Abstract
The Sclerotium tuber rot fungus (Sclerotium rolfsii Sacc.) represents a serious problem for Jerusalem artichoke (JA) tubers during storage periods. The aim of this study was to investigate an alternative preservation method using a natural essential oil to inhibit the fungal growth, increase storability, and keep nutritive value of JA tubers under storage conditions. In vitro antifungal activity was assessed using two essential oils; caraway and spearmint at concentrations of 2, 3, 4 and 5 %. Among the tested treatments, caraway oil at 2 % resulted in complete inhibition of the fungal growth. In the storage experiment, two preservation methods were applied using caraway oil. In the first method, JA tubers were treated with caraway oil at 2 %, kept in perforated polyethylene bags and stored at 4 °C and 90 % relative humidity (RH). In the second method, JA tubers were treated with caraway oil at 2 %, kept between peat moss layers and stored at room temperature (25/10 °C, day /night) and 70 % RH. Comparing with the infected-untreated control, tubers infected with S. rolfsii and treated with caraway oil which kept in peat moss exhibited lower severity of Sclerotium tuber rot, sprouting percentage and weight loss. On the other hand, this treatment led to the highest dry matter and contents of carbohydrates, protein, inulin and total phenols as well as the activity of peroxidase and polyphenol oxidase enzymes. Based on the obtained results we recommend the use of caraway oil and peat moss when storing JA tubers at room temperature due to its eco-safety and saving of the cooling energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From a biorefinery point of view, Jerusalem artichoke or sunchoke (Helianthus tuberosus L.) is a perennial plant which has a high economic value. In addition to be used traditionally as a human food and livestock fodder due to its high nutritive value, its tubers have been used for the production of biofuels and some functional food ingredients such as inulin, oligofructose and fructose. The carbohydrates in the tubers were found to have a potential for production of platform chemicals, e.g., succinic acid. Moreover, some bioactive metabolites from its leaves and stems have been used in some pharmaceutical applications (Johansson et al. 2015; Yang et al. 2015).
Jerusalem artichoke tubers are subjected to attack by several phytopathogenic fungi, causing rust, southern blight, and tuber rots diseases that can limit its yield production. Under storage conditions, approximately 20 pathogens causing storage rots have been isolated from JA tubers, which vary in their disease potentiality and economic significance (Kays and Nottingham 2008). Tuber and stem rot, caused by Sclerotium rolfsii Sacc., is one of the most important diseases of JA infecting the tubers during field and/or storage stages. It can be a serious disease causing a severe yield loss, especially under higher storage temperatures (Sennoi et al. 2010). Although fungal spoilage of tubers can start in the field, actual proliferation and substantial spoilage occur in the postharvest period when the main plant defenses are reduced or eliminated (Jin et al. 2013). Therefore, simple and economic storage technologies of JA to reduce losses are demanded.
Storage JA tuber rots can be controlled by various means including; low temperature, removal of diseased tubers and minimizing mechanical damage or application of chemical fungicides. Another applied method in the high cold climatic regions which is the over-wintering (keeping tubers in the soil). Unfortunately, many fungi can grow at low temperatures and cause substantial damage, especially when storage extended for long periods (Tesio et al. 2011). A common solution, is the use of synthetic chemical fungicides, however, their use is accompanied by threatening human health and the environment by supporting the emergence of resistant pathogens and by contamination of food with the pesticide deposits (Zhang et al. 2013).
Recently, the natural alternatives such as plant essential oils provide a promising control of plant diseases because they virtually constitute a rich source of bioactive chemicals such as phenols, flavonoids, quinones, tannins, alkaloids, saponins and sterols (Combrinck et al. 2011). Moreover, these natural alternatives can also maintain the biochemical constituents of tubers during storage, they are biodegradable to nontoxic products, and are potentially suitable for use in integrated pest management programs.
Caraway (Carum carvi L.), also known as meridian fennel, is a biennial medicinal plant in the family Apiaceae. It was used in folk medicine as a digestive, carminative and lactogenic. Different researches have been previously studied its antimicrobial, anticancer, antioxidant, antidiabetic, analgesic, gastrointestinal and many other pharmacological activities (Seidler-Lozykowska et al. 2013; Al-Snafi 2015).
Spearmint (Mentha spicata L.), also known as bush mint, is a perennial plant that grows in temperate climates. Its essential oil is used in perfume, cosmetics, air fresheners, drink flavorings, candies and medications. It has been reported to possess antibacterial, antifungal, antiviral, insecticidal and antioxidant properties (Hussain et al. 2010)
The present work was planned to investigate the most important rot causing fungal pathogens affecting JA tubers, find out an alternative preservation method for controlling tuber and stem rot disease of JA under storage conditions, with keeping the nutritive value and quality of the tubers using the combinations of essential oils of caraway and spearmint at room temperature and cooling conditions.
Materials and methods
Isolation of tuber rot associated fungi
Rotted JA tubers obtained from the refrigerated storage were separately washed, surface-disinfected for 3 min in 0.5 % sodium hypocholorite and rinsed with sterilizing water. Sections (1 cm) of the tubers were placed on potato dextrose agar (PDA) plates (Difco, USA), supplemented with antibacterial agent (L-chloramphenicol; 5 mg/L and streptomycin sulphate; 5 mg/L) and incubated at 25 °C for 5–7 days. Hyphal tip and single spore techniques were used to obtain pure cultures of the isolated fungal pathogens. The recovered isolates were maintained onto slants of potato, carrot agar medium and kept at 4 °C for further studies. The isolated fungi were identified according to their cultural, morphological and microscopical characteristics as described by Domsch et al. (1980) and Watanable (2002).
Pathogenicity test
Pathogenicity test was conducted to determine the rotting potential of the isolated fungi. Disease-free tubers of Fuseau cultivar, obtained from Vegetable Research Department, Horticulture Research Institute, Agricultural Research Center, Giza, Egypt, were washed and surface disinfested as described. Inoculation was carried out under sterilized conditions by making a small hole in the tuber using a sterile 5 mm Cork-borer and inserting a 5 mm disc of 5-days-old culture of the tested fungus in the hole. Inoculated tubers were then placed under moist conditions in covered glass containers at 25 °C. Latter, selected pathogenic isolates were tested for their rotting potentiality at 4, 15 and 25 °C.
The severity (%) of the tuber rot was evaluated based on our proposed scale; where: 0 = no symptoms of rot, 1 = 1–10 % of tuber rot, 2 = 11–20 % of tuber rot, 3 = 21–30 % of tuber rot, 4 = 31–40 % of tuber rot, 5 = 41–50 % of tuber rot, 6 = 51–60 % of tuber rot, 7 = 61–70 % of tuber rot, 8 = 71–80 % of tuber rot and 9 ≥ 81 % of tuber rot. The severity was then calculated using the formula;
Where; S = severity of tuber rot (%); Σn = sum of the individual ratings; N = total number of potato tubers assessed and 9 is the highest score on the severity scale.
Essential oils extraction
Essential oils were extracted separately from (200 g) of caraway seeds and spearmint dry leaves. Extraction was done by hydro-distillation of the plant materials for 150 min using Clevenger apparatus as described Charles and Simon (1990). The purified extracted essential oils were then stored in clean dark glass bottles at 4 °C until used.
In vitro assessment for antifungal activity
Antifungal activity of the two essential oils was evaluated. Suitable volumes of the plant essential oils were separately added to 100 ml Erlenmeyer flasks containing 20 ml sterilized PD broth and 0.5 % Tween-20 to obtain the proposed concentrations of 2, 3, 4 and 5 % (v/v). Discs (5 mm diameter) from 5-days-old culture of S. rolfsii were used to inoculate the flasks, and then the flasks were incubated in a dark at 25 ± 2 °C for 7 days. Untreated medium was used as a control. Triplicate flasks were used for each treatment. At the end of the incubation period, the mycelial mats were harvested, washed several times with distilled water, and oven dried to constant weight at 80 °C. The antifungal activity was expressed as the reduction percentage in the mycelial dry weight.
Gas-liquid chromatography – mass spectrometry (GLC-MS)
After extraction, the most efficient antifungal essential oil was analyzed to identify and quantify its basic constituents. Identification of the constituents of the essential oil was performed by gas-liquid chromatography coupled to mass spectrometry (Schimadzu QP-5000 GCMS), using an Autosystem XL, equipped with a DB-1 fused silica column (30 m × 0.25 mm film thickness 0.25 m; J&W Scientific Inc.) connected to a Perkin-Elmer Turbomass. The oven temperature was programmed from 50 to 200 °C in increments of 3 °C min−1. On reaching 200 °C, the temperature was kept isothermal for 10 min, the temperature of the transfer line, 230 °C; the temperature of the ionization chamber 200 °C, helium carrier gas, adjusted to a linear velocity of 30 cm s−1; split-flow ratio 1:40, ionization energy 70 eV, ionization current, 60 A; mass range, 40–300 U, scan time 1 s. A standard solution of n-alkanes (C9-C21) was used to obtain the retention indices. The compounds were identified by comparison of their retention indices with those reported in literature and also in the Wiley Registry of Mass Spectral Data, 6th Edition (Wiley Interscience, New York). (Andrade et al. 2015).
Evaluation of the caraway essential oil and peat moss application under storage conditions
Healthy JA tubers (~20 g each) were washed by water, surface disinfected in 0.5 % (v/v) sodium hypochlorite for 3 min, then rinsed in sterilized water and left to dry. For essential oil application, JA tubers were soaked in caraway essential oil at 2 % concentration for 20 min, then left to dry about 2 h before the storage. For inoculum preparation, 250 ml Erlenmeyer flask containing 50 ml of sterilized PD broth medium and inoculated with a disc (5 mm diameter) from 5-days-old culture of S. rolfsii was incubated in the dark at 25 ± 2 °C for 10 days. The mycelial mat was then harvested and washed gently with sterile distilled water. Twenty grams of the fungal mat were taken, mixed thoroughly and blended with 1000 ml of distilled water to produce a homogenized suspension and adjusted at a concentration of 5 × 104 cfu mL−1. Artificial infection was done by spraying the tubers with the fungal suspension until run-off.
The experiment was divided into two groups according to the storage method. In the first group, the tubers were kept in 10-kg capacity perforated polyethylene bags (0.075 mm thickness), and stored at 4 °C at a relative humidity (RH) of 90–95 %. In the other group, the tubers were stored at 25 ± 2 °C in carton boxes (50 × 30 × 20 cm) with moistened peat moss layers (SAB Syker Agrarberatungs und Handels GmbH & Co., Germany, pH 3.5 and RH 75 %) at the rate of peat moss: JA tubers (1.5: 1, Kg/Kg). The treatments applied to each group can be summarized as follows: untreated control (C), infected with S. rolfsii (P), treated with caraway oil (O), and treated with caraway oil and infected with S. rolfsii (O + P). For each treatment, 30 kg of tubers were used.
Monthly and along 120 days of storage, tuber rot severity, percentage of sprouting, weight losses and dry matter of tubers were evaluated. The stored tubers were also analyzed for carbohydrate, inulin and protein contents according to and Dubois et al. (1956), Winton and Winton (1958) and Robinson (1973), respectively. The defense related enzymes (peroxidase and polyphenoloxidase) were assessed according to the methods described by Maria et al. (1981) and Maxwell and Bateman (1967). Total phenol was determined according to Diaz, and Martin (1972).
Statistical analysis
All experiments were arranged in randomized block design. Statistical analysis, including, analysis of variance and Duncan test, as well as the standard deviation were performed using CoStat (CoHort Software, U.S.A) version 6.4 at level of probability (P) ≤ 0.05.
Results and discussion
Tuber rot associated fungi
Occurrence percentages of the fungal species associated with rotted JA tubers which stored under cold conditions are illustrated in Fig. 1. Seventeen fungal species belonging to 12 genera were isolated and identified with varied occurrence percentages. The highest occurrence was recorded for S. rolfsii (61.7 %), followed by Fusarium incarnatum (22 %), whereas Geotrichum candidum was the lowest recovered fungus, recording 2.7 %. A thin, easily penetrated surface layer and high sugar content make JA tubers more susceptible to infection by a wide range of fungi. Several fungi associated with rotted JA tubers have been reported in the literature e.g. S. rolfsii, Sclerotinia sclerotiorum (Snowden 2010), Botrytis cinerea, Rhizopus stolonifera, Penicillium and Fusarium species (Kays and Nottingham 2008) and Rhizoctonia solani (Ezzat et al. 2015).
Pathogenicity test
All isolated fungal strains were subjected to the pathogenicity test at different temperatures (4, 15 and 25 °C) to determine the most aggressive fungus causing tuber rot and its best growth temperature (Table 1). Great variation in the pathogenic potential ranged between 0 and 100 % was recorded for the tested fungi according to the temperature and the fungal species. Some fungi have the pathogenic potential even under cooling temperature. The pathogenic potential, if present, increased with the temperature increment. Among the tested fungi, S. rolfsii was the only pathogen that recorded 100 % disease severity at 25 °C. However, its pathogenic potential remained relatively high at 4 and 15 ° C (53.3 and 73.3 %, respectively). Sclerotinia sclerotiorum came next with disease severity of 70, 93.3 and 25.7 % at 4, 15 and 25 °C, respectively. On the other hand, six fungal species showed low to moderate pathogenic potentials, while, nine fungi recorded no pathogenic potential at any temperature.
The obtained results are in accordance with that of Koike (2004) and Sennoi et al. (2013) who reported that, S. rolfsii is considered as a serious pathogen of JA tubers in both field and storage. Jerusalem artichoke tuber rot caused by S. rolfsii was reported to occur in temperature range of 2 to 20 °C. The pathogen sclerotia have the ability to survive at low temperature (−10 °C), and can germinate under a wide range of relative humidity (Kays and Nottingham 2008; Snowden 2010). On the other hand, watery soft rot of JA tubers caused by S. sclerotiorum was reported to develop at low temperature (Snowden 2010). Blue mold rot and Fusarium rot, caused by several Penicillium and Fusarium species, respectively, were reported to infect JA tubers only at relatively high temperatures (Kays and Nottingham 2008). Based on the obtained results, S. rolfsii was selected for the next tests.
In vitro assessment for antifungal activity of the essential oils
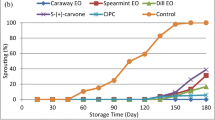
In vitro antifungal activity of the caraway and spearmint essential oils was evaluated against S. rolfsii (Fig. 2). The obtained data revealed that, caraway essential oil completely inhibited the growth of the pathogen even at the lowest concentration (2 %), while, spearmint essential oil showed a slight reduction of the fungal growth. These results are in agreement with those of Abdel-Kader et al. (2011) who reported the inhibitory effects of caraway oil against the mycelial growth of F. solani, R. solani, S. rolfsii and Macrophomina phaseolina under in vitro conditions. The antifungal activity of caraway essential oil may be attributed to some antifungal phytochemicals that constitute a large fraction of the oil like carvone, limonene, carveol, pinen and thujone (Seidler-Lozykowska et al. 2013; Darougheh et al. 2014).
Constituents of caraway essential oil
GLC analysis of caraway oil revealed that, monoterpenes is the main component of the oil. Ten compounds were determined in caraway essential oil. The main components were carvone and limonene (57.7 and 35.5 %, respectively). According to other researchers, the percentage of carvone and limonene in caraway essential oil ranges from 92.2 to 99.8 %. The overall quality of caraway fruits is considered to correlate with its carvone / limonene ratio; the higher the ratio, the better the quality (Seidler-Lozykowska et al. 2010). In addition, the essential oil contains myrcene, α-Terpinolene, trans-limonene oxide, trans -dihydro carvone, trans -carvol, perilla alcohol, carvacrol and β-caryophyllene. In this study, these constituents were present in small amounts.
Evaluation of the caraway essential oil and peat moss application under storage conditions
Disease severity
The mean data of rot disease severity of JA tubers treated with caraway essential oil and infected with S. rolfsii using the two storage methods are presented in Table 2. Although the disease severity increased with the increase of storage period using the two storage methods, the storage of the infected JA tubers treated with caraway essential oil (O + P) in peat moss layer at 25 °C significantly reduced the disease severity compared with the cooling method even after 120 days of storage. Storage of the infected-untreated JA tubers (P) in peat moss layer at 25 °C significantly reduced the disease severity for 60 days of storage compared with the storage under cooling method, after which, the tubers were fully deteriorated. On the other hand, storage of the untreated-uninfected JA tubers (C) in peat moss layer at 25 °C significantly reduced the disease severity (caused by reasons other than S. rolfsii) compared with the storage under cooling method. These results are in accordance with those obtained by Al-Mughrabi et al. (2013) against potato storage pathogens (F. coeruleum, F. sambucinum, F. avenaceum, F. oxysporum, Alternaria solani, R. solani, Phytophthora infestans, P. erythroseptica and Phoma exigua). The antimicrobial effects of monoterpenes, carvone and limonene, presented in the caraway essential oil may be due to the increase of the permeability of the fungal cell membrane, inactivation of some structures and energy production enzymes and/or the reduction of conidial germination, ultimately resulting in death of the fungus (Ma et al. 2015). In addition, the use of essential oils in managing JA storage diseases may provides several positive advantages, including, they are eco-friendly, biodegradable, multifunctional, and non-persistent natural products. Moreover, these essential oils may tend to reduce the risk of pathogen resistance build up against chemicals because they contain two or more stereo-isomers that may be targeted on multi-sites on the pathogen’s cell membrane (Lambert et al. 2001).
One of the valuable applications for peat moss is the traditional use in food preservation (Taskila et al. 2016). The antifungal property of peat mosses has been reported by many researchers against Candida albicans, Cryptococcus albidus, Aspergillus niger, A. flavus, A. terreus, A. nidulans, A. spinulosus, and Trichophyton rubrum (Zaitseva 2009). The antimicrobial activity in peat moss may be attributed to some of its bioactive components like sphagnan, a pectin-like polymer, that inhibit microbial growth via electrostatic immobilization of extracellular enzymes and/or nitrogen deprivation, phenolics that inhibit the activity of extracellular enzymes of microbes or other constituents like sterols and polyacetylenes (Borsheim et al. 2012).
Sprouting, weight loss and dry matter of JA tubers
The mean data of sprouting, weight loss and dry matter weight of JA tubers treated with caraway essential oil and infected with S. rolfsii using the two storage methods are presented in Table 3.
Results indicated that, the treatment of healthy JA tubers with caraway essential oil completely prevented the tubers sprouting and weight loss, but recorded the highest dry mater weight percentage along the storage period compared with the untreated-uninfected control using the two methods. Even after 4 months of storage, the treatment of the infected JA tubers with caraway essential oil led to a lower sprouting and weight loss and higher dry matter weight for JA tubers stored in peat moss at 25 °C than those stored in polyethylene bags at 4 °C when compared with the infected-untreated tubers. On the other hand, storage of the untreated-uninfected JA tubers in peat moss at 25 °C increased the sprouting, decreased the weight loss and lowered the reduction of dry matter weight of the tubers compared with the storage in polyethylene bags at 4 °C. These results are in agreement with those obtained by Gomez-Castillo et al. (2013) on potato tubers. The active compounds, limonene and carvone, in caraway oil are known to suppress sprouting of tubers by the inhibition of mitochondrial respiration and reducing carbohydrate degradation sugar. Carvone may play a more specific role in the sprout growth of potato tubers, such as the inhibition of key enzyme in the mevalonate pathway, which is the main pathway of gibberellin biosynthesis (Oosterhaven et al. 1995). On the other hand, peat moss has a relatively high water retention capacity; their cells can hold 16–25 times their dry weight of water (Taskila et al. 2016). This property gives an advantage for its use in the preservation of JA tubers by providing a relative humidity around the tubers and blocking heat transfer within the peat moss leading to the decrease of the water loss even at 25 °C. Cabezas et al. (2002) reported that dry matter content in JA tubers depends on many factors, such as storage conditions, and keeping tubers for 30 days at 18 °C, this leads to loosing water above 20 %.
Biochemical characters of JA tubers
Mean data of carbohydrates content, inulin and protein of JA tubers treated with caraway essential oil and then infected with S. rolfsii using the two storage methods are shown in Table 4. The obtained results showed that the treatment of the uninfected JA tubers with caraway essential oil recorded the highest total carbohydrates, inulin and protein contents compared with the untreated-uninfected control in both methods. Along 120 days of storage, the treatment of infected JA tubers with caraway essential oil effectively lowered the reduction of carbohydrate, inulin and protein contents compared with the infected-untreated tubers in both methods. A fresh JA tuber contains 80 % water, 15 % carbohydrates, mainly in the form of inulin, and about 2 % protein (Brkljaca et al. 2014). For long term storage of JA tubers, there are various changes, i.e. microbiological, enzymatic and biochemical which may lead to tuber damage. To inhibit these biochemical activities, natural or artificial drying products are widely used (Norkulova and Safarov 2015). Davies (1990) reported that the basic constituents of caraway oil (monoterpenes) tend to delay the breakdown of carbohydrates and protein associated with the enzymatic system as well as respiration and energy metabolism enzyme keeping the internal biochemical enzymatic activities in minimum level.
Peroxidase, polyphenoloxidase enzymes and phenol content in JA tubers
Data presented in Table 5 show the mean activities of peroxidase, polyphenoloxidase enzymes and phenol content of JA tubers treated with caraway essential oil and infected with S. rolfsii using the two storage methods. Results revealed that infection with S. rolfsii led to higher total phenol and the activity of peroxidase and polyhenoloxidase enzymes in JA tubers than those of the uninfected control in the two storage methods. On the other hand, the treatment with caraway essential oil of infected/uninfected JA tubers increased these enzymes and phenol content compared with the untreated-uninfected tubers in both methods. These results are in agreement with those obtained by Afify et al. (2012) who reported an increase in peroxidase and polyhenoloxidase activities in potato tubers when treated with caraway essential oil. Although most enzymatic reactions are drastically reduced at low temperature, JA tubers metabolism could continue at a slow rate even at 2 °C during storage (Saengthobpinit and Sajjaanantakul 2005). The enzymatic activation due to caraway essential oil treatment could be directly related to its content of carvone.
In conclusion, this study has presented an eco-safe and energy saving method for preserving JA tubers for 120 days of storage. From the obtained results, it can be concluded that caraway oil contains bioactive compounds able to inhibit the growth of the pathogen under laboratory and storage environments as well as improve the quality parameters of JA tubers and its storability. Treatment of JA tubers with caraway oil and preserving them in between peat moss layers can provide an effective protection against tuber rot for 120 days of storage even at 25 °C (the favored growth temperature by the pathogen).
References
Abdel-Kader, M., El-Mougy, N., & Lashin, S. (2011). Essential oils and Trichoderma Harzianum as an integrated control measure against Faba bean root rot pathogens. Journal of Plant Protection Research, 51(3), 306–313.
Afify, A. M. R., El-Beltagi, H. S., Aly, A. A., & El-Ansary, A. E. (2012). Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pacific Journal of Tropical Biomedicine, 2012, S772–S780.
Al-Mughrabi, K. I., Coleman, W. K., Vikram, A., Poirier, R., & Jayasuriya, K. E. (2013). Essential oils and their combinations with aluminum starch octenylsuccinate on potato storage pathogens. Journal of Essential Oil Bearing Plants, 16(1), 23–31.
Al-Snafi, A. E. (2015). The chemical constituents and pharmacological effects of Carum carvi—a review. Indian Journal of Pharmaceutical Science and Research, 5(2), 72–82.
Andrade, M. A., Cardoso, M. D., Gomes Mde, S., de Azeredo, C. M., Batista, L. R., Soares, M. J., et al. (2015). Biological activity of the essential oils from Cinnamodendron dinisii and Siparuna guianensis. Brazilian Journal of Microbiology, 46(1), 189–194.
Borsheim, K. Y., Christensen, B. E., & Painter, T. (2012). Preservation of fish by embedment in Sphagnum moss, peat, or holocellulose: experimental proof of the oxopolysaccharidic nature of the preservative substance and its antimicrobial and tanning action. Innovative Food Science and Emerging Technologies, 2(1), 63–74.
Brkljaca, J., Bodroza-Solarov, M., Krulj, J., Terzic, S., Mikic, A., & Marjanovic-Jeromela, A. (2014). Quantification of inulin content in selected accessions of Jerusalem artichoke (Helianthus tuberosus L.). Helia, 37(60), 105–112.
Cabezas, M. J., Rabert, C., Bravo, S., & Shene, C. (2002). Inulin and sugar contents in Helianthus tuberosus and Cichorium intybus tubers: effect of post harvest storage temperature. Journal of Food Science, 67, 2860–2865.
Charles, D. J., & Simon, J. E. (1990). Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. Journal of the American Society for Horticultural Science, 3, 458–462.
Combrinck, S., Regnier, T., & Kamatou, G. P. P. (2011). In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Industrial Crops and Products, 33, 344–349.
Darougheh, F., Barzegar, M., & Sahari, M. (2014). Antioxidant and anti-fungal effect of caraway (Carum Carvi L.) essential oil in real food system. Current Nutrition & Food Science, 10(1), 70–76.
Davies, H. V. (1990). Carbohydrate metabolism during sprouting. American Potato Journal, 67, 815–827.
Diaz, D. H., & Martin, G. C. (1972). Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. Journal of the American Society for Horticultural Science, 97, 651–654.
Domsch, K. H., Gams, W., & Anderson, T. H. (1980). Compendium of soil fungi. Vols. 1, 2.. New York: Academic.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Ezzat, A. E. S., Ghoneem, K. M., Saber, W. I. A., & Al-Askar, A. A. (2015). Control of wilt, stalk and tuber rots diseases using arbuscular mycorrhizal fungi, Trichoderma species and hydroquinone enhances yield quality and storability of Jerusalem artichoke (Helianthus tuberosus L.). Egyptian Journal of Biological Pest Control, 25(1), 11–22.
Gomez-Castillo, D., Cruz, E., Iguaz, A., Arroqui, C., & Virseda, P. (2013). Effects of essential oils on sprout suppression and quality of potato cultivars. Postharvest Biology and Technology, 82, 15–21.
Hussain, A. I., Anwar, F., Shahid, M., Ashraf, M., & Przybylski, R. (2010). Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint (Mentha spicata L.) from Pakistan. Journal of Essential Oil Research, 22(1), 78–84.
Jin, S., Liu, L., Liu, Z., Long, X., Shao, H., & Chen, J. (2013). Characterization of marine Pseudomonas spp. antagonist towards three tuber-rotting fungi from Jerusalem artichoke, a new industrial crop. Industrial Crops and Products, 43, 556–561.
Johansson, E., Prade, T., Angelidaki, I., Svensson, S.-E., Newson, W. R., Gunnarsson, I. B., & Persson Hovmalm, H. (2015). Economically viable components from Jerusalem artichoke (Helianthus tuberosus L.) in a biorefinery concept. International Journal of Molecular Sciences, 16(4), 8997–9016.
Kays, S. J. & Nottingham, S. F. (2008). Biology and chemistry of Jerusalem artichoke: Helianthus tuberosus L. - Boca Raton - Abingdon - Oxon - New York: CRC Press, Taylor and Francis Group.
Koike, S. T. (2004). Southern blight of Jerusalem artichoke caused by Sclerotium rolfsii in California. Plant Disease, 88, 769.
Lambert, R. J. W., Sjandamis, P. N., Coote, P. J., & Nychas, G. J. E. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology, 91, 453–462.
Ma, B., Ban, X., Huang, B., He, J., Tian, J., Zeng, H., Chen, Y., & Wang, Y. (2015). Interference and mechanism of dill seed essential oil and contribution of carvone and limonene in preventing Sclerotinia rot of rapeseed. PLoS ONE, 10(7), e0131733.
Maria, A., Galeazzi, M., Valdemo, C., Garbieri, S., & Spiros, M. (1981). Isolation, purification and physicochemical of polyphenol oxidase (PPO) from a dwarf variety of banana. Journal of Food Science, 46(1), 150–155.
Maxwell, D. P., & Bateman, D. F. (1967). Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyl in relation to lesion maturation. Phytopathology, 57, 132.
Norkulova, K. T., & Safarov, J. E. (2015). Research of sorption characteristics of tubers Jerusalem artichoke (Helianthus tuberosus). Journal of Food Processing and Technology, 6(6), 453–454.
Oosterhaven, K., Hartmans, K. J., & Scheffer, J. J. C. (1995). Inhibition of potato sprout growth by carvone enantiomers and their bioconversion in sprout. Potato Research, 38, 219–230.
Robinson, R. G. (1973). Element composition and response to nitrogen of sunflower and corn. Agronomy Journal, 66, 313.
Saengthobpinit, W., & Sajjaanantakul, T. (2005). Influence of harvest time and storage temperature on charecteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biology and Technology, 37(1), 93–100.
Seidler-Lozykowska, K., Kedzia, B., Karpinska, E., & Bocianowski, J. (2013). Microbiological activity of caraway (Carum carvi L.) essential oil obtained from different origin. Acta Scientiarum Agronomy, 35, 495–500.
Seidler-Lozykowska, K., Baranska, M., Baranski, R., & Krol, D. (2010). Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. Journal of Agricultural and Food Chemistry, 58, 5271–5275.
Sennoi, R., Jogloy, S., Saksirirat, W., Kesmala, T., & Patanothai, A. (2013). Genotypic variation of resistance to southern stem rot of Jerusalem artichoke caused by Sclerotium rolfsii. Euphytica, 190(3), 415–424.
Sennoi, R., Jogloy, S., Saksirirat, W., & Patanothai, A. (2010). Pathogenicity test of Sclerotium rolfsii, a causal agent of Jerusalem artichoke (Helianthus tuberosus L.) stem rot. Asian Journal of Plant Sciences, 9, 281–284.
Snowden, A. L. (2010). A colour allas of post-harvest diseases and disorders of fruits and vegetables: volume 2: vegetables, Manson Publishing CRC Press, p. 416.
Taskila, S., Särkelä, R., & Tanskanen, J. (2016). Valuable applications for peat moss. Biomass Conversion and Biorefinery, 6, 115–126.
Tesio, F., Weston, L. A., & Ferrero, A. (2011). Allelochemicals identified from Jerusalem artichoke (Helianthus tuberosus L.) residues and their potential inhibitory activity in the field and laboratory. Scientia Horticulturae Amsterdam, 129, 361–368.
Watanable, T. (2002). Pictorial atlas of soil and seed fungi: Morphologies of cultured fungi and key to species. 2nd. Boca Raton: CRC Press.
Winton, A. L., & Winton, K. B. (1958). The analysis of foods. London: Wiley. 857p.
Yang, L., He, Q. S., Corscadden, K., & Udenigwe, C. C. (2015). The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnology Reports, 5, 77–88.
Zaitseva, N. (2009). A polysaccharide extracted from Sphagnum moss as antifungal agent in archaeological conservation Master’s Thesis. Queen’s University, Kingston, Ontario, Canada.
Zhang, Y., Li, S., Jiang, D., Kong, L., Zhang, P., & Xu, J. (2013). Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. Journal of Agricultural and Food Chemistry, 61, 1521–1524.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-VPP-327.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghoneem, K.M., Saber, W.I.A., El-Awady, A.A. et al. Alternative preservation method against Sclerotium tuber rot of Jerusalem artichoke using natural essential oils. Phytoparasitica 44, 341–352 (2016). https://doi.org/10.1007/s12600-016-0532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0532-3