake, S. G. J. N., Hemachandra, P. V., & Ratnasekera, D. (2011). Resistance of Oryza nivara and Oryza eichingeri derived lines to brown planhopper, Nilaparvata lugens (Stal). Journal of National Science Foundation of Sri Lanka, 39, 175–181.
Maffei, E. M., & Bossi, S. (2006) Electrophysiology and plant responses to biotic stress. In Plant Electrophysiology—Theory and Methods; Volkov, A., Ed.; springer-Verlag: Berlin, Germany, pp. 461–481.
Maffei, E. M., Mithöfer, A., & Boland, W. (2007). Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. PhytoChem and Biosub, 68, 2946–2959.
Marklund, S., & Marklund, G. (1974). Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 169–174.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490–498.
Nakano, Y., & Asada, K. (1987). Purification of ascorbate peroxidases in spinach chloroplasts: Its inactivation in ascorbata depleted medium and reactivation by monodehydro ascorbata radical. Plant and Cell Physiology, 28, 131–140.
Ni, X., Quisenberry, S. S., Heng-Moss, T., Markwell, J., Sarath, G., & Klucas, R. (2001). Oxidative responses of resistant and susceptible cereal leaves to symptomatic and nonsymptomatic cereal aphid (Hemiptera: Aphididae) feeding. Journal of Economic Entomology, 94, 743–751.
Pathak, P. K., Saxena, R. C., & Heinrichs, E. A. (1982). Parafilm sachet for measuring honeydew excretion by Nilaparvata lugens on rice. Journal of Economic Entomology, 75, 194–195.
Reddy, B. N., Lakshmi, V. J., Maheswari, T. U., Ramulamma, A., & Katti, G. R. (2016). Studies on antibiosis and tolerance mechanism of resistance to brown planthopper, Nilaparvata lugens (Stal) (Hemiptera: Delphacidae) in the selected rice entries. The Ecoscan, 10, 269–275.
Shannon, L. M., Kay, E., & Lew, J. Y. (1966). Peroxidase isozymes from horse radish roots I. Isolation and physical properties. The Journal of Biological Chemistry, 241, 2166–2172.
Simova-Stoilova, L., Demirevska, K., Petrova, T., Tsenov, N., & Feller, U. (2009). Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties subjected to long-term field drought. Plant Growth Regulation, 58, 107–117.
Sinha, A. K. (1971). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Sofo, A., Scopa, A., Nuzzaci, M., & Vitti, A. (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Sciences, 16, 13561–13578.
Taggar, G. K., Gill, R. S., Gupta, A. K., & Sandhu, J. S. (2012). Fluctuations in peroxidase and catalase activities of resistant and susceptible black gram (Vigna mungo (L.) Hepper) genotypes elicited by Bemisia tabaci (Gennadius) feeding. Plant Signalling Behaviour, 7, 1321–1329.
Thamarai, M., & Soundararajan, R. P. (2017). Evaluation of antibiosis resistance to brown planthopper, Nilaparvata lugens (Stal) in rice. Journal of Entomology and Zoology Studies, 5, 954–957.
Torres, M. A. (2010). ROS in biotic interactions. Physiologia Plantarum, 138, 414–429.
Udayasree, M., & Rajanikanth, P. (2018). Non-preference/antixenosis and antibiosis mechanism contributing to BPH resistance in certain identified elite rice genotypes. International Journal of Current Microbiology and Applied Sciences, 7, 1908–1914.
Waetzig, G. H., Sobczak, M., & Grundler, F. M. W. (1999). Localization of hydrogen peroxide during the defence response of Arabidopsis thaliana against the plant-parasitic nematode Heterodera glycine. Nematology, 1, 681–686.
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hussain, B., Ignacimuthu, S., & Sharma, H. C. (2012). Mechanisms of plant defense against insect herbivores. Plant Signalling Behaviour, 7, 1306–1320.
War, A. R., Paulraj, M. G., Ignacimuthu, S., & Sharma, H. C. (2013). Defensive responses in groundnut against chewing and sap sucking insects. Plant Growth Regulation, 3, 259–272.
Yang, L., Han, Y., Li, F., Ali, S., & Hou, M. (2017). Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Scientific Reports, 7, 4232.
Zhao, H., Sun, X., Xue, M., Zhang, X., & Li, Q. (2016). Antioxidant enzyme responses induced by whiteflies in tobacco plants in defense against aphids: Catalase may play a dominant role. PLoS One, 11(10), e0165454.
Acknowledgements
The authors appreciate the financial support of INSPIRE fellowship by Department of Science and Technology (DST), Government of India, for conducting this research work. We are also grateful to the Incharge Rice section, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana for providing infrastructure for conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sandhu, R.K., Sarao, P.S. & Sharma, N. Antibiosis in wild rice accessions induced by Nilaparvata lugens (Stål) feeding. Phytoparasitica 48, 801–812 (2020). https://doi.org/10.1007/s12600-020-00835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-020-00835-2