Abstract
The predatory bug, Anthocoris minki Dohrn (Hem.: Anthocoridae), is an important predator of ash psyllid, Psyllopsis repens Loginova (Hem.: Psyllidae) and is very abundant on ash trees (Fraxinus excelsior L.) in Mashhad region, Iran. Functional response parameters, handling time and searching efficiency are of particular value in understanding feeding behavior of a predator. The type of the functional response and the rates of its parameters are influenced by different factors. In this study, the functional responses of A. minki to densities of P. repense at different temperatures (15, 24 and 30 °C), prey instar (2nd and 4th instar nymphs), developmental stage of predator (4th nymph and adult) and sex of predator (male and female) were investigated. The effects of predator densities and interference on searching efficiency were estimated. Logistic regression and nonlinear least- square regression were used to estimate the type of the functional response and its parameters, respectively. In order to determine the per capita searching efficiency and interference coefficient, Nicholson’s model and linear regression were used. The results showed a type II functional response on all experiments except of adult predators to 2nd instar nymphs of prey which exhibited a type III functional response. The greatest value of attack rate and the shortest handling time were seen at 30 °C. The per capita searching efficiency decreased as densities increased but there was no significant difference. The laboratory results suggest that A. minki has potential to control P. repens on ash trees but further research is needed under field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional response of predators to prey densities and mutual interference among predators are two important behaviors which can be used to determine their efficacy as biocontrol agents (Huffaker and Messenger 1976; Butler and O’Neil 2008). In general, the functional response of a predator to a prey can be classified in three response models: type I, II, and III (e.g., Holling 1961; Timms et al. 2008). Each type has a characteristic curve with different consequences for population dynamics (Petchey et al. 2008; Petchey et al. 2010). Whereas a type I response with linear increase in consumption rate, implies a density-independent predator attack rate, a type II response with decelerating rate leads to inverse density-dependent predation and the type III functional response with a sigmoid shape encompasses density dependence; at low densities of prey has an accelerating phase (Hassell et al. 1977; Hassell 1978; Berryman 1999; Bernstein 2000).
The type of functional response and the size of its parameters (attack rate and handling time) is affected by factors such as the species of predators or its prey, the variety and phenology of host plants, insecticides, predator age and other biotic and abiotic factors (Coll and Ridgway 1995; Messina and Hanks 1998; Fathipour et al. 2001; Gitonga et al. 2002; Pervez and Omkar 2005; Fathipoour et al. 2006; Sarmento et al. 2007; Pakyari et al. 2016; Mercado et al. 2017;).
The functional response of several species of anthocorids to different prey species has been studied. Jalalizand et al. (2011) studied the effect of host plant morphological features on the functional response of Orius niger Wolff (Hemiptera: Anthocoridae) to Tetranychus urticae Koch (Acari: Tetranychidae). The effect of plant varieties on the functional response of Anthocoris nemoralis Fabricius (Hem.: Anthocoridae) to Cacopsylla pyricola (Foerster) (Hem.: Psyllidae) was investigated by Emami et al. (2014).
Allied to a prey-density response of a predator are effects of changes in predator density. Generally, a higher density of predators leads to a lower feeding rate because predators interfere among them (Rotheray 1989). As the density of conspecifics increases, each individual predator spends less time searching for prey and more time interacting with other conspecifics (Hassell 1971). According to Hassell and Varley (1969), Hassell (1971) and Delong and Vasseur (2011), mutual interference is known as interference competition which occurs when access to resources is negatively affected by the presence of other individuals. For example, searching rate of Coccinella undecimpunctata Linnaeus and Hippodamia tredecimpunctata Linnaeus (Coleoptera: Coccinellidae) for Aphis gossypii (Glover) and Aphis punicae (Shinji) (Hemiptera: Aphididae) declined with increasing predator density (Al-Deghairi et al. 2014).
The ash tree, Fraxinus excelsior Linnaeus (Oleaceae), is the second most abundant shade tree in many urban green spaces in Iran and is the host of several herbivores, including a common psyllid in the genus Psyllopsis Loew (Hem.: Psyllidae). Psyllopsis repens Loginova, is the most abundant species of this genus on ash in Mashhad region, Iran. The psyllid induces galls in the host plant, characterized by rolled leaf margins that presumably protect this herbivore (Khandehroo 2015; Loginova 1963, 1968).
Anthocoris minki Dohrn (Hem.: Anthocoridae) is an important predator of P. repens and is very abundant on ash trees in the study area, but little is known about its predation rate on P. repens. Consequences of A. minki densities on its feeding behavior also are lacking. The present study investigated feeding behavior of A. minki to P. repens. Specifically the functional response parameters (e.g., handling times and attack rate/searching efficiency) of A. minki to densities of P. repens at different a) temperatures, b) prey instar, c) developmental stage of predator, d) sex of predator, and e) the effects of predator densities (via interference) on searching efficiency were investigated.
Materials and methods
The study insects
A laboratory colony of A. minki was established in June 2016 by collecting psyllid- and predator-infested leaf galls from Fraxinus excelsior. Predators were kept in plastic cages (8 cm in diameter and 11 cm high) and reared on ash psyllid nymphs in climate cabinets (L16: D8 photoperiod, 24 ± 1 °C and 65–70% RH). Twice a week leaves with galls and psyllid nymphs were replaced and leaves with anthocorid eggs were moved to new cages to produce equal age cohorts of the predator.
The prey species, P. repens was collected from insecticide-free ash trees. Prey were kept on ash leaves in the laboratory at a constant environmental condition (L16: D8 photoperiod, 24 ± 1 °C and 65–70% RH).
Functional response
Assays were conducted to determine the effect of A) temperature (15, 24 and 30 °C fed on 4th instar nymphs of prey), B) prey instar (2nd and 4th instar nymphs), C) predator sex (male and female fed on 4th instar nymphs of prey), D) developmental stage of predator (4th instar nymphs and adult females of predator fed on 2nd instar nymphs of prey) and E) predator density (one, two and three) on the functional response and its parameters under laboratory conditions (24 ± 1 °C, L16:D8 and 65–70% RH). Experiments were performed in small Petri dishes (9 cm diameter) ventilated with a 2 cm diameter mesh-covered hole in the lid. Leaf discs of ash were used for experiments. To provide water and support for leaves the floor of the petri dishes were covered with 1% agar. The leaves were collected from the field and checked for cleanness immediately before their use in the experiments. Female A. minki of 5–7 days in age were used in all experiments, except for experiments ‘C’ and ‘D’ in which 4th instar nymphs and 5–7 day old males were also examined. Predators were starved for 24 h before each assay to standardize hunger levels.
Prey size was standardized as much as possible to exclude the influence of this variable on the choices made by the predators. Prey densities were 5, 10, 15, 30, 60, and 100. Prey were allowed to settle on substrate for 20 min before adding a predator into each Petri dish. The lid of the Petri dish was sealed with Parafilm to prevent escape of prey. Ten replicates were made per prey density tested. A control treatment (five replicates) without predator was made to assess natural prey mortality. The number of prey consumed within 24 h was recorded.
To determine if increased density of predators depresses their feeding rate, three predator densities were tested (1, 2 and 3 individuals). In each Petri dish, 30 similar-sized nymphs of P. repens were offered to the 5–7 day-old predators. The number of prey consumed after 24 h was recorded. Each treatment was replicated 8 times. The experiment was conducted at the same environmental conditions as described above.
Data analysis
Functional response
The functional responses of A. minki were analyzed in two steps, model selection and parameters estimation (Juliano 2001). The type of functional response was described by determining how well the data fitted to a type I, II, or III functional responses. A polynomial logistic regression of the proportion of prey consumed (Na/N0) was used as follows (Eq. 1):
where Na is the number of prey consumed, N0 is the initial prey density, and the parameters P0, P1, P2, and P3 are the constant, linear, quadratic, and cubic parameters related to the slope of the curve. The types of functional response were determined by examining the signs of P1 and P2. If P1 was positive and P2 was negative, a type III functional response was evident. However, if P1 was negative the functional response type was a type II (Juliano 2001). The type of the functional response was selected using FRAIR (Functional Response Analysis in R, version 0.5.100) package in the R statistical environment. The FRAIR test function of this package was used for selection of response type based on sign and significance of terms in Eq. 1 (Pritchard et al. 2017a; Pritchard et al. 2017b).
Because the experiments allowed prey depletion, the random predator equation (Eq.: 2) (Rogers 1972) for Type II and the integral Hassell equation (Eq.: 3) (Hassell 1978) for type III functional response were used to estimate the handling time (Th) and searching efficiency or attack rate (a) as follows:
Where Na is the number of prey eaten by the predator, N0 is the initial prey density, a is attack rate, T is the time that predator and prey are exposed to each other (24 h) and Th is handling time.
Where T is the total time available for search (24 h), Th is the handling time. The parameters b, c and d are constants from the function that relates the a and N0 in type III functional response (Eq.: 4) (Hassell 1978):
Parameters of Type II (Eq. 2) and III (Eq. 3) functional responses were estimated using the FRAIR response function provided in FRAIR package version 0.5.100 (Pritchard et al. 2017a; Pritchard et al. 2017b).
The parameters of functional response (a and Th) were calculated by fitting data to eq. 2 for type II and to eq. 3 for type III functional responses. Differences between treatments for the estimated predator handling time and searching rate were considered as significant when their 95% confidence intervals did not overlap.
Mutual interference
The following equation was used to calculate the per capita searching efficiency (a) of the predator at different predator densities (Nicholson 1933) (Eq. 5):
Where Nt is the total number of prey available (=30), Na is the total number of prey eaten, P is the number of predators, and T is the duration of the experiment (set to 1.0 for one day). Searching efficiency was plotted against predator density on a logarithmic scale. The points were fitted to a linear regression by least squares analysis according to the inductive model proposed by Hassell and Varley (1969) (Eq. 6).
Where a is the searching efficiency of the predator, Q is the quest constant, and m is the mutual interference constant. In this model, m includes only the component of interference due to behavioral interactions between predators, not pseudo interference resulting from patch exploitation (Free et al. 1977). A negative value for the regression slope would indicate an inverse relationship between predator density and per capita searching efficiency (a).
Results
Functional response
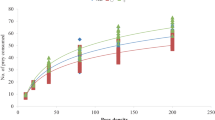
The estimated linear coefficients (P1) of the logistic regressions were negative in all the experiments except in experiments ‘prey instar’ and ‘developmental stage of predator’ in which the linear coefficient (P1) and quadratic coefficient (P2) were positive and negative, respectively (Table 1). These results indicate that A. minki exhibited a type II functional response to density of P. repense at all temperatures, to 4th instar nymphs of the prey, for both females and males of the predator, for nymphs of predator, and type III functional responses in cases of the adult predator to 2nd instar nymphs of prey in experiments ‘prey instar’ and ‘developmental stage of predator’ (Fig. 1). No natural prey mortality was observed in the control treatment.
The shortest handling time (Th) estimated in experiment ‘temperature’ was 1.4848 h whereas the greatest searching rate (a) was 0.0401 h−1. Both were obtained at the 30 °C treatment. Estimated handling times of A. minki at all temperatures (15, 24 and 30°c) were significantly different whereas differences on searching rates were only observed for 15 and 30 °C treatments. In experiment ‘prey instar’, A. minki had a shorter handling time (Th = 1.7684 h) when preying on 2nd instar nymphs of P. repens but differences were not significant. The attack rate of the predator to 4th instar of nymphs of prey (a = 0.0274 h−1) was significantly greater than that to 2nd instar nymphs (a = 0.0032 h−1) (Table 2). In experiment ‘predator sex’ both handling times and attack rates of female and male predators were not significantly different. In experiment ‘developmental stage of the predator’, 4th instar nymphs of the predator had a slightly shorter handling time (Th = 1.601 h) than adult predators (Th = 1.768 h). Their attack rate (a = 0.0269 h−1) was nevertheless greater than that of adult predators (a = 0.0032 h−1). Searching rates of nymph and adult predators were significantly different but no differences on handling times were observed (Table 2).
Mutual interference
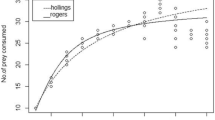
Per capita predation (prey mortality) was significantly higher with one predator (7 ± 0.378a) than with two (4.75 ± 0.575b) and three predators (3.46 ± 0.188b) (F2, 21 = 18.96, P < 0.0001). Also, per capita searching efficiency was significantly higher with one predator (0.267 ± 0.0168a) than with three (0.142 ± 0.0097b) (F2, 21 = 7.97, P < 0.0027). When two predators were released (0.197 ± 0.033a, b), per capita searching efficiency was similar to one and three predators.
The linear regression between the logarithm of per capita searching efficiency and logarithm of predator density in the mutual interference analysis resulted in the equation: Log a = −0.564–0.575 Log P (F1, 1 = 95.20, P = 0.0650). The estimated interference coefficient (slope of the regression line) was m = −0.575 ± (0.059) indicating an inverse relationship between predator density and per capita searching efficiency (Fig. 2).
Discussion
Functional response
In this study the predatory bug, A. minki showed two types of functional responses to density of its prey depending on experimental conditions. This predator exhibited a type II functional response to density of P. repense at all temperatures, to 4th instar nymphs of the prey, for both females and males of the predator, for nymphs of predator, and type III functional responses in cases of the adult predator to 2nd instar nymphs of prey.
Whereas a type II functional response has been reported by several authors for some species of different genus of anthocorids including Orius Wolff, Xylocoris Dufour and Anthocoris Fallen to their prey (McCaffrey and Horsburgh 1986; Singh and Arbogast 2008; Rahman et al. 2009; Zamani et al. 2009; Hemerik and Yano 2010; Gholami Moghaddam et al. 2012; Emami et al. 2014), a type III response was inhibited by O. albidipennis to T. urticae on both cucumber and strawberry substrates (Jalalizand et al. 2011). A type III response was originally considered characteristic of vertebrate predators (Holling 1959). However, a number of invertebrate predators and parasitoids, if presented with cryptic, relatively small and immobile prey, show a type III response (Hassell 1978). The three postulated mechanisms for type III functional responses in predators are as follows: (1) the concentration of a predator’s hunting efforts in a high-density patch (Hertlein and Thorarinsson 1987), (2) switching in a multiple prey system (Murdoch 1969), and (3) learning (Holling 1965; Murdoch and Oaten 1975). It seems that a type III response for A. minki in this study could be induced by smaller size of 2nd instar nymphs of prey as mentioned by Hassell (1978).
In this study, the handling time decreased and attack rate increased with increasing temperature indicating that the predator was more effective at higher temperatures under our conditions (24 h searching). Similarly, Ding-Xu et al. (2007) showed that temperature had significant effects on the predation and functional response of the predatory thrips, Scolothrips takahashii Priesner (Thys.: Thripidae) against the hawthorn spider mite, Tetranychus viennensis Zacher (Acari: Tetranychidae). Such strong influence of temperature on parameters of the functional response which can lead to important changes in predator–prey interactions, population dynamics, and food-web connectance has been reported by other researchers (Vasseur and McCann 2005; Englund et al. 2011; Vucic-Pestic et al. 2011; Sentis et al. 2012).
Females of A. minki were more effective in feeding on smaller than on larger nymphs. In line with the present study, Evans (1976a) showed that in A. nemorum Linnaeus (Hem.: Anthocoridae), the larger the individual predator was in relation to its prey, Acyrthosiphon pisum (Harris) (Hom.: Aphididae), the greater was the searching efficiency. However, Rotheray (1983) found that in cases of two syrphid species namely Melanostoma scalare (Fabricius) and Syrphus ribesii (Linnaeus) (Dip.: Syrphidae), small larvae did best on small aphids and large larvae did best on large aphids. As an explanation, large aphids were not so readily captured by small predators because these aphids were able to defend themselves.
The results of the present study showed that the value of the functional response parameters could be affected by the developmental stage of the predator. The handling time for adult predators was similar to that of nymph predators but the attack rate of the nymph predators was greater than that of adults.
The present study showed that the attack rates of both female and male of A. minki were almost equal, indicating that they had similar feeding behaviors. In contrast to our results, the handling time of Cosmoclopius nigroannolatus Stal. (Hem.: Reduviidae) on Spartocera dentiventris (Berg) (Hem.: Coreidae) was higher in males than females (Rocha and Redaelli 2004).
The per capita predation and per capita searching efficiency of A. minki decreased significantly with increasing predator density. The same effect has been documented for some coccinellid predators in relation to their prey (Abd El-Fattah et al. 1987; Abd El-Kareim 1998; Bayoumy and Michaud 2012). Abd El-Fattah et al. (1987) reported that intraspecific competition among Coccinella undecimpunctata individuals reduced their searching rate. Increasing density of two predators, C. undecimpunctata and Hippodamia tredecimpunctata fed Aphis gossypii and Aphis punicae, caused a reduction in foraging efficiency of the predators (Al-Deghairi et al. 2014). The per capita parasitism and per capita searching efficiency of Diaeretiella rapae (M’Intosh) (Hym.: Braconidae) to Brevicoryne brassicae (Linnaeus) (Hom.: Aphididae) decreased significantly with increasing parasitoid density. According to Hassell and Varley (1969), there was an adverse influence of predator density on searching efficiency in the same experimental arena. As the density of conspecifics increases, each individual predator spends less time looking for prey and more time interacting with other conspecifics (i.e., mutual interference) (Hassell 1971). Commonly when one predator meets another they spend time interacting and may disperse from the site of the interaction rather than staying to feed. For instance, adult anthocorids may emit an alarm pheromone on contact with each other and this may cause them to redistribute among their prey (Evans 1976b).
In conclusion, this is the first study in evaluating the foraging behavior of A. minki on P. repens. These laboratory results suggest that A. minki has the potential of depression the prey populations and can be considered as augmentative biocontrol agent in green space ecosystems. Exhibiting a type III functional response by the adult predator on the second nymphal stage of the prey implies that the application of adult predators when the density of the second nymphal stage of the prey is higher, especially at high temperatures, can improve the results of biological control. However, further studies should be carried out in field.
References
Abd El-Fattah, M. I., El-Nabawi, A., & Hendi, A. (1987). Effect of intra and inter-specific larval competition on the development of certain aphidophagous predators (Coleoptera: Coccinellidae). Bulletin of the Entomological Society of Egypt, 65, 73–79.
Abd El-Kareim, A. I. (1998). Searching rate and potential of some natural enemies as bioagents against the cotton whitefly, Bemisia tabaci Genn. (Hom., Aleyrodidae). Journal of Applied Entomology, 122, 487–492.
Al-Deghairi, M. A., Abdel-Baky, N. F., Fouly, A. H., & Ghanim, N. M. (2014). Foraging behavior of two Coccinellid species (Coleoptera: Coccinellidae) fed on aphids. Journal of Agricultural and Urban Entomology, 30, 12–24.
Bayoumy, M. H., & Michaud, J. P. (2012). Parasitism interacts with mutual interference to limit foraging efficiency in larvae of Nephus includens (Coleoptera: Coccinellidae). Biological Control, 62, 120–126.
Bernstein, C. (2000). Host-parasitoid models: The story of successful failure. In M. Hochberg & A. Ives (Eds.), Population biology of host-parasitoid interactions (pp. 41–57). Princeton: Princeton University Press.
Berryman, A. A. (1999). The theoretical foundations of biological control. In B. A. Hawkins & H. V. Cornell (Eds.), Theoretical approaches to biological control (pp. 3–21). Cambridge: Cambridge University Press.
Butler, C. D., & O’Neil, R. J. (2008). Voracity and prey preference of insidious flower bug (Hemiptera: Anthocoridae) for immature stages of soybean aphid (Hemiptera: Aphididae) and soybean thrips (Thysanoptera: Thripidae). Environmental Entomology, 37, 964–972.
Coll, M., & Ridgway, R. L. (1995). Functional and numerical response of Orius insidiosus (Heteroptera: Anthocoridae) to its prey in different vegetable crops. Annals of the Entomological Society of America, 88, 732–738.
Delong, J. P., & Vasseur, D. A. (2011). Mutual interference is common and mostly intermediate in magnitude. BMC Ecology, 11, 1–8.
Ding-Xu, L., Juan, T., & Zuo-Rui, S. (2007). Functional response of the predator Scolothrips takahashii to hawthorn spider mite, Tetranychus viennensis: Effect of age and temperature. BioControl, 52(1), 41–61.
Emami, M. S., Shishehbor, P., & Karimzadeh-Esfahani, J. (2014). Functional response of Anthocoris nemoralis (Hemiptera: Anthocoridae) to the pear psylla, Cacopsylla pyricola (Hemiptera: Psyllidae): Effect of pear varieties. Journal of Crop Protection, 3(Suppl., 597–609.
Englund, G., Ohlund, G., Hein, C. L., & Diehl, S. (2011). Temperature dependence of the functional response. Ecology Letters, 14, 914–921.
Evans, A. F. (1976a). The role of predator size ration in determining the efficiency of capture by Anthocoris nemorum and the escape reactions of the prey, Acyrthosiphum pisum. Ecological Entomology, 1, 85–90.
Evans, A. F. (1976b). Mutual interference between predatory anthocorids. Ecological Entomology, 1, 283–286.
Fathipoour, Y., Hosseini, A., Talebi, A. A., & Moharramipour, S. (2006). Functional response and mutual interference of Diaeretiella rapae (Hymenoptera: Aphidiidae) on Brevicoryne brassicae (Homoptera: Aphididae). Entomologica Fennica, 17, 90–97.
Fathipour, Y., Kamali, K., Khalghani, J., & Abdollahi, G. (2001). Functional response of Trissolcus grandis (Hym., Scelionidae) to different egg densities of Eurygaster integriceps (Het., Scutelleridae) and effects of wheat genotypes on it. Applied Entomology and Phytopathology, 68, 123–136.
Free, C. A., Beddington, J. R., & Lawton, J. H. (1977). On the inadequacy of simple models of mutual interference for parasitism and predation. Journal of Animal Ecology, 46, 543–554.
Gholami Moghaddam, S., Hosseini, M., Modarres Awal, M., & Allahyari, H. (2012). Effect of leaf surface characteristics of wheat cultivars on functional response of Orius albidipennis (Reuter) to barely aphid Sipha maydis (Passerini). Biological Control of Pest and Plant Diseases, 2, 73–85.
Gitonga, L. M., Overholt, W. A., Lohr, B., Magambo, J. K., & Mueke, J. M. (2002). Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biological Control, 24, 1–6.
Hassell, M. P. (1971). Mutual interference between insect parasites. Journal of Animal Ecology, 40, 473–486.
Hassell, M. P. (1978). The dynamics of arthropod predator-prey systems. Monographs in population biology. Princeton: Princeton University Press.
Hassell, M. P., & Varley, G. C. (1969). New inductive population model for insect parasites and its bearing on biological control. Nature, 223, 1133–1137.
Hassell, M. P., Lawton, J. H., & Beddington, J. R. (1977). Sigmoid functional response by invertebrate predators and parasitoids. Journal of Animal Ecology, 46, 249–262.
Hemerik, L. & Yano, E. (2010). A simulation model for the functional response of Orius sauteri on eggplant leaves with Thrips palmi: Implications for biological control. Proceeding of the Netherlands entomological society meeting, 21, 61–74.
Hertlein, M. B., & Thorarinsson, K. (1987). Variable patch times and the functional response of Leptopilina boulardi (Hymenoptera: Eucoilidae). Environmental Entomology, 16(3), 593–598.
Holling, C. S. (1959). Some characteristics of simple types of predation and parasitism. Canadian Entomology, 7, 385–399.
Holling, C. S. (1961). Principles of insect predation. Annual Review of Entomology, 6, 163–182.
Holling, C. S. (1965). The functional response of predators to prey density and its role in mimicry and population regulation. Memoirs of the Entomological Society of Canada, 97(45), 1–60.
Huffaker, C. B., & Messenger, P. S. (1976). Theory and practical of biological control. New York, USA: Academic.
Jalalizand, A., Modaresi, M., Tabeidian, S. A. & Karimy, A. (2011). Functional response of Orius niger niger to Tetranychus urticae (Acari: Tetranychidae): Effect of host plant morphological feature. International Conference on Food Engineering and Biotechnology IPCBEE. 9. IACSIT Press, Singapoore, 92–96.
Juliano, S. A. (2001). In S. M. Scheiner & J. Gurevitch (Eds.), Design and Analysis of Ecological Experiments Nonlinear curve fitting: Predation and functional response curve (pp. 178–216). New York: Oxford University Press.
Khandehroo, F. (2015). Survey on species diversity of arthropods associated with ash trees (Fraxinus excelsior L.) in Mashhad and vicinity (Master's thesis). Ferdowsi University of Mashhad, Mashhad, Iran.
Loginova, M. M. (1963). Psyllids of the genus Psyllopsis Loew. Acta Entomologica Musei Nationalis Paragae, 35, 183–196.
Loginova, M. M. (1968). New data on the fauna and biology of the Caucasian Psylloidea. Trudy Vsesoyuznogo Entomologicheskogo Obshchestva, Moskva-Leningrad, 52, 275–328.
McCaffrey, J. H., & Horsburgh, R. L. (1986). Functional response of Orius insidiosus (Hemiptera: Anthocoridae) to the European red mite, Panonychus ulmi (Acari: Tetranychidae), at different constant temperatures. Environmental Entomology, 15, 532–535.
Mercado, V. E. T., Maza, M. E. Z., & Pantoja, A. M. S. (2017). Functional response of Cydnodromus picanus (Acari: Phytoseiidae) on two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Acta Agron, 6(2), 275–281.
Messina, F. J., & Hanks, J. B. (1998). Host plant alters the shape of functional response of an aphid predator (Coleoptera: Coccinellidae). Environmental Entomology, 27, 1196–1202.
Murdoch, W. W. (1969). Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecological Monographs, 39(4), 335–364.
Murdoch, W. W., & Oaten, A. (1975). Predation and population stability. Advances in Ecological Research C., 9, 1–131.
Nicholson, A. J. (1933). The balance of animal populations. Journal of Animal Ecology, 2, 132–178.
Pakyari, H., Kasirloo, F., & Arbab, A. (2016). Effect of sublethal doses of Abamectin and fenpropathrin on functional response of Cryptolaemus Montrouzieri (Coleoptera: Coccinellidae) predator of Planococcus citri (Hemiptera: Pseudococcidae). Journal of Entomology and Zoology Studies, 4(1), 469–473.
Pervez, A., & Omkar, L. (2005). Functional responses of coccinellid predators: An illustration of a logistic approach. Journal of Insect Science, 5, 1–6.
Petchey, O. L., Beckerman, A. P., Riede, J. O., & Warren, P. H. (2008). Size, foraging, and food web structure. Proceedings of the National Academy of Sciences USA, 105, 4191–4196.
Petchey, O. L., Brose, U., & Rall, B. C. (2010). Predicting the effects of temperature on food web connectance. Philosophical Transactions of the Royal Society B, 365, 2081–2091.
Pritchard, D. W., Barrios-O'Neill, D., Bovy, H. & Paterson, R. (2017a). frair-package: Tools for Functional Response Analysis in R. URL: https://github.com/dpritchard/ frair.
Pritchard, D. W., Paterson, R. A., Bovy, H. C., & Barrios-O’Neill, D. (2017b). Frair: An R package for fitting and comparing consumer functional responses. Methods in Ecology and Evolution, 8, 1528–1534.
Rahman, M. M., Islam, W., & Ahmed, K. N. (2009). Functional response of the predator Xylocoris flavipes to three stored product insect pests. International Journal of Agriculture and Biology, 11, 316–320.
Rocha, L., & Redaelli, L. R. (2004). Functional response of Cosmoclopius nigroannolatus (hem.: Reduviidae) to different densities of Spartocera dentiventris (hem.: Coreidae). Brazilian Journal of Biology, 64(2), 309–316.
Rogers, D. J. (1972). Random search and insect population models. Journal of Animal Ecology, 41, 369–383.
Rotheray, G. E. (1983). Feeding behavior of Syrphus ribesii and Melanostoma scalare on Aphis fabae. Entomologia Experimentalis et Applicata, 34, 148–154.
Rotheray, G. E. (1989). Aphid predators: Cambridge Naturalists' Handbooks 7. Cambridge: Cambridge University Press.
Sarmento, R. A., Pallini, A., Venzon, M., De Souza, O. F. F., Molina-Rugama, A. J., & De Oliveira, C. L. (2007). Functional response of the predator Eriopis connexa (Coleoptera: Coccinellidae) to different prey types. Brazilian Archives of Biology and Technology, 50(1), 121–126.
Sentis, A., Hemptinne, J. L., & Brodeur, J. (2012). Using functional response modeling to investigate the effect of temperature on predator feeding rate and energetic efficiency. Oecologia, 169, 1117–1125.
Singh, S. E., & Arbogast, R. T. (2008). Predatory response of Xylocoris flavipesto bruchid pests of stored food legumes. Entomologia Experimentalis et Applicata, 126, 107–114.
Timms, J. E., Oliver, T. H., Straw, N. A., & Leather, S. R. (2008). The effects of host plant on the coccinellid functional response: Is the conifer specialist Aphidecta obliterata (L.) (Coleoptera: Coccinellidae) better adapted to spruce than the generalist Adalia bipunctata (L.) (Coleoptera: Coccinellidae)? Biological Control, 47, 273–281.
Vasseur, D. A., & McCann, K. S. (2005). A mechanistic approach for modeling temperature-dependent consumer resource dynamics. American Naturalist, 166, 184–198.
Vucic-Pestic, O., Ehnes, R. B., Rall, B. C., & Brose, U. (2011). Warming up the system: Higher predator feeding rates but lower energetic efficiencies. Global Change Biology, 17, 1301–1310.
Zamani, A. A., Vafaei, S., Vafaei, R., Goldasteh, S., & Kheradmand, K. (2009). Effect of host plant on the functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Tetranychus urticae (Acari: Tetranychidae). IOBC/WPRS Bulletin, 50, 125–129.
Acknowledgments
This study is a part of PhD thesis of the first author which was carried out at Ferdowsi University of Mashhad. The authors are grateful to Professor Mehdi Nassiri Mahallati at Ferdowsi university of Mashhad, for helping with R software and to Dr. Horton at United States Department of Agriculture, for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanzadeh-Avval, M., Sadeghi-Namaghi, H. & Fekrat, L. Factors influencing functional response, handling time and searching efficiency of Anthocoris minki Dohrn (Hem.: Anthocoridae) as predator of Psyllopsis repens Loginova (Hem.: Psyllidae). Phytoparasitica 47, 341–350 (2019). https://doi.org/10.1007/s12600-019-00739-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-019-00739-w