Abstract
Bactrocera zonata is a devastating invasive pest of tropical and subtropical horticultural crops. Since its detection in Sudan in 2011, almost no information has emerged regarding its bio-ecology. This study aimed to determine the pest’s range and potential distribution in Sudan, it’s relative abundance, infestation level, associated indigenous natural enemies and assess their role in its natural control. The infestation levels of B. zonata and B. dorsalis were assessed in fruit orchards between 2014 and 2016. MaxEnt software was used to predict the distribution of both species countrywide using occurrence points. Out of eighteen states, B. zonata was recorded coexisting with B. dorsalis in nine states, with relative abundance ranging between 0.2–100%. This co-occurrence was also confirmed by MaxEnt that showed high climate suitability in these states, with the mean annual temperature being the most important variable affecting the distribution of both species. Fruits infested with B. zonata included mango, guava, grapefruit, oranges and papaya. Three parasitioids; Tetrastichus giffardianus, Agonaspis sp. and Psytallia sp. were found associated with B. zonata. Our results provide evidence that the pest is widely spread across the country and poses a significant threat of invasion into neighboring countries and beyond unless early detection and eradication programs are applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Bactrocera (Diptera: Tephritidae) comprises aggressive invasive fruit flies species that impact negatively on fruit and vegetable production worldwide. Three Bactrocera species, namely the oriental fruit fly, Bactrocera dorsalis (Hendel), the Solanum fruit fly, Bactrocera latifrons (Hendel) and the peach fruit fly, Bactrocera zonata (Saunders) invaded Africa in recent years and their impact has been enormous in fruit and vegetable production in the continent (Lux et al. 2003; Mwatawala et al. 2007; De Meyer et al. 2007). Among the above, B. zonata is still restricted in North Africa, but there are fears that the pest might eventually find its way into Northeast Africa, East Africa and Southern Africa (Ni et al. 2012).
Despite its common name, the peach fruit fly, B. zonata is extremely polyphagous and attacks over 50 host plants in a number of major plant families (White and Elson-Harris 1994; EPPO 2013; Allwood et al. 1999). The host range is reportedly growing with new records of host plants being documented. For example, in Mauritius where B. zonata is already established, watermelon, Citrullus lanatus (Thunb) (Cucurbitaceae); avocado, Persea americana Mill (Lauraceae); java apple, Syzygium samarangense (Blume) (Myrtaceae) and bottle gourd, Lagenaria leucaritha Molina (Cucurbitaceae), are some of the plants previously not recorded as host plants (Quilici et al. 2008). Among the most preferred host plants are commercially important fruits such as mango, Mangifera indica L (Anacardiaceae), peaches, Prunus persica (L) Batsch (Rosaceae), guava, Psidium guajava L (Myrtaceae) and fig, Ficus carica L (Moraceae) (Qureshi et al. 1991; Mosleh et al. 2011). In a choice test, it has been demonstrated that B. zonata preferred mango fruits followed by peach which allows 84.53% and 81.09, respectively of immature stages to develop to adults (Sarwar et al. 2013). Prior to its detection in Sudan, B. zonata was reported in the Arabian Peninsula in countries including the United Arab Emirates, Saudi Arabia, Oman, and Yemen, (White 2006; EPPO 2010). The pest has also been recorded on potatoes in Egypt where it widely occurs (El-Samea and Fetoh 2006; Darwish et al. 2014).
Bactrocera zonata also occurs widely in Vietnam, India, Sri Lanka, Bhutan, Laos, Thailand, Bangladesh, Pakistan and most likely other countries in greater Asia (White and Elson-Harris 1994; Kapoor 1993; Drew and Romig 2013; Sarwar et al. 2014). Some studies (i.e. White and Elson-Harris 1994; EPPO 2010) reported that B. zonata occurs in Indonesia. However, Drew and Romig (2013) stated that intensive surveys were conducted throughout the country for B. zonata but no specimen was found, thus, the occurrence of this pest in Indonesia should be considered doubtful. Away from Asia, the pest was once detected in California, USA in 1984, 1987 (Spaugy 1988; Foote et al. 1993) and recently in Miami in 2010 (Steck 2010). Bactrocera zonata remains a real threat even to major trading blocs such as the European Union, thus in efforts aimed at quarantining the pest from Europe, the European and Mediterranean Plant Protection Organization (EPPO) categorized B. zonata as an A1 phytosanitary threat to horticulture in Europe (Kapoor 1993). The pest is presumed to be more competitive and invasive than other invasive pests such as the Medfly Ceratitis capitata (Wiedemann), which is already established in Europe.
In Africa, B. zonata was intercepted for the first time in Egypt in 1914 (Efflatoun 1924) and declared widespread in the same country by the year 2002 (EPPO 2002). The pest has also been reported in Libya and the Indian Ocean islands of Mauritius and Reunion (White et al. 2000). In Egypt alone, annual losses amounting to 190 million Euro due to infestation by the pest on mango, peach, guava and apricot Prunus armeniaca L. (Dicotyledonae) have been recorded while similarly, the Middle East countries lose in excess of 320 million Euro worthy of earnings (EPPO 2005). In addition to the direct losses, countries, where the pest has been recorded continue to lose potential revenue due to quarantine restrictions imposed by lucrative export markets.
In July 2011, B. zonata was detected in Central Sudan on mango and guava, but no data is available to quantify actual and potential losses attributable to the pest (Salah et al. 2012). Data from detection and surveillance traps set in Gezira and Sennar states showed higher trap catches for B. zonata than B. dorsalis (Salah et al. 2012) which could mean that the former inhabit niches which are unexploited by the later or may be more aggressive and devastating. This is quite alarming given the fact that horticultural production in the country is already overburdened by the heavy infestation attributed to B. dorsalis. Presence and the inability to adequately manage B. dorsalis has already resulted in the loss of exports to Gulf countries and other international markets. This could be compounded by B. zonata, which will impact rural communities that rely on agriculture for their livelihood.
Several control methods have been used to manage B. zonata in fruit orchards. These includes the male annihilation technique using methyl eugenol attractant, filed sanitation and pesticides application on foliar and soil to target adults and pupae (Ghanim et al. 2010; Al-Eryan et al. 2018). In addition, some parasitoids such as Dirhinus giffardii Silvestri, Trybliographa daci Weld, Diachasmimorpha longicaudata Ashmead, Fopius vandenboschi Fullaway and Psyttalia makii Sonan were identified in the pest native region as the potential natural enemies (Stibick 2004; Sarwar et al. 2015). However, being an invasive pest, classical biological control might be an ideal option for its suppression as has been proven for other invasive fruit flies species. Nevertheless, classical biological control should be informed and guided by extensive knowledge on whether indigenous natural enemies capable of suppressing B. zonata adequately are available locally. In light of B. zonata being a potential menace to fruit and vegetable production in Sudan, the objective of the current study was to determine the occurrence, relative abundance, magnitude of infestation and indigenous natural enemies’ fauna of B. zonata in selected states in Sudan.
Materials and methods
Study sites
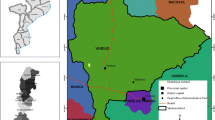
The study was conducted from September 2014 to April 2016 in different fruit producing states representing agro-ecological zones of Sudan. These included Northern, River Nile, Khartoum, Gezira, Sennar, Kassala, Gedarif, White Nile, Blue Nile, North Kordofan, South Kordofan, West Darfur, North Darfur and East Darfur, with several localities within the states being demarcated for study (Table 1). In each State, three to five sites were randomly selected as sampling sites. This selection was based on the availability of the fruit orchards in the state because fruits production is rarely practiced in some states. Areas close to the borders of Ethiopia, Eritrea and Republic of South Sudan were also included in the study. GPS coordinates for all sites where fruits were sampled and traps were set, were recorded.
Fruit flies trapping using methyl eugenol baited traps
Modified Lynfield traps equipped with cotton wicks soaked in a mixture of methyl eugenol (Farma Tech International Corporation, Fresno, CA) and Malathion 57 EC (Cheminova India Ltd., Mumbai, Maharashtra, India) in the ratio 3:1 were randomly distributed in study orchards at a density of 4–8 traps/Ha. Traps were suspended on trees at two meters above the ground level using galvanised wire and were placed 50 m away from each other. The wire was coated with a thin layer of Tree Tanglefoot® glue (Canada) to prevent predatory ants from entering the traps. Cotton wicks were changed after every six weeks and fruit flies caught in the traps were removed weekly for the entire duration of the study. Flies were then preserved in 70% ethanol and transported to the laboratory at the Agricultural Research Corporation (ARC), Wad Medani, Sudan for sorting and identification following descriptions and keys by (Virgilio et al. 2014; Drew and Romig 2016).
Fruit sampling
Mango, papaya Carica papaya L (Caricaceae), citrus, Citrus sp. (Rutaceae), grapefruit, Citrus × paradisi (Rutaceae), banana, Musa sp. (Musaceae) date palm and guava fruits were sampled from the same farms in which traps were set for monitoring. The sampled fruits were placed in khakhi bags then transported to the Agricultural Research Corporation (ARC) laboratory where they were weighed and incubated in plastic containers (20 × 20 × 30 cm) placed over a 5 cm layer of sterilized sand which acted as pupating media for larvae popping out from the fruits. A rectangular piece (10 × 10 cm) had been cut out from the side of the container and replaced with fine mesh cloth fitted tightly over the resulting space in order to provide aeration.
Puparia were picked from the sand using a pair of soft forceps and placed in Petri dishes (8.6 cm diameter) then introduced in Perspex cages (50 × 50 × 50 cm) for adult fruit flies to emerge. Emerged adult flies were fed on an artificial diet consisting of a mixture of sugar and ultrapure grade enzymatic yeast hydrolysate (USB Corporation, Cleveland, Ohio, USA) at the ratio of 3:1 by volume and were provided with water in a Petri dish (8.6 cm diameter) with a layer of pumice. They were left for 48 h to allow the development of full coloration and were then killed by placing them in a freezer (-18 °C) for 30 min.
Emerging parasitoids were fed on honey streaked on the topmost part of the cage and also killed in the same manner. Thereafter, the flies and parasitoids were preserved in 70% ethanol and kept for identification later. Parasitoids were identified following the description and keys of (LaSalle and Wharton 2002; Wharton and Yoder 2020).
Indigenous parasitoid species associated with B. zonata
Parasitoids emerging from fruits in which B. zonata was the only emerging fruit fly species were counted and percent parasitism calculated. Since parasitoids were emerging from fruits with mixed infestations of B. zonata, B. dorsalis, Ceratitis cosyra (Walker), and Ceratitis capitata (Wiedemann), an independent study was conducted at Fadasi, in Gezira State where fruits were exclusively infested with B. zonata, to determine parasitoid species specifically parasitizing the pest. This was from November 2015 to May 2016.
Data analysis
Flies captured in methyl eugenol baited traps were identified, counted and the daily capture rate computed using the formula: Fruit Flies per trap per day (FF/T/D) = F/(T × D) where F = total number of flies; T = number of serviced traps and D = average number of days traps were exposed in the field (IAEA 2003). The relative abundance of each fruit fly species was estimated as: the number of flies of the same species/ the total number of all species caught × 100. Infestation level of various fruits was expressed as the number of fruit flies per kg of sampled fruit. Percent parasitism was expressed as the number of emerged parasitoids/ total number of puparia × 100. Percent relative abundance (PRA) and fruit flies captured per trap per day (FF/T/D) of B. zonata and B. dorsalis from methyl eugenol traps were subjected to one-way analysis of variance to determine differences of the same in various states. All analyses were performed using R software version 3.1.1 (R Core Team 2017).
GPS coordinates collected for the occurrence of B. zonata and B. dorsalis were used to predict the distribution of the two pests in Sudan using MaxEnt—software. MaxEnt evaluates the probability distribution for target species using presence data through the maximum entropy function (Phillips et al. 2006). In our study, 19 bioclimatic variables were obtained from Worldclim database (http://www.worldclim.org/), with a spatial resolution of 2.5 arc sec to predict both fruit fly species distribution. We used 70% of the fruit fly occurrence data to develop the model, while the remaining 30% were used to test the accuracy of the model. The model accuracy was evaluated using the Area Under the Curve (AUC) of the receiver operating characteristics curve. The contribution of each environmental variable to the model performance was evaluated by the jackknife procedure. The most important environmental variables were those that produced the highest contribution to the model.
Results
Fruit flies catches from methyl Eugenol baited traps
Bactrocera zonata was recovered from methyl eugenol traps in nine out of the 14 surveyed states (Fig. 1). These are Northern, River Nile, Khartoum, Gezira, White Nile, Kassala, Gedarif, North Kordofan and South Kordofan states. Mean percent relative abundance of B. zonata was significantly higher in Khartoum state (F = 3.78; df 13,52; p < 0.0001), followed by Northern state and being lowest in South, West and Central Darfur states (Table 2). Similarly, mean trap catches were significantly higher (F = 3.25; df 13,52; p < 0.001), in Khartoum and Northern State than in any other sampled states, again being lowest in South, West and Central Darfur states. On the other hand, mean percent relative abundance of B. dorsalis was significantly higher (F = 4.29; 13,52; p < 0.0001), in River Nile, Sennar, Blue Nile, Gedarif, South, West and Central Darfur states compared to the other seven states (Table 2). Mean trap catches were significantly higher (F = 2.57; 13,52; p < 0.008), in Sennar State compared to 13 other states. Overall mean percent relative abundance was four times higher for B. dorsalis compared to B. zonata while mean FF/T/D was 13 times higher for the former than the later (Table 2).
Fruit flies species recovered from fruits sampled from various states
Apart from B. zonata, other fruit flies species were recovered from sampled fruit in some of the 14 states in Sudan (Table 3). These were B. dorsalis, Ceratitis cosyra and Ceratitis capitata. Bactrocera zonata was mainly recovered from mango, grapefruit, guava, oranges and papaya while B. dorsalis was only recovered from mango, guava and oranges. Ceratitis cosyra was recovered from mango and guava while C. capitata was recovered from guava only. In the Northern and River states, B. zonata was the only species recovered from the sampled fruits (with up to 10.2 fly/kg of fruit), except at one site (Argou 2), where few C. capitata (1.5 fly/kg of fruit) were also recovered (Table 3). Conversely, in Khartoum State, at Elfaki Hashim, four fruit flies were recovered from the sampled fruit with B. zonata being the highest in number (201.1 fly/kg of fruit) which was approximately three fold of that of B. dorsalis, while C. cosyra was the least recovered species (12.4 fly/kg of fruit). However, within the same State at Elkabashi, B. zonata and B. dorsalis were recovered in almost similar proportions (Table 3). In Gezira State, only the two Bactrocera species recovered from all sampled sites. Furthermore, the highest number of B. dorsalis fruit flies per kilogram of fruit was recorded in this State, specifically at Fadasi in guava (Table 3).
Fruits sampled from South Kordofan State, yielded both B. zonata and B. dorsalis, though the former species was reared in very low numbers, being higher in the central part of the State (Abu Jubyhah) with 4.8 fly/kg of fruit (Table 3). On the other hand, all fruits collected from Sennar, Blue Nile, Kassala, Gedarif and North Kordofan states yielded no B. zonata. The only fruit fly recovered from Gedarif was B. dorsalis with up to 1246.9 flies/kg from mango and 1086.6 flies/kg from guava sampled (Table 3).
Indigenous parasitoid species associated with B. zonata
Out of the eleven states where fruit samples were collected, fruit fly parasitoids were recovered only from Khartoum, Gezira and White Nile states. These were the gregarious Tetrastichus giffardianus Silvestri (Hymenoptera: Eulophidae) and an unidentified species of Aganaspis and Psyttalia. From fruits at Fadasi, Gezira State where B. zonata was the sole fruit fly species, percent parasitism by T. giffardianus ranged from 0.8% to 58.1% (Fig. 2). Mean number of parasitoid wasps emerging from a single puparium ranged from 1.3 to 6.5.
Distribution of B. zonata and B. dorsalis in Sudan
The model evaluation provided good performance with AUC of 0.90, 0.95 and 0.89 for B. zonata, B. dorsalis and the occurrence of both species, respectively (Fig. 3). The bioclimatic variables which provided the highest contribution to the potential distribution of B. zonata were the annual mean temperature and precipitation of coldest quarter with values of 78.8 and 15.2% respectively (Table 4). While the annual mean temperature, max temperature of the warmest month, mean temperature of the coldest quarter and the precipitation of warmest quarter were the most important variables contributing to the B. dorsalis distribution model, accounting for 45.1, 20.8, 13.8 and 9.7%, respectively (Table 4). For the occurrence of both species in the same location, annual mean temperature, precipitation of coldest quarter and mean temperature of warmest quarter were the most contributed variables with 77.3, 14.9 and 3.2%, respectively (Table 4). The model predicted that the most suitable areas for B. zonata were Kassala, Gezira, Sennar, Khartoum, Northern, Gedarif, River Nile and White Nile states, with the highest probability of occurrence for this species (Fig. 4A). On the other hand, the most suitable areas for B. dorsalis were less than that of B. zonata, with the highest probability predicted in Kassala, Gezira, Sennar and Gedarif states (Fig. 4B). Similarly, the most suitable habitat for the occurrence of both species was predicted in Kassala, Gezira, Sennar, Khartoum, Northern, Gedarif, River Nile and White Nile states (Fig. 4C).
Discussion
The study reports the widespread occurrence of B. zonata in Sudan, with the pest being confirmed in nine out of the 14 states through methyl eugenol traps and further by fruit sampling. We may attribute its absence especially in South, West and Central Darfur states to fewer (only one) sampling sites, although this may not be the case in Sennar and Blue Nile where we had five and seven sampling sites respectively. Our sampling period was not continuous and depended on availability of fruits. The absence of B. zonata from the three states of Darfour, could further be explained by the fact that this region is separated from the other major fruit producing regions by a massive belt of the Great Sahara that acts as a natural barrier for insect movement. However, it is expected that eventually, the pest could find its way to this region through fruit trade, unless strong legislation of restricting movement of fruits among the states is strictly enforced an adhered to in order to maintain this region as B. zonata free zone. In addition, as suggested by Zhang et al. (2007), ecosystem services and disservices highly affect the dispersal of insects. Thus, the spread of B. zonata may be influenced by several factors such as habitat complexity, habitat fragmentation and habitat connectivity (Chidawanyika et al. 2019). On the negative side, habitat complexity due to highly fragmented cropping systems, unavailability of quality resources, and poorly connected habitats pose serious challenges and impediments to the dispersal and successful establishment of insect pests. However, when suitable resources are available, cropping systems continuous, and predation fairly low and ineffective, there is more room for dispersal and establishment.
In Sennar, Blue Nile and Greater Darfur State, B. zonata was neither detected in methyl eugenol baited traps nor recovered from fruits sampled from these states. It is quite intriguing, to note that B. zonata could not be trapped in methyl eugenol baited traps nor be recovered from fruits sampled in Sennar, yet it was accidentally detected for the first time in Sudan in this State from a survey targeting the other invasive species B. dorsalis (Salah et al. 2012). The reason for its possible disappearance from this State is not clear, especially considering that the State is a major producer of guava and mango, (IFAD 2012) both of which are preferred host plants for B. zonata (Sarwar et al. 2013; Rauf et al. 2013; Rizk et al. 2014).
Interestingly, the neighboring State of Gezira, where the pest was detected at the same time as that of Sennar State (in 2011 survey), B. zonata was recorded in lure traps as well as sampled fruits. The occurrence of B. zonata and B. dorsalis in the Northern State in traps indicates that the pest is well established in this State, and possibly it has been there for a long time but remained undetected. This also suggest that the invasion by B. zonata in Sudan could be as a result of southwards movement of this pest from Egypt, where it has been reported as a major pest of guava, mango and peach, apricot and fig across several Governorates in this country (Shehata et al. 2008; Mosleh et al. 2011). Although this invasion may have been through the natural movement of the pest through the orchards on the River Nile banks across the borders of the two countries, there is also an active trans-boundary fruits trade between the two countries, a sizable consignment of which very often not passing through strict quarantine inspection. Nevertheless, the actual invasion routes of this pest into Sudan need to be determined through molecular tools, a study that we are currently undertaking. In the White Nile and South Kordofan states, B. zonata seems to be a recent invasion as B. dorsalis is generally still dominating in both traps and fruits.
In the recent past, the potential distribution of B. zonata worldwide was projected using CLIMEX model (Ni et al. 2012). The model predicted the main mango and guava producing states in Sudan to be marginally suitable for B. zonata. Similarly, Stephens et al. (2007) and De Villiers et al. (2016) predicted Sudan as unsuitable area for B. dorsalis. In our study however, the major fruit producing states in Sudan were predicted by MaxEnt to be highly suitable for B. zonata and B. dorsalis, which matched closely with the currently known distribution of both species in the country. The differences between these studies could be as a result of different dataset especially the species occurrence points, different types and spatial resolutions of climatic data, as well as the levels of complexity in model fitting. Besides, CLIMEX model also uses the detailed knowledge of species physiological tolerances to climate factors as well as the collective indicator of the species growth rate to predict potential areas for the species establish (Kriticos et al. 2015; Byeon et al. 2017). This differences in the modelling approach between CLIMEX and MaxEnt could be also contributed to the discrepancy between these studies. In the present study, the predicted suitable areas for B. zonata was wider than that of B. dorsalis, which demonstrate a high threat of B. zonata to the fruit industry in Sudan. The annual mean temperature was the most important variable that influenced the distribution of both species. Since temperature plays a crucial role in the distribution and population growth of insects (Azrag et al. 2018; Bale et al. 2002), the variation in the population abundance of B. dorsalis and B. zonata we obtained in this study could be linked to the differences in the climatic variables as demonstrated by the MaxEnt model.
The report of the occurrence of B. zonata in eastern states of Kassala (specifically, Elsawagi South) and El-Gadarif (specifically, Hawata), with high suitability of these areas for this pest poses a serious risk of its invasion into neighboring Ethiopia and Eritrea. The detection of the pest at Makhaleef and Abu Jubyhah, a border region with the Republic of South Sudan, even though at low level, is quite alarming and raises concern of the risk of invasion of Kenya and Uganda by this pest considering the continuous wild plantations of mango and guava, where no management or even monitoring of this pest is currently being undertaken. In general, the pest has been found to displace the native Ceratitis species such as Ceratitis cosyra and Ceratitis capitata that were the dominant species (Deng 1990) before B. zonata invasion. Similar findings of Ceratitis displacement were reported in Egypt following the invasion by the same alien pest (Shehata et al. 2008; Amro and Abdel-Galil 2008). Displacement of native fruit flies by invasive ones has been reported by several other authors. For example, (Ekesi et al. 2009; Rwomushana et al. 2009) demonstrated the displacement of C. cosyra by B. dorsalis in Kenya. In fact, the former species is at the verge of disappearing at lower altitude (≤ low 200 masl) (Ekesi et al. 2009). Likewise, (Mwatawala et al. 2009; Isabiriye et al. 2015; Cugala et al. 2016) reported displacement of the Ceratitis species by B. dorsalis, in Tanzania, Uganda and Mozambique, respectively. In the present study, the abundance of B. zonata and B. dorsalis were inconsistent depending on sampling sites and it may be too early to speak of displacement. Though B. dorsalis was mostly absent in the Northern, Khartoum states and in Gezira State, its displacement is unlikely as it is considered highly polyphagous and a better invader than B. zonata especially considering that it has been many years since the former was reported in Egypt and it is still restricted up north Africa. On the other hand, B. dorsalis has spread to many countries conquering various habitats and ecological zones within a very short space of time (Goergen et al. 2011).
Bactrocera dorsalis had earlier displaced C. cosyra and C. capitata following its invasion and subsequent extensive spread in Sudan in 2005. Similar scenarios of successive displacement of various flies’ species has been documented in La Reunion and Mauritius, where the indigenous Ceratitis catoirii was displaced by C. capitata which was in turn displaced by C. rosa which was later displaced by B. zonata (White et al. 2000; Duyck et al. 2004; Duyck et al. 2006a)] Outside Africa, examples of competitive displacement include that of the earlier invader C. capitata by B. dorsalis from the Hawaiian coastal zone (Bess 1953), following the invasion by the latter in 1940s (Van Zwaluwenberg 1947) and the displacement of the former species by Queensland fruit fly Bactrocera tryoni, (Froggatt) from Eastern Australia (Vera et al. 2002).
In the current study, few parasitoids species were recovered only from Khartoum, Gezira and White Nile States. Two parasitoid species were considerably low in number, while the generalist gregarious Eulophid, Tetrastichus giffardianus was the most abundant species. Nevertheless, none of these parasitoid species is quite specific to this pest. The scarcity of indigenous fruit fly parasitoid found to be associated with B. zonata as well as their very low diversity is understandable considering the fact that B. zonata is alien to the African continent and none of these parasitoid species shared a co-evolutionary history with this pest. In Egypt, five parasitoid species, Aganaspis daci (Weld.), Fopius arisanus (Sonan), Diachasmimorpha kraussii (Fullaway), Diachasmimorpha tryoni (Cameron) and Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae), have been imported from Hawaii for testing and final release against this pest. Based on the laboratory evaluation, the former species was found to be very promising candidate. Thus, it was released in El-Arish district, North Sinai Governorate and later recovered with 9.7% parasitism (Mohamed et al. 2016). Similarly, in the Indian Ocean island of La Réunion, F. arisanus was also imported from Hawaii and released in the island against the same pest (Rousse et al. 2006) where it proved to be quite promising with percent parasitism ranging between 70 and 80 on tropical almond Terminalia catappa L (Combretaceae) where B. zonata is the dominant fruit fly species (Quilici et al. 2008).
Conclusions
The findings of this study provide a clear indication that B. zonata is fairly widely spread in Sudan and it represents a looming danger to the neighboring countries given the fact that the pest has been detected at the border areas with Eretria, Ethiopia and the Republic of South Sudan. This in turn poses a serious potential threat of invasion by this pest to the entire sub-Saharan and Sahel regions. Therefore, these findings should serve as an early warning to the countries in these regions. Thus, this calls for an urgent need for enforcing phytosanitary and quarantine measures and deployment of systematic surveillance targeting B. zonata for its early detection and possible containment and/or eradication. Indeed, the modelling projection by (Ni et al. 2012) indicates that a large part of the continent is climatically suitable for B. zonata establishment. Being an alien pest in Sudan and the continent at large and lacking specific efficient natural enemies, it represents an ideal target for classical biological control. Currently efforts are underway to import F. arisanus and the larval-prepupal parasitoid, D. longicaudata from the International Centre of Insect Physiology and Ecology icipe, Nairobi, Kenya into Sudan for testing and subsequent release for biological control of B. zonata.
References
Al-Eryan, M. A. S., El-Minshawy, A. M., & Awad, A. I. (2018). Suppression program of the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae) depend on male annihilation and bait application techniques in northern coast of Egypt. Acta Scientific Agriculture, 2(8), 92–98.
Allwood, A. J., Chinajariyawong, A., Kritsaneepaiboon, S., Drew, R. A. I., Hamacek, E. L., Hancock, D. L., Hengsawad, C., Jipanin, J. C., Jirasurat, M., Krong, C. K., & Leong, C. T. S. (1999). Host plant records for fruit flies (Diptera: Tephritidae) in Southeast Asia. Raffles Bulletin of Zoology, 47, 1–92.
Amro, M. A., & Abdel-Galil, F. A. (2008). Infestation predisposition and relative susceptibility of certain edible fruit crops to the native and invading fruit flies (Diptera: Tephritidae) in the New Valley oases, Egypt. Assiut University bulletin for environmental researches, 11, 89–97.
Azrag, A. G., Pirk, C. W., Yusuf, A. A., Pinard, F., Niassy, S., Mosomtai, G., & Babin, R. (2018). Prediction of insect pest distribution as influenced by elevation: Combining field observations and temperature-dependent development models for the coffee stink bug, Antestiopsis thunbergii (Gmelin). PLoS One, 13, e0199569.
Bale, J. S., Masters, G. J., Hodkinson, I. D., Awmack, C., Bezemer, T. M., Brown, V. K., Butterfield, J., Buse, A., Coulson, J. C., Farrar, J., & Good, J. E. (2002). Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology., 8, 1–16.
Bess, H. A. (1953). Status of Ceratitis capitata in Hawaii following the introduction of Dacus dorsalis and its parasites. Proceedings of the Hawaii Entomological Society, 15, 221–234.
Byeon, D. H., Jung, J. M., Lohumi, S., Cho, B. K., Jung, S., & Lee, W. H. (2017). Predictive analysis of Metcalfa pruinosa (Hemiptera: Flatidae) distribution in South Korea using CLIMEX software. Journal of Asia-Pacific Biodiversity, 10, 379–384.
Chidawanyika, F., Mudavanhu, P., & Nyamukondiwa, C. (2019). Global climate change as a driver of bottom-up and top-down factors in agricultural landscapes and the fate of host-parasitoid interactions. Parasitoids’ ecology and evolution. Frontiers in Ecology and Evolution, 7, 80. https://doi.org/10.3389/fevo.2019.00080.
Cugala, D.R., De Meyer, M., Canhanga, L. J. (2016) Integrated management of fruit flies–case studies from Mozambique, In S. Ekesi et al (eds), fruit fly research and development in Africa - towards a sustainable management strategy to improve horticulture. Springer International Publishing Switzerland, pp. 531–552.
Darwish, D. Y., Rizk, M. M., Abdel-Galil, F. A., & Temerak, S. A. (2014). Seasonal population trend of the peach fruit fly (PFF), Bactrocera zonata (Saunders) (Diptera: Tephritidae) in Assiut, northern upper Egypt. Archives of Phytopathology and Plant Protection, 47, 1158–1165.
De Meyer, M., Mohamed, S.A, White, I.M. (2007). Invasive fruit fly pests in Africa. 2007. Available onlin: http://www.africamuseum.be/fruitfly/AfroAsia.htm ().
De Villiers, M., Hattingh, V., Kriticos, D. J., Brunel, S., Vayssières, J. F., Sinzogan, A., Billah, M. K., Mohamed, S. A., Mwatawala, M., Abdelgader, H., Salah, F. E. E., & De Meyer, M. (2016). The potential distribution of Bactrocera dorsalis: Considering phenology and irrigation patterns. Bulletin Entomological Research, 106, 19–33.
Deng, A. L. (1990). Study on the behaviour, host range, seasonal abundance and chemical control of the Mediterranean fruit fly (Ceratitis capitata Wiedemann) in Sudan. M. Sc. Thesis, University of Khartoum, Sudan.
Drew, R.A., Romig, M.C. (2013). Tropical fruit flies (Tephritidae Dacinae) of South-East Asia: Indomalaya to north-West Australasia. CABI, Wallingford.
Drew, R.A.; Romig, M.C. (2016). Keys to the tropical fruit flies (Tephritidae: Dacinae) of South-East Asia: Indomalaya to north-West Australasia; CABI: Oxfordshire, UK,
Duyck, P. F., David, P., & Quilici, S. (2004). A review of relationships between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecological Entomology, 29, 511–520.
Duyck, P. F., David, P., & Quilici, S. (2006a). Climatic niche partitioning following successive invasions by fruit flies in La Réunion. Journal of Animal Ecology, 75, 518–526.
Efflatoun, H. C. (1924). A monograph of Egyptian Diptera, part II., Fam. Trypaneidae. Mémoires Society Entomology Egypte., 2, 1–132.
Ekesi, S., Billah, M. K., Nderitu, P. W., Lux, S. A., & Rwomushana, I. (2009). Evidence for competitive displacement of Ceratitis cosyra by the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement. Journal of Economic Entomology, 102, 981–991.
El-Samea, S. A. A., & Fetoh, B. E. A. (2006). New record of Bactrocera zonata (Saunders) (Diptera: Tephritidae) on potatoes in Egypt. Egyptian Journal of Agricultural Research, 84, 61–63.
EPPO (2002) Introduction of Bactrocera zonata into the EPPO region (situation in 2002). Bactrocera zonata: introduction into the EPPO region and its identification by Dr Ian M. White EPPO Workshop on Bactrocera zonata; Paris, 5–6 March 2002. https://www.eppo.int/ACTIVITIES/plant_quarantine/shortnotes_qps/bactrocera_zonata. Accessed 19 March 2020.
EPPO. (2010). Bactrocerazonata: Procedure for official control. Bulletin OEPP/EPPO Bulletin, 40, 390–395.
EPPO. (2013). PM 7/114 (1) Bactrocera zonata. Bulletin OEPP/EPPO Bulletin., 43, 412–416.
EPPO. (2005). Bactrocera zonata. Bulletin OEPP/EPPO Bulletin, 35, 371–373.
Foote, R. H., Blanc, F. L., & Norrbom, A. L. (1993). Handbook of the fruit flies (Diptera: Tephritidae) of America north of Mexico. Ithaca, USA: Comstock.
Ghanim, N. M., Moustafa, S. A., El-Metwally, M. M., Afia, Y. E., Salman, M. S., & Mostafa, M. E. (2010). Efficiency of some insecticides in male annihilation technique of peach fruit fly, Bactrocera zonata (Saunders) under Egyptian conditions. Egyptian Academic Journal of Biological Sciences, F. Toxicology and Pest Control, 2, 13–19.
Goergen, G., Vayssières, J. F., Gnanvossou, D., & Tindo, M. (2011). Bactrocera invadens (Diptera: Tephritidae), a new invasive fruit fly pest for the Afrotropical region: Host plant range and distribution in west and Central Africa. Environmental Entomology, 40, 844–854.
IAEA (2003). Trapping Guidelines for Area-Wide Fruit Fly Programmes. International Atomic Energy Agency, Vienna, Austria.2003. Available onlin at http://www-naweb.iaea.org/nafa/ipc/public/Trapping-guideline-(002).pdf (accessed on 22 August 2019).
IFAD (2012). Supporting the small-scale traditional rain fed producers in Sinnar state (SUSTAIN) design report: Main report and annexes near east and North Africa division programme management department. Republic of the Sudan. URL:https://www.ifad.org/documents/10180/d90ce55a-719d-41cf-bb02-2925d5809050 (accessed on 22 August 2019).
Kapoor, V.C. (1993). Indian fruit flies (Insecta: Diptera: Tephritidae). Oxford and IBH Publishing Company, New Delhi.
Kriticos, D. J., Maywald, G. F., Yonow, T., Zurcher, E. J., Herrmann, N. I., & Sutherst, R. (2015). Exploring the effects of climate on plants, animals and diseases. CLIMEX Version, 4, 184.
LaSalle, J., & Wharton, R. A. (2002). The identity and recognition of African Tetrastichus species (Hymenoptera: Eulophidae) associated with fruit flies (Diptera: Tephritidae). African entomology, 10(2), 297–304.
Lux, S. A., Copeland, R. S., White, I. M., Manrakhan, A., & Billah, M. K. (2003). A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. International Journal of Tropical Insect Science, 23, 355–361.
Mohamed, S.A., Ramadan, M.M., & Ekesi S. (2016). In and out of Africa: Parasitoids used for biological control of fruit flies. In S. Ekesi et al (eds), fruit fly research and development in Africa - towards a sustainable management strategy to improve horticulture. Springer International Publishing Switzerland; pp. 325–368.
Mosleh, Y.Y., Moussa, S.F.M; Mohamed, L.H.Y. (2011). Comparative toxicity of certain pesticides to peach fruit fly, Bactrocera zonata Saunders (Diptera: Tephritidae) under laboratory conditions. Plant Protection Science, 47, 115–120.
Mwatawala, M., De Meyer, M., White, I. M., Maerere, A., & Makundi, R. H. (2007). Detection of the solanum fruit fly, Bactrocera latifrons (Hendel) in Tanzania (Dipt., Tephritidae). Journal of Applied Entomology, 131, 501–503.
Mwatawala, M. W., De Meyer, M., Makundi, R. H., & Maerere, A. P. (2009). An overview of Bactrocera (Diptera: Tephritidae) invasions and their speculated dominancy over native fruit fly species in Tanzania. Journal of Entomology, 6, 18–27.
Ni, W. L., Li, Z. H., Chen, H. J., Wan, F. H., Qu, W. W., Zhang, Z., & Kriticos, D. J. (2012). Including climate change in pest risk assessment: The peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). Bulletin of Entomological Research, 102, 173–183.
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259.
Quilici, S. P., Rousse, J.P., Deguine, C., Simiand, A., Franck, F., Gourdon, T., & Harris, E.J. (2008). Successful acclimatization of the ovo-pupal parasitoid Fopius arisanus in Réunion island for the biological control of the Peach fruit fly, Bactrocera zonata. In Communication presented at the 1st Meeting of TEAM. Tephritid Workers of Europe Africa and the Middle East, pp. 7–8.
Qureshi, Z. A., Hussain, T., & Siddiqui, Q. H. (1991). Relative preference of mango varieties by Dacus zonatus (Saunders) and D. dorsalis Hendel. Pakistan Journal of Zoology, 23, 85–87.
R Core Team (2017). A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. Available onlinee at https://www.R-project.org/.
Rauf, I., Ahmad, N., Rashdi, S. M. S., Ismail, M., & Khan, M. H. (2013). Laboratory studies on ovipositional preference of the peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephiritidae) for different host fruits. African Journal of Agricultural Research, 8, 1300–1303.
Rizk, M. M., Abdel-Galil, F. A., Temerak, S. A., & Darwish, D. Y. (2014). Relative preference of peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae) to some fruit and vegetables under laboratory conditions. Archives of Phytopathology and Plant Protection, 47, 1376–1380.
Rousse, P., Gourdon, F., & Quilici, S. (2006). Host specificity of the egg pupal parasitoid Fopius arisanus (Hymenoptera: Braconidae) in La Reunion. Biological Control, 37, 284–290.
Rwomushana, I., Ekesi, S., Ogol, C. K., & Gordon, I. (2009). Mechanisms contributing to the competitive success of the invasive fruit fly Bactrocera invadens over the indigenous mango fruit fly, Ceratitis cosyra: The role of temperature and resource pre-emption. Entomologia Experimentalis et Applicata, 133, 27–37.
Salah, F.E.E., Abdelgader, H., De Villiers, M. (2012). The occurrence of the peach fruit fly, Bactrocera zonata (Saunders) (Tephritidae) in Sudan. In TEAM 2nd International Meeting: Biological Invasions of Tephritidae: Ecological and Economic Impact. Kolymbari Crete.
Sarwar, M., Ahmad, N., Rashid, A., & Shah, S. M. M. (2015). Valuation of gamma irradiation for proficient production of parasitoids (Hymenoptera: Chalcididae and Eucoilidae) in the management of the peach fruit-fly, Bactrocera zonata (Saunders). International journal of pest management, 61, 126–134.
Sarwar, M., Hamed, M., Rasool, B., Yousaf, M., & Hussain, M. (2013). Host preference and performance of fruit flies Bactrocera zonata (Saunders) and Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) for various fruits and vegetables. International Journal of Scientific Research Environmental Science, 1, 188–194.
Sarwar, M., Hamed, M., Yousaf, M., & Hussain, M. (2014). Monitoring of population density and fruit infestation intensity of tephritid fruit flies (Diptera: Tephritidae) in Citrus reticulata Blanco orchard. Journal of Zoological Science., 2, 1–5.
Shehata, N. F., Younes, M. W., & Mahmoud, Y. A. (2008). Biological studies on the peach fruit fly, Bactrocera zonata (Saunders) in Egyptian. Journal of Applied Science and Research, 4, 1103–1106.
Spaugy, L. (1988). Fruit flies: Two more eradication projects over. Citrograph, 73, 168.
Steck, G.J. (2010). A peach fruit fly, Bactrocera zonata (Saunders)(Tephritidae). Florida Department of Agriculture and Consumer Service, Available online: http://www.freshfromflorida.com/content/download/65230/1532266/PESTALERTPeachFruit_Fly_Bactrocera_Zonata.pdf (accessed on 22 August 2019).
Stephens, A. E. A., Kriticos, D. J., & Leriche, A. (2007). The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Bulletin of Entomological Research., 97, 369–378.
Stibick, J.N. (2004). Natural enemies of true fruit flies (Tephritidae) (p. 86). United States Department of Agriculture, Animal and Plant Health Inspection Serv.
Van Zwaluwenberg, R. H. (1947). Notes and exhibitions. Proceedings of the Hawaii Entomological Society, 13, 8.
Vera, M. T., Rodriguez, R., Segura, D. F., Cladera, J. L., & Sutherst, R. W. (2002). Potential geographical distribution of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), with emphasis on Argentina and Australia. Environmental Entomology, 31, 1009–1022.
Virgilio, M., White, I., & De Meyer, M. (2014). Set of multi-entry identification keys to African frugivorous flies (Diptera, Tephritidae). ZooKeys, 428, 97–108.
Wharton, RA., Yoder, MJ. (2020). Parasitoids of fruit-infesting Tephritidae. http://paroffit.org. Accessed on Thu mar 19 11:13:08 -0500 2020.
White, I. M. (2006). Taxonomy of the Dacina (Diptera:Tephritidae) of Africa and the Middle East. African Entomology, 2, 1–156.
White, I.M., De Meyer, M.D., Stonehouse, J. (2000). A review of native and introduced fruit flies (Diptera, Tephritidae) in the Indian Ocean islands of Mauritius, Réunion, Rodrigues and Seychelles. In Proceedings of the Indian Ocean Commission, Regional Fruit Fly Symposium, Flic en Flac, Mauritius, 5th-9th June. Pp. 15-21.
White, I.M, Elson-Harris, M.M. (1994). Fruit flies of economic significance: Their identification and bionomics. CAB International: Wallingford, UK.
Zhang, W., Ricketts, T. H., Kremen, C., Carney, K., & Swinton, S. M. (2007). Ecosystem services and dis-services to agriculture. Ecological Economics, 64, 253–260. https://doi.org/10.1016/j.ecolecon.2007.02.024.
Acknowledgments
Authors would like to thank the staff of the Agricultural Research Corporation at all stations in Sudan for Technical and kind support in the Laboratory and field.
Funding
This research was funded by the Department for International Development (DFID), through the program “Support to International Agricultural Research Centres ARIES Code 202748. Additional support was obtained from icipe’s institutional funding from the UK’s Department for International Development (DFID), the Swedish International Development Cooperation Agency (SIDA), the Swiss Agency for Development and Cooperation (SDC), and the Kenyan Government.
Author information
Authors and Affiliations
Contributions
The work was conceptualized by: [Mohammedelnazeir Elfadil Mahmoud, Samira Abuelgasim Mohamed and Sunday Ekesi]; methodology: [Mohammedelnazeir Elfadil Mahmoud and Samira Abuelgasim Mohamed]; software: [Abdelmutalab G. A. Azrag]; validation: [Mohammedelnazeir Elfadil Mahmoud, Samira Abuelgasim Mohamed, Abdelmutalab G. A. Azrag and Shepard Ndlela]; formal analysis: [Shepard Ndlela, Abdelmutalab G. A. Azrag, Samira Abuelgasim Mohamed and Mohammedelnazeir Elfadil Mahmoud]; investigation: Mohammedelnazeir Elfadil Mahmoud, Mohamed A. E. Bashir]; resources: [Samira Abuelgasim Mohamed and Sunday Ekesi] data curation: [Mohammedelnazeir Elfadil Mahmoud, Samira Abuelgasim Mohamed and Shepard Ndlela]; writing—original draft preparation: [Mohammedelnazeir Elfadil Mahmoud, Samira Abuelgasim Mohamed, Samira Abuelgasim Mohamed, Abdelmutalab G. A. Azrag and Shepard Ndlela]; writing—review and editing: [Shepard Ndlela, Abdelmutalab G. A. Azrag, Samira Abuelgasim Mohamed and Shepard Ndlela]; supervision: [Samira Abuelgasim Mohamed and Fathiya M. Khamis]; project administration: [Samira Abuelgasim Mohamed]; funding acquisition: [Samira Abuelgasim Mohamed and Sunday Ekesi].
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoud, M.E.E., Mohamed, S.A., Ndlela, S. et al. Distribution, relative abundance, and level of infestation of the invasive peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephritidae) and its associated natural enemies in Sudan. Phytoparasitica 48, 589–605 (2020). https://doi.org/10.1007/s12600-020-00829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-020-00829-0