Abstract
Conyza bonariensis is an invasive weed of increasing importance in subtropical and warm-temperate regions worldwide, both in non-agricultural habitats and in annual and perennial crops, especially under no-till management. To gain insigths on basic life cycle processes determining the demographic success of C. bonariensis, we studied for this species during two seasons seedling emergence patterns, survival to the adult stage and fecundity in a ruderal Mediterranean habitat in which C. bonariensis was a component of the plant community. The influence of emergence date on survival and fecundity was studied using four successive sowing dates, i.e. cohorts, encompassing the favorable season for plant establishment. The mean rate of seedling emergence was 61%. Emergence patterns were characterized by high initial emergence rates, which were highly dependent on rainfall. The mean rate of survival to the adult stage was 33%. Fecundity reached a mean value of 86,066 achenes and presented density-dependent regulation.. Plant survival and fecundity were positively related to cohort earliness and thus earlier cohorts should preferably be targeted for an effective management of C. bonariensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conyza bonariensis (L.) Cronquist is an annual Asteraceae native to South America and currently distributed in tropical and warm temperate areas worldwide (Karlsson and Milberg 2007; Prieur-Richard et al. 2000). The invasive behavior of Conyza species is contributed by a very high plant fecundity and ability for long-distance seed dispersal through wind (Dauer et al. 2007). Long-distance dispersal of seeds and pollen has been suggested to explain the low levels of geographic structure of the high genetic variation found in Conyza, i.e. most of this genetic variation resides within-populations (Ming-Xun et al. 2010). In addition, Conyza seeds germinate easily under favorable conditions (Nandula et al. 2006; Zambrano-Navea et al. 2013). These traits identify C. bonariensis as a colonizer species of transient, highly disturbed, habitats. As such, it mainly behaves as a ruderal plant inhabiting road verges, field boundaries and open ground. In addition, it is considered as a weed in at least 40 crops in 70 countries (Holm et al. 1997), and it has become one of the most difficult weeds to control in different agricultural systems, particularly under soil conservation management (Wu et al. 2010; Zambrano-Navea et al. 2013). Substantial yield reduction has been reported in different crops (e.g. Trezzi et al. 2015). The adoption of measures to keep its populations in check is essential to prevent serious crop losses. Population control is frequently based on herbicide treatments, but this tactic is becoming jeopardized due to the appearance of resistance to different active ingredients, including glyphosate (Urbano et al. 2007).
It is widely accepted that knowledge of weed biology is essential for setting up plans for the integrated management of weed populations (Cousens and Mortimer 1995). While there is considerable information on the biology of the congeneric Conyza canadensis, little is known on demographic aspects of C. bonariensis (Shrestha et al. 2008) and most of the information available is mainly limited to laboratory studies. For instance, Wu et al. (2007) reported that seedling emergence of C. bonariensis occurs only from shallow depth and, under the seasonal climate of the study locality, from autumn to earlywinter, with only a small fraction emerging in spring. Karlsson and Milberg (2007) and Vivian et al. (2008) estimated base, optimum and maximum temperatures for germination at 4.2, 20 and 35 °C, respectively. Yamashita and Guimarães (2010) found that the percentage and rate of germination of C. bonariensis seeds were reduced when soil water potential decreased below −0.2 MPa.
This work was aimed to gain insights on the demographic behavior of C. bonariensis in ruderal, Mediterranean habitats. Identification of particularly vulnerable life history processes may contribute to design more rational control strategies of populations of this weed species.
Materials and methods
Study site
The study was conducted in an experimental field at the Instituto de Investigación y Formación Agraria y Pesquera (IFAPA), Cordoba, southern Spain (37° 51′ 40” N, 4° 47′ 56”W, 117 m asl). The climate is Mediterranean with a mean annual rainfall of approximately 600 mm, mainly distributed from autumn to mid spring. The mean maximum and minimum annual temperature is 27 °C and 10 °C, respectively. The soil is loamy with a pH of 8.5 and an organic matter content of 1.1%.
Experimental design
Three field experiments were carried out between October and August during two consecutive seasons (2010–2011 and 2011–2012) aimed to estimate the demographic rates governing stage transitions in annual plants, including emergence rate, survival rate to the adult stage and fecundity.
To determine the emergence rate and the temporal pattern of seedling emergence, at the onset of the rainy season, in October, six microplots of 0.3 × 0.3 m were each superficially sown with 200 achenes (hereafter seeds) randomly selected from a single batch collected each year from at least 30 individuals of the local C. bonariensis population. For this purpose, the soil was previously ploughed and, subsequently, we added a fine layer of inert substrate (peat) with which the seeds were lightly mixed to prevent them from being blown away. Counts of emerged seedlings were made every three days until end of emergence. After each count, seedlings were removed.
A second experiment was performed with the aim of assessing the influence of emergence timing on survival to the adult stage and on fecundity of surviving adults. In each study year four different cohorts were established by sowing at four dates evenly spaced throughout the rainy season, 20 October, 13 December, 7 February and 4 April. At the beginning of each study season, 16 plots of 1 × 1 m were established and four plots were randomly assigned to each cohort. The soil of each plot was ploughed prior to sowing. At sowing, a large number of C. bonariensis seeds were mixed with peat to form a 1 cm depth layer at the soil surface. If necessary because of lack of rain, watering was done during 3–4 days after sowing to promote seed germination. Once emerged, 30 seedlings were randomly selected and marked in each plot, and any other plant was periodically suppressed at emergence, thus maintaining low plant density (Shrestha et al. 2010). Survival censuses were made weekly until the onset of seed production. In addition, at the onset of flowering three plants were randomly selected in each plot. At fruiting, one capitulum was randomly obtained from each plant at a stage immediately before the beginning of seed dispersal, and the seed content was counted. The total number of capitula produced by each plant was counted at end cycle. Fecundity of surviving adults was estimated as the product of the number of capitula produced per plant by the mean seed content per capitulum.
A survival analysis based on the Log Rank test was carried out using SIGMAPLOT v.11 to test for cohort effects on survival to the adult stage. Multiple pairwise comparisons among cohorts were based on the Holm-Sidak test. The effect of cohort on fecundity was analyzed by the non-parametric Kruskall-Wallis test and the pairwise comparison test using STATISTIX v.9.
To quantify the effect of intraspecific density on fecundity seven sites forming a gradient of plant densities were identified in May 2012, at the onset of seed production, within the local natural population of C. bonariensis. Density values were 1, 55, 122, 277, 555, 777 and 1111 plants m−2. For each site, three plants were randomly selected and the number of capitula produced per plant was counted at end cycle. In addition, the number of seeds per capitulum was counted in a random sample of 10 capitula per site. Mean plant fecundity in each site was estimated as above.
The functional relationship between intraspecific density and fecundity was established through a hyperbolic model
where Y is the number of seeds produced per plant, X is the density (plants m-2) of C. bonariensis, fo represents the number of seeds produced per plant when the population density approaches zero and a is a parameter measuring the species-specific susceptibility of fecundity to plant density. To fit the model, the non-linear regression module of SIGMAPLOT v. 11 was used. Goodness of fit was assessed using the root mean square error (RMSE) and pseudoR2. Lower RMSE values and pseudoR2 values closer to 1 indicate better model fit.
Results
Emergence rate and pattern
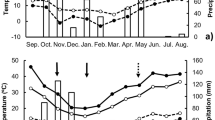
The average emergence rate for the two study seasons was 0.61 ± 0.16. Figure 1 depicts the emergence patterns of C. bonariensis in the two years of the experiment. In 2010 (Fig. 1a) there was a rapid emergence at the beginning of the experiment, with 90% emergence reached 21 days after sowing (das). In contrast, in autumn 2011, there was a slow initial emergence (5% at 7 das), which 50% and 90% emergence reached at 20 and 35 das, respectively (Fig. 1b). The rainfall in autumn 2010 was more abundant and more regularly distributed than in the autumn of 2011.
Survival rate to adult stage
The mean survival rate of C. bonariensis seedlings to the adult stage over the two studied seasons was 0.30 ± 0.20.
In the 2010–2011 season, the first three cohorts exhibited similar survival rates, with values ranging between 0.34 and 0.44. In contrast, the latest cohort showed a substantially lower survival rate (0.13) (Table 1). The log-rank analysis confirmed a significant effect of cohort on survival (χ2 = 10.157; gl = 3; p = 0.017; Fig. 2a), with a significantly lower survival rate of the latest cohort compared to the earliest cohort (Holm-Sidak test, p < 0.01; Table 2).
In the 2011–2012 season, the three latest cohorts showed similar survival rates, with values ranging between 0.26 and 0.29, approximately half the rate of the earliest cohort (0.53, Table 1). The log-rank analysis indicated a significant effect of cohort on survival (χ2 = 25.261; gl = 3; p < 0.001; Fig. 2b), and the survival curve of the earliest cohort was statistically different (Holm-Sidak test. p < 0.01) from curves of the three later cohorts, which showed non-significant differential behavior (Table 2).
Fecundity
The number of capitula produced per plant tended to decrease from the first to the third cohort (Fig. 3a). Plants of the latest cohort did not reach the flowering stage, eventually dying in vegetative phase, and thus this cohort was not included in further analyses. These differences in fecundity among the first three cohorts were statistically significant both in 2010–2011 (Kruskal-Wallis test, χ2 = 21.64; gl = 29; p < 0.01) and 2011–2012 seasons (χ2 = 23.24; gl = 35; p < 0.01). Individual plants of the first, second and third cohort produced 201 ± 107, 83 ± 24 and 30 ± 9 capitula, respectively, during the first study year, and 184 ± 68, 58 ± 31 and 38 ± 21, respectively, during the second year. In both study years, a significant difference between the first and third cohort was found (Fig. 3a).
Number of capitula per plant (a) and achenes/capitulum (b) of Conyza bonariensis for each cohort (October, December and February) in the seasons of 2010–2011 (dark colour) amd 2011–2012 (pale colour). Bars followed by the same letter in the same season did not present any significant differences (p < 0.05) in agreement with the pairwise comparisons test. The vertical lines indicate the standard error
The mean seed content per capitulum slightly decreased with the lateness of the cohort, both in the first study year (272 ± 30, 257 ± 41 and 241 ± 38 for the first, second and third cohort, respectively) and in the second year (273 ± 43, 247 ± 50 and 249 ± 47). Nevertheless, these differences among cohorts were non-significant (Kruskal-Wallis test: 2010–2011, χ2 = 1.52; gl = 29; p = 0.46; 2011–2012, χ2 = 2.41; gl = 35; p = 0.29) (Fig. 3b).
Fecundity of C. bonariensis plants, estimated as the product of the number of capitula produced by individual plants by the mean seed content per capitulum, was significantly dependent on cohort (Kruskal-Wallis test, χ2 = 22.57; gl = 29 p < 0.01) and in 2011–2012 (χ2 = 22.99; gl = 35; p < 0.01), appearing positively related to its earliness (Fig. 4). In the first study year, individual plants of the first, second and third cohort produced 70,288 ± 29,651, 18,206 ± 8747 and 8218 ± 3794 seeds, respectively, whereas in the second year they produced 51,066 ± 23,893, 14,930 ± 9305 and 9424 ± 5380 seeds (Fig. 4). In both seasons, differences in fecundity between the first and third cohort were significant (Fig. 4).
Mean fecundity of the different cohorts (October, December and February) of Conyza bonariensis in the 2010–2011 (dark colour) and 2011–2012 seasons (pale colour). Bars followed by the same letter in the same season do not present any statistically significant differences (p < 0.05) in agreement with the pairwise comparisons test. The vertical lines indicate the standard error
Effect of density on fecundity
Density-dependence of fecundity was adequately explained by the hyperbolic model (Eq. 1: pseudoR2 = 0.99, RMSE = 2809.7, gl = 6) (Fig. 5). The values estimated for model parameters were fo = 87,274 achenes/plant (SE = 3300) and a = 0.06 (SE = 0.01).
Relationship between fecundity and density of a Conyza bonariensis population. The line corresponds to the fit of the hyperbolic model (Eq. 1). The points correspond to the values observed
Discussion
With the aim of understanding and predicting the population dynamics of weeds, we have to be able to relate changes in population size with a series of demographic variables, such as emergence, mortality and fecundity. In the studied ruderal population of C. bonariensis the average recruitment of seedlings was 61%, with individual values ranging from 35% to 87%. A similar average emergence rate of 64% has been reported for this species in Australia (Green 2010).
Final rates and temporal patterns of seedling emergence are dependent on seed distribution in the soil profile (Gramshaw and Stern 1977). In our emergence experiments, the seeds were shallowly buried in the soil (<1 cm), as occurs with the natural seed bank of this species (Wu et al. 2007). This situation promotes a complete and rapid seedling appearance, giving rise to high emergence rates under favorable environmental conditions, as has been the case.
The emergence pattern of C. bonariensis was characterized by high initial emergence fluxes, which appear to be highly dependent on the amount and distribution of rainfall (Fig. 1). The effects of seasonal distribution of rainfall on seedling emergence patterns have been reported in other weed species (Mulugeta and Stoltenberg 1997; Puricelli et al. 2002; Izquierdo et al. 2009). Seed germination of C. bonariensis is particularly sensitive to scant moisture conditions, and, as no dormancy is present, favorable environmental conditions such as rainfall or irrigation significantly promote emergence (Yamashita and Guimarães 2010; Nandula et al. 2006; Wu and Walker 2004).
Seed production in Mediterranean populations of C. bonariensis is highly protracted from early summer up to late autumn. As a consequence, the full crop of ready-to-germinate, non-dormant seeds gives rise to continuous seedling cohorts potentially experiencing contrasting environmental conditions (Mokhtassi-Bidgol et al. 2013). To take into account the demographic consequences of this behavior, we monitored different sequentially established cohorts. In seasonal climates, the influence of emergence time on seedling survival rates has been consistently documented in different species (Fernandez-Quintanilla et al. 1986; Verdú and Mas 2006; Borger et al. 2009). These studies have generally shown that early emergence is associated with higher survival rates. Our results for a ruderal population of C. bonariensis support these findings. Across study years, mean survival rate of the earliest cohort was 0.45 and this value progressively decreased down to 0.21 for the latest cohort.
Differences in survival rates among cohorts were similar to those reported by Buhler and Owen (1997) and Regehr and Bazzaz (1979) for the congeneric and ecologically similar species C. canadensis. These authors reported survival rates of plants emerging in autumn of over 41%, and of 36% in those emerging in the spring.
Plants of the earliest cohort consistently showed the highest fecundity. These plants were taller and more branched compared to later emerging plants, which probably resulted in a higher reproductive capacity. Similar results have been found for other weed species in seasonal climates (Verdú and Mas 2006; Kiegel 1995; Gallart et al. 2010) and also for other populations of C. bonariensis. For instance, Green (2010) found a reduction of 29.6% in fecundity of C. bonariensis plants emerging in spring compared with plants emerging at the end of the previous autumn. Torra and Recasens (2008) reported a progressive reduction in fecundity of the weed Papaver rhoeas from the earliest to the latest cohorts. This reduction in seed production in plants of later cohorts has been related to the smaller accumulation of biomass due to a shortened period for vegetative growth (Bosnic and Swanton 1997; Knezevic and Horak 1998; Norris 1996).
Plant fecundity in the studied ruderal population ranged between 96,712 and 75,420 seeds, with an average value of 86,066 seeds, fairly similar to the 85,074 seeds/plant found by Green (2010) for this species in Australia. However, other studies have reported much higher fecundities for the study species under favorable laboratory/greenhouse conditions, with mean values of 119,100 (Wu et al. 2007) and 266,753 seeds (Kempen and Graf 1981). The abiotic stress mainly resulting from water shortage during the summer, imposed by the Mediterranean climate, may account for this relatively limited fecundity we have found in the study population. Limited fecundity can result from reduced production of capitula and/or seed content per capitulum. The maximum number of capitula produced per plant we have recorded for the study population is similar to values reported for this species by Kempen and Graf (1981) and Green (2010), who found 290 and 232 capitula per plant on average, respectively. However, 400 capitula per plant have been reported for C. bonariensis under laboratory conditions (Wu et al. 2007). Similarly, maximum values of seed content per capitulum in the study population appeared to be lower than the 366 seeds/capitulum found by Green (2010). In general, C. bonariensis has a high reproductive capacity which undoubtedly favours its expansion.
Regulation of population growth results from negative density-dependence of at least one demographic rate controlling stage transition. Our results indicate a regulatory effect of density at the level of fecundity in C. bonariensis. This type of density-dependent regulation has also been observed in different weed species including Agrostemma githago (Firbank and Watkinson 1986) and Avena sterilis (Gonzalez-Andujar and Fernandez-Quintanilla 1991, 1993). In C. canadensis, Palmblad (1968) and Bhowmik and Bekech (1993) reported reductions in fecundity of 50–68% when intraspecific density was increased from 55 to 200 plants m−2, in line with 65% reduction found in this work for a similar change in plant density. With the establishment of conservation systems in agriculture, species of the genus Conyza have become an important weed problem for different crops (Buhler 1995). Mediterranean woody crops (e.g. olive orchards and fruit tree crops) are among those most affected. Control measures are mainly based in herbicide applications with the highest effectiveness achieved at early growth stages. However, long-lasting control reliance on a few active ingredients has triggered the appearance of populations resistant to different herbicides (Urbano et al. 2007), so that it would be recommendable to adopt integrated strategies for their management (Widderick et al. 2012). The results obtained in this work will help to establish those strategies, for which a thorough knowledge of the biology and demography of the species is of major importance.
Individual plants of Conyza species produce large numbers of wind-dispersed seeds. High ability for long-distance dispersal through wind of this seed (Dauer et al. 2007) indicates that effectiveness of control measures of a within-field population of any of these species, including herbicide-resistant biotypes, can benefit from a landscape perspective, such that coordinated actions of neighboring farmers are required to limit arrival of seeds from extra-field sources (Gonzalez-Andujar et al. 2001). These include not only populations established in adjacent fields but also ruderal populations inhabiting field and road margins. As our results show, plants of C. bonariensis in Mediterranean ruderal habitats can exhibit high fecundity. Through extensive dispersal, produced seeds can reach adjacent or even distant fields. Adequate management of field margins, including increased width and reduced disturbance can promote the establishment of species-rich, high-cover vegetation types, corresponding to more advanced successional stages (Marshall 1989; Schippers and Joenje 2002), from which pioneer species, such as Conyza spp., are competitively excluded (Thébaud et a. 1996; Prieur-Richard et al. 2000).
In conclusion, in the Mediterranean ruderal habitat studied earlier cohorts of C. bonariensis contributed most to the following generation, and, therefore, they should be preferably targeted when designing control strategies.
References
Bhowmik, P. C., & Bekech, M. M. (1993). Horseweed (Conyza canadensis) seed production, emergence, and distribution in no-tillage and conventional tillage corn (Zea mays). Agronomy, 1, 67–71.
Borger, C. P., Scott, J. K., Walsh, H. M., & Powles, S. B. (2009). Demography of Salsola australis populations in the agricultural region of south-west Australia. Weed Research, 49, 391–399.
Bosnic, A. C., & Swanton, C. J. (1997). Influence of barnyardgrass (Echinochloa crusgalli) time of emergence and density on corn (Zea mays). Weed Science, 45, 276–282.
Buhler, D. D. (1995). Influence of tillage systems on weed population dynamics in corn and soybean in the central USA. Crop Science, 35, 1247–1258.
Buhler, D. D., & Owen, M. D. (1997). Emergence and survival of horseweed (Conyza canadensis). Weed Science, 45, 98–101.
Cousens, R., & Mortimer, M. (1995). Dynamics of weed populations (p. 332). New York: Cambrige University Press.
Dauer, J. T., Mortensen, D. A., & Vangessel, M. J. (2007). Temporal and spatial dynamics of long-distance Conyza canadensis seed dispersal. Journal of Applied Ecology, 44, 105–114.
Fernandez-Quintanilla, C., Navarrete, L., Gonzalez-Andujar, J. L., Fernandez, A., & Sanchez, M. J. (1986). Seedling recruitment and age-specific survivorship and reproduction in populations of Avena sterilis ssp. ludoviciana. Journal of Applied Ecology, 23, 945–955.
Firbank, L. G., & Watkinson, A. R. (1986). Modelling the population dynamics of an arable weed and effects upon crop yield. Journal of Applied Ecology, 23, 147–159.
Gallart, M., Mas, M. T., & Verdú, A. M. (2010). Demography of Digitaria sanguinalis: Effect of the emergence time on survival, reproduction, and biomass. Weed Biology and Management, 10, 132–140.
Gonzalez-Andujar, J. L., & Fernandez-Quintanilla, C. (1991). Modelling the population dynamics of Avena sterilis in winter wheat production under dry-land cereal cropping systems. Journal of Applied Ecology, 28, 16–27.
Gonzalez-Andujar, J. L., & Fernandez-Quintanilla, C. (1993). Strategies for the control of Avena sterilis in winter wheat production systems in central Spain. Crop Protection, 12, 617–623.
Gonzalez-Andujar, J. L., Plant, R. E., & Fernandez-Quintanilla, C. (2001). Modeling the effect of farmers’ control decisions on the population dynamics of winter wild oat (Avena sterilis ssp ludoviciana) in an agricultural landscape. Weed Science, 49, 414–422.
Gramshaw, D., & Stern, W. R. (1977). Survival of annual ryegrass (Lolium-rigidum-gaud) in a mediterranean type environment. 1. Effect of summer grazing by sheep on seed numbers and seed-germination in autumn. Australian Journal of Agricultural Research, 28, 81–91.
Green, T. D. (2010). The ecology of fleabane (Conyza spp.). PhD Thesis. University of New England. Australia.
Holm, L. G., Doll, J., Holm, E., Pancho, J., & Herbeger, J. (1997). World weeds: natural histories and distribution (p. 1129). Toronto: Wiley.
Izquierdo, J., Gonzalez-Andujar, J. L., Bastida, F., Lezaun, J. A., & Sanchez del Arco, M. J. (2009). A Thermal Time Model to Predict Corn Poppy (Papaver rhoeas) emergence in cereal fields. Weed Science, 57, 660–664.
Karlsson, L. M., & Milberg, P. (2007). Comparing after-ripening response and germination requirements of Conyza canadensis and Conyza bonariensis (Asteraceae) through logistic functions. Weed Research, 47, 433–441.
Kempen, H. M., & Graf, J. (1981).Weed seed production. Proc.West. Soc. Weed Sci. 17–19 March. San Diego, California (pp. 78–81).
Kiegel, J. (1995). Seed germination in arid and semiarid regions. In J. Kiegel (Ed.), Seed Dormancy and Germination (pp. 645–700). New York: Marcel Dekker, Inc..
Knezevic, S. Z., & Horak, M. J. (1998). Influence of emergence time and density on redroot pigweed (Amaranthus retroflexus). Weed Science, 46, 665–672.
Marshall, E. J. P. (1989). Distribution Patterns of Plants Associated with Arable Field Edges. Journal of Applied Ecology, 26, 247–257.
Ming-Xun, R., Xiao-Qiong, L., & Jian-Qing, D. (2010). Genetic variation and spread pattern of invasive Conyza sumatrensis around China’s Three Gorges Dam. Acta Oecologica, 36, 599–603.
Mokhtassi-Bidgol, I. A., Navarrete, L., Aghaalikhani, M., & Gonzalez-Andujar, J. L. (2013). Modelling the population dynamic and management of Bromus diandrus in cereal. Weed Research, 43, 128–133.
Mulugeta, D., & Stoltenberg, D. E. (1997). Seed bank characterization and emergence of a weed community in a moldboard plow system. Weed Science, 45, 54–60.
Nandula, V. K., Eubank, T. W., Poston, D. H., Koger, C. H., & Reddy, K. N. (2006). Factors affecting germination of horseweed (Conyza canadensis). Weed Science, 54, 898–902.
Norris, R. F. (1996). Morphological and phenological variation in barnyardgrass (Echinochloa crus-galli) in California. Weed Science, 44, 804–814.
Palmblad, I. G. (1968). Competition in experimental populations of weeds with emphasis on the regulation of population size. Ecology, 49, 26–34.
Prieur-Richard, A., Lavorel, S., Grigulis, K., & Dos Santos, A. (2000). Plant community diversity and invasibility by exotics: invasion of Mediterranean old fields of Conyza bonariensis and Conyza canadensis. Ecology Letters, 3, 412–422.
Puricelli, E., Orioli, G., & Sabbatini, M. R. (2002). Demography of Anoda cristata in wide- and narrow-row soyabean. Weed Research, 42, 456–463.
Regehr, D. L., & Bazzaz, F. A. (1979). The population dynamics of Erigeron canadensis, a successional winter annual. Journal of Ecology, 67, 923–933.
Schippers, P., & Joenje, W. (2002). Modelling the effect of fertiliser, mowing, disturbance and width on the biodiversity of plant communities of field boundaries. Agriculture, Ecosystems & Environment, 93, 351–365.
Shrestha, A., Hembree, K., & Wright, S. (2008). Biology and management of horseweed and hairy fleabane in California. University of California, ANR Publication 8314. pp. 1–9.
Shrestha, A., Hanson, B. D., Fidelibus, M. W., & Alcorta, M. (2010). Growth, phenology, and intraspecific competition between glyphosate-resistant and glyphosate-susceptible horseweeds (Conyza canadensis) in the San Joaquin Valley of California. Weed Science, 58, 147–153.
Thébaud, C., Finzi, A. C., Affre, L., Debussche, M., & Escarré, J. (1996). Assessing why two introduced Conyza differ in their ability to invade Mediterranean old fields. Ecology, 77, 791–804.
Torra, J., & Recasens, J. (2008). Demography of corn poppy (Papaver rhoeas) in relation to emergence time and crop competition. Weed Science, 56, 826–833.
Trezzi, M. M., Vidal, R. A., Patel, F., Miotto Junior, E., Debastiani, F., Balbinot Junior, A. A. & Mosquen, R. (2015). Impact of Conyza bonariensis density and establishment period on soyabean grain yield, yield components and economic threshold. Weed Res, 55, 34–41
Urbano, J. M., Borrego, A., Torres, V., Leon, J. M., Jimenez, C., Dinelli, G., & Barnes, J. (2007). Glyphosate-resistant hairy fleabane (Conyza bonariensis) in Spain. Weed Technology, 21, 396–401.
Verdú, A. M., & Mas, M. T. (2006). Cohort-dependent seedling recruitment, survival and reproductive capacity of Tribulus terrestris. Weed Research, 46, 371–378.
Vivian, R., Gomes, J. R., Chamma, H. M., Silva, A. A., Fagan, E. B., & Riuz, S. T. (2008). Efeito da luz e da temperatura na germinacao de Alternathera tenella, Conyza bonariensis e Digitaria ciliaris. Planta Daninha, 26, 507–513.
Widderick, M., Walker, S., & Cook, T. (2012). Flaxleaf fleabane (Conyza bonariensis)-strategic solutions using best management practice. Pakistan Journal of Weed Science Research, 18, 687–693.
Wu, H., & Walker, S. (2004). Fleabane biology and control. In S. R. Walker, M. Widderick, & H. Wu (Eds.), Fleabane: workshop proceedings (pp. 5–6). Toowoomba: CRC for Australian Weed Management.
Wu, H., Walker, S., Rollin, M. J., Tan, D. K., Goeuff, R., & Werth, J. (2007). Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biology and Management, 7, 192–199.
Wu, H., Walker, S., Robinson, G., & Coombers, N. (2010). Control of Flaxleaf Fleabane (Conyza bonariensis) in Wheat and Sorghum. Weed Technology, 24, 102–107.
Yamashita, O. M., & Guimarães, S. C. (2010). Germinação das sementes de Conyza canadensis e Conyza bonariensis em função da disponibilidade hídrica no substrato. Planta Daninha, 28, 309–317.
Zambrano-Navea, C., Bastida, F., & Gonzalez-Andujar, J. L. (2013). A hydrothermal seedling emergence model for Conyza bonariensis. Weed Research, 53, 213–220.
Acknowledgements
This work was supported by grant AGL2009-7883 of the FEDER (European Regional Development Funds) and of the Spanish Ministry of Economy and Competitiveness (MINECO). CZ-N acknowledges a scholar grant from CDCH-UCV (Council of Scientific and Humanistic-Central University of Venezuela).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zambrano-Navea, C., Bastida, F. & Gonzalez-Andujar, J.L. Demography of Conyza bonariensis (Asteraceae) in a ruderal Mediterranean habitat. Phytoparasitica 46, 263–272 (2018). https://doi.org/10.1007/s12600-018-0647-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-018-0647-9