Abstract
The replacement of liquid electrolyte with solid electrolyte can significantly improve the safety and power/energy density of lithium batteries. 70Li2S–30P2S5 is one of the most promising solid electrolytes with high conductivity for solid–state batteries. In this work, the ionic conductivity and stability toward moisture and lithium metal of 70Li2S–30P2S5 were enhanced by introducing the different amounts of Li2O additives. 65Li2S–30P2S5–1%Li2O delivered the highest conductivity, while 65Li2S–30P2S5–5%Li2O showed the best moisture stability and improved lithium compatibility. Solid-state batteries using 65Li2S–30P2S5–5%Li2O electrolyte and high-voltage LiNi0.6Mn0.2Co0.2O2 cathode exhibited low initial discharge capacity (100 mAh·g−1) and Coulombic efficiency (69%). Li3InCl6 electrolytes were introduced both in the cathode mixture to replace sulfide electrolyte and in the interface layer to improve the cathode compatibility for the solid-state batteries, showing enhanced discharge capacity (175 mAh·g−1) and improved initial Coulombic efficiency (86%). Moreover, it also exhibited good performance at − 20 °C.
Graphical abstract

摘要
用固体电解质替代液体电解质可以显著提高锂电池的安全性和功率/能量密度。70Li2S–30P2S5是最有前途的高导电性固体电解质之一。在这项工作中, 通过引入不同量的 Li2O 添加剂, 70Li2S–30P2S5 的离子电导率和对空气及锂金属的稳定性得到了增强。其中, 组成为65Li2S–30P2S5–1%Li2O 的电导率最高, 而 组成为65Li2S–30P2S5–5%Li2O 则表现出最好的空气稳定性和改善的锂金属兼容性。使用 65Li2S–30P2S5–5%Li2O 电解质和高电压 LiNi0.6Mn0.2Co0.2O2 正极的固态电池展示出较低的初始放电比容量(100 mAh·g−1)和库仑效率(69%) 。随后, 在正极混合物中引入 Li3InCl6 电解质添加剂替代硫化物电解质, 并在界面层加入该电解质隔绝直接接触, 提高了固态电池正极兼容性, 显示出更高的放电比容量(175 mAh·g−1)和初始库仑效率(86%) 。此外, 该电池还可在− 20 ℃ 下展现出良好的性能。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid-state batteries have attracted significant attentions due to their increased energy and power density and improved safety compared to the current lithium-ion batteries using liquid organic electrolytes [1,2,3,4,5]. Exploration of solid electrolytes with high ionic conductivity and high stability toward electrode materials can promote the development of solid-state batteries [6,7,8]. Intensive research efforts have been devoted to exploring and designing new kinds of solid electrolytes which can be applied in solid-state batteries [9]. Recently, sulfide solid electrolytes have exhibited great potential as one of the most promising candidates of solid electrolytes for solid-state batteries due to their high ionic conductivity and mechanical softness [10,11,12,13,14,15]. High ionic conductivity of solid electrolyte provides fast lithium-ion migration, and good mechanical softness property enables good interface contact with electrode materials, both of which enable the possibility to fabricate solid-state batteries with excellent electrochemical performances [16, 17].
Li2S–P2S5 system was reported as the most famous sulfide solid electrolytes with high ionic conductivity over 1 × 10–4 S·cm−1 at room temperature [18]. The ionic conductivities of Li2S–P2S5 electrolytes are highly dependent on the ratio of Li2S and P2S5 in the mixture of raw materials. The Li2S–P2S5 amorphous glass solid electrolytes are usually synthesized via the high-energy mechanical milling process, and their ionic conductivity can be further enhanced after an annealing process [19]. Among those different compositions, the annealed 70Li2S–30P2S5 solid electrolyte showed a metastable phase with high ionic conductivity up to 1 × 10–3 S·cm−1 at room temperature, which can promote the development of solid-state batteries [20,21,22,23,24]. However, sulfide electrolytes suffer from the low chemical stability with moisture to generate H2S, which increases the cost to fabricate solid-state batteries using 70Li2S–30P2S5 solid electrolytes. The low moisture stability was associated with S atoms in the structure, which can be easily reacted with H2O to generate H2S. Therefore, the replacement of S with O in the 70Li2S–30P2S5 solid electrolyte exhibited the possibility to enhance the moisture stability [25,26,27,28,29,30,31]. Moreover, sulfide electrolytes have redox reactive in contact with pristine high-voltage active material due to the great electrochemical potential differences [32, 33]. Surface modifications are the common route to avoid the direct contact between sulfide electrolytes and high-voltage cathode in the cathode mixture. However, homogenous coatings surround the whole particle are quite important. Another possible route to improve the compatibility of sulfide electrolytes toward high-voltage cathode is to replace the unstable sulfide electrolytes with high-stability electrolytes, such as lithium halide [34]. It has been reported that lithium halide solid electrolytes showed excellent compatibility with pristine high-voltage cathode materials [35, 36]. Therefore, the introduction of lithium halide additive in the cathode mixture and the interface layer to avoid the direct contact between sulfide electrolytes and pristine high-voltage cathode is a promising solution to enhance the electrode compatibility of sulfide electrolytes. Furthermore, solid-state batteries exhibited poor electrochemical performances at low temperature due to the decreased ionic conductivity and the slow kinetics of lithium-ions transport between the interface of electrode materials and solid electrolytes [37,38,39,40,41]. To fabricate solid-state batteries with acceptable performances and unravel the resistance evaluations during cycling at low temperature can promote the development of solid-state batteries.
In this work, we introduced Li2O additives to partly replace Li2S in 70Li2S–30P2S5 electrolyte to improve the moisture stability and lithium metal compatibility. The amounts of Li2O additives were optimized to achieve high ionic conductivity and good stability toward moisture and lithium metal. The prepared solid electrolytes after modified by the Li2O additives were combined with high-voltage cathode LiNi0.6Mn0.2Co0.2O2 and Li-In anode to fabricate solid-state batteries. Furthermore, Li3InCl6 electrolyte was introduced both in the cathode mixture to replace the sulfide electrolyte and in the interface of the above solid-state batteries to improve the electrochemical performance at different temperatures.
2 Experimental
2.1 Material synthesis
The solid electrolytes in this work were prepared through mechanical milling followed by a sintering process described in previous work [42,43,44]. Reagent-grade Li2S (99.9%, Sigma-Aldrich), P2S5 (99%, Macklin), and Li2O (99.9%, Sigma-Aldrich) powders were mixed with the required amount ratio using ZrO2-coated stainless steel with ZrO2 balls. The total weight ratio of ZrO2 balls and the mixture of raw materials were fixed at 20/1. The total amount of the starting materials was 2.0 g for each jar. The mixture was first ball milled with a rotation speed of 500 r·min−1 for 12 h. The mixture obtained after the milling process was sealed in a quartz tube and annealed at 270 °C for 3 h to get the final solid electrolytes. Li3InCl6 was prepared by mechanical milling of the required amount of LiCl and InCl3 with a rotation speed of 500 r·min−1 for 24 h followed by annealing the milled mixture at 260 °C for 5 h to obtain the final powder.
2.2 Material characterization
X-ray diffractometer (XRD) patterns of the annealed (70–x)Li2S–30P2S5–x%Li2O (x = 1, 2, 5, 10) solid electrolytes were collected using Cu Kα radiation from a SmartLab-SE Powder instrument over a 2θ range of 10°–70° to identify the phases. Morphology and energy-dispersive spectroscopy (EDS) mapping of the solid electrolytes were observed by scanning electron microscopy (SEM, Nova NanoSEM 450). Lithium-ion conductivities of the obtained solid electrolytes were characterized by pelletizing 100 mg target powder into a pellet (diameter of 10 mm) using stainless steel as the blocking electrodes. Alternating current (AC) impedance spectrum was performed with an impedance analyzer (Solartron, 1260) in the frequency range of 1 Hz to 10 MHz with an applied voltage of 0.02 V. Li/solid electrolyte/Li batteries were constructed to evaluate the lithium compatibility of 70Li2S–30P2S5 and 65Li2S–30P2S5–5%Li2O as a function of the storage time. The ionic conductivities of the Li3InCl6/65Li2S–30P2S5–5%Li2O bilayer solid electrolyte at different temperatures were characterized the same as the above description. The amounts of H2S generated from the prepared solid electrolytes were measured by pelletizing 100 mg powder into a pellet with a diameter of 10 mm. The pressed pellet was transferred in a sealed plastic jar with a volume of 4 L, which was filled by moist air with a relative humidity of 60%–70%. The amount of H2S was tested using an H2S gas sensor as a function of time.
2.3 Solid-state battery fabrication and electrochemical measurement
For LiNi0.6Mn0.2Co0.2O2/65Li2S–30P2S5–5%Li2O/Li-In solid-state battery, 10 mg cathode mixture and 80 mg 65Li2S–30P2S5–5%Li2O electrolyte were pressed together by applying a pressure of 624 MPa, and then a piece of Li-In alloy was attached on the other side of the above bilayer pellet to form a triple-layer pellet using a pressure of 62.4 MPa. The cathode mixture for this battery was obtained by mixing the LiNi0.6Mn0.2Co0.2O2 with the 65Li2S–30P2S5–5%Li2O solid electrolyte with a weight ratio of 7/3. To fabricate the LiNi0.6Mn0.2Co0.2O2/Li3InCl6/65Li2S–30P2S5–5%Li2O/Li-In solid-state battery, 30 mg Li3InCl6 electrolyte and 50 mg 65Li2S–30P2S5–5%Li2O electrolyte were first pressed together with the same pressure and then combined the same amount of new cathode mixture, which consist of LiNi0.6Mn0.2Co0.2O2 and prepared Li3InCl6 electrolyte with the weight ratio of 7/3 to fabricate the final solid-state battery. The assembled solid-state batteries were charged/discharged at 0.1C between 2.4 and 3.7 V (vs. Li-In) at both room temperature and − 20 °C to evaluate the electrochemical performances. EIS before and after different cycles at different temperatures were measured in the frequency range of 1 Hz to 10 MHz with an applied voltage of 0.02 V.
3 Results and discussion
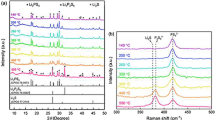
Li7P3S11 solid electrolytes with different amounts of Li2O additives were prepared by mechanical milling followed by an annealing process. XRD patterns of the prepared samples are shown in Fig. 1a. As illustrated, all the diffraction peaks of the obtained samples with different Li2O additives were indexed to the pure phase of Li7P3S11. Moreover, typical diffraction peaks due to Li7P3S11 located at 18°, 24° and 30° once again confirmed that all those prepared electrolytes displayed similar structure as a function of the increasing Li2O additives amount. The peak located at ~ 22° is attributed to the plastic film used for isolating moisture from the electrolytes. The ionic conductivities of Li7P3S11 electrolytes with different amounts of Li2O additives were characterized by temperature-dependent AC impedances. The lithium-ion conductivity of Li7P3S11 was 1.0 × 10–3 S·cm−1, increased to 1.2 × 10–3 S·cm−1 for 69Li2S–30P2S5–1%Li2O (1@7311), and then decreased to 8.4 × 10–4 S·cm−1 for 68Li2S–30P2S5–2%Li2O (2@7311), 9.9 × 10–4 S·cm−1 for 65Li2S–30P2S5–5%Li2O (5@7311), and 5.5 × 10–4 S·cm−1 for 60Li2S–30P2S5–10%Li2O (10@7311). The ionic conductivity of Li7P3S11 first increased with the introduction of a small amount of Li2O additive and then decreased with the introduction of a large amount of Li2O additive. The ionic conductivities of those solid electrolytes with different amounts of Li2O additive at different temperatures also confirmed this result, as shown in Figs. 1b, S1. 1@7311 electrolyte showed the highest ionic conductivity, and 10@7311 electrolyte displayed the lowest ionic conductivity among those solid electrolytes at different temperatures. As shown in Fig. 1c, the activation energy for conduction of those solid electrolytes first decreased with the introduction amount of Li2O additives and then increased at a large introduction amount, such as 10%.
a XRD patterns of Li7P3S11 solid electrolytes with different amounts of Li2O additives; b Arrhenius plots of corresponding prepared solid electrolytes; c changes of activation energy of solid electrolytes as a function of amount of Li2O additives; d H2S amount generated from pelletized Li7P3S11 solid electrolytes with different amounts of Li2O additives after exposition to air at room temperature for different storage durations
To validate the effect of introducing Li2O on the air stability of Li7P3S11 solid electrolyte, H2S amounts generated from the prepared samples were investigated. Figure 1d displays the amounts of H2S generated from the pelletized (70–x)Li2S–30P2S5–xLi2O (x = 0, 1, 2, 5, 10) solid electrolytes. The addition of Li2O additives plays a key point in the generation of H2S gas from solid electrolytes after exposition to air at room temperature. H2S gas generation rate and amount decreased after the introduction of Li2O additives, indicating that introduction of Li2O additives in Li7P3S11 solid electrolyte is an effective modification route to improve the moisture stability. Owing to the higher stability of O than S in contact with moisture, partial replacement of S with O by the introduction of Li2O additive during the synthesis can significantly enhance the moisture stability of sulfide solid electrolytes. The amount of H2S gas increased as a function of the exposition time for all samples until reached the limit of the H2S gas sensor. 5@7311 electrolyte showed the slowest H2S gas generation rate among those solid electrolytes, suggesting the highest stability to moisture. Considering the acceptable lithium-ion conductivity and the best moisture stability of 5@7311 among those solid electrolytes, it was chosen as the solid electrolyte in this work. SEM images and EDS mapping images of the prepared 5@7311 solid electrolytes are performed and presented in Fig. 2. For comparison, SEM and EDS mapping images of Li7P3S11 solid electrolyte are shown in Figure S2a–c. The electrolyte showed particles with diameter of larger than 10 μm and homogenous distribution of P, S and O. Previous research showed that the introduction of O can improve the stability of sulfide electrolyte toward lithium metal. To verify the lithium compatibility of the electrolyte before and after adding the Li2O additives, Li/5@7311/Li and Li/Li7P3S11/Li batteries were constructed. As shown in Fig. 3a, b, the resistance of Li/ Li7P3S11/Li battery increased as a function of the storage time with an extra resistance due to the chemical instability between lithium metal and Li7P3S11 electrolyte. In contrast, the resistance showed slight changes with storage time increasing for the Li/5@7311/Li battery, suggesting a higher lithium metal compatibility than that of the bare 70Li2S–30P2S5 electrolyte. This also can be confirmed by the change of interfacial resistances for the assembled two batteries as a function of storage time, as illustrated in Fig. 3c. It has been reported that the chemical and electrochemical stability against lithium metal of Li6PS5Br electrolyte was enhanced with O doping [45]. The above results suggested that lithium metal compatibility and moisture stability of Li7P3S11 electrolyte can be enhanced by the introduction of the Li2O additives. To elucidate the inner mechanism of the improved compatibility between Li metal and Li7P3S11 electrolyte, the XPS P 2p and S 2p detail spectra of Li7P3S11 and 5@7311 were carried out. The ratio of PS43− and P2S74− groups was enhanced after introducing Li2O in terms of P 2p results, as illustrated in Fig. 3d, e. Additionally, the S 2p result of 5@7311 showed impaired P2Sx peak (Fig. 3f, g), which is consistent with the P 2p result. Therefore, the ameliorative Li metal compatibility of Li7P3S11 electrolyte could be attributed to the reduced P–S–P bonding.
All solid-state batteries based on Li7P3S11 and 5@7311 solid electrolytes in combination with the coated LiNi0.6Mn0.2Co0.2O2 and Li-In alloy were constructed and charged/discharged at 0.1C between 2.4 and 3.7 V (vs. Li-In) at room temperature. As shown in Fig. 4a, the LiNi0.6Mn0.2Co0.2O2/Li7P3S11/In-Li battery delivered an initial charge capacity of 88 mAh·g−1 and discharge capacity of 54 mAh·g−1 with a low Coulombic efficiency of 61%. The LiNi0.6Mn0.2Co0.2O2/5@7311/In-Li delivered an initial charge capacity of 144 mAh·g−1 and discharge capacity of 100 mAh·g−1 with an enhanced Coulombic efficiency of 69%, as shown in Fig. 4b. After 10 cycles, the former maintained a discharge capacity of 33 mAh·g−1 with a discharge capacity retention of 61%, while the latter maintained a discharge capacity of 92 mAh·g−1 with a discharge capacity retention of 92%, as illustrated in Fig. 4c, d. EIS spectra before and after 10 cycles of above batteries were compared, as shown in Fig. 4e, f. The half-circle in the low frequency of the spectrum for cycled battery represents the resistance from the electrode and the solid electrolyte interfaces. The LiNi0.6Mn0.2Co0.2O2/5@7311/In-Li battery showed lower resistance compared to the LiNi0.6Mn0.2Co0.2O2/Li7P3S11/In-Li battery, suggesting the improved compatibility of Li7P3S11 electrolyte by introducing O atoms. Whereas Li7P3S11 and 5@7311 solid electrolyte can directly contact with the pristine high-voltage LiNi0.6Mn0.2Co0.2O2 because the coated-LiNi0.6Mn0.2Co0.2O2 applied here was not homogenously coated, yielding interface reaction. The redox activity of sulfide electrolyte in the cathode mixture will cause a side reaction between the active material and electrolyte, leading to the formation of side reaction products at the interface with high interfacial resistance. The poor charge/discharge capacities and low initial Coulombic efficiency for the assembled solid-state battery here were due to the incompatibility between the coated-LiNi0.6Mn0.2Co0.2O2 high-voltage cathode material and electrolyte.
Initial charge/discharge curves of a LiNi0.6Mn0.2Co0.2O2/Li7P3S11/In-Li and b LiNi0.6Mn0.2Co0.2O2/5@7311/In-Li solid-state batteries cycled at 0.1C between 2.4 and 3.7 V at room temperature; c corresponding cycling performances and d Coulombic efficiency comparison; e, f EIS of above solid-state batteries before and after 10 cycles
Lithium halide solid electrolytes have been reported to exhibit excellent stability and compatibility with pristine high-voltage cathode material. To improve the stability of the cathode mixture, Li3InCl6 was synthesized and introduced to replace the high-voltage cathode instable 5@7311 electrolyte in the cathode mixture. Moreover, to separate the direct contact between the coated-LiNi0.6Mn0.2 Co0.2O2 and the prepared 5@7311 electrolyte layer, a thin layer of Li3InCl6 was introduced in the new designed solid-state battery. As shown in Figure S3a, a pure phase of Li3InCl6 (ICSD: 17638) was prepared by mechanical milling followed by an annealing route. The AC impedance spectra at different temperatures were performed using stainless steel as the blocking electrode, as shown in Figure S3b. The resistance of the prepared Li3InCl6 decreased as a function of the increasing testing temperature. It showed a room temperature ionic conductivity of 5.4 × 10–4 S·cm−1 with an activation energy of 0.40 eV for lithium-ion conduction, as illustrated in Figure S3c. SEM image and EDS mapping results exhibited large particle size with homogenous distribution of In and Cl (Figure S3d). To investigate the ionic conductivities of the 5@7311 and Li3InCl6 bilayer solid electrolytes, 30 mg Li3InCl6 and 50 mg 5@7311 electrolytes were pressed as a pellet using the same process with the same pressure. The resistance of this bilayer solid electrolytes increased with testing temperatures decreasing (Fig. 5a), delivering an ionic conductivity of 6.2 × 10–4 S·cm−1 at room temperature and 5.5 × 10–5 S·cm−1 at − 20 °C, respectively. The conductivities of 5@7311 and Li3InCl6 are 1.32 × 10–4 and 2.83 × 10–5 S·cm−1 at − 20 °C, respectively, as shown in Figure S4. As illustrated, the activation energy for lithium-ion conduction of the Li3InCl6/5@7311 bilayer solid electrolytes was 0.33 eV (Fig. 5b), which was lower than the activation energy of the Li3InCl6 electrolyte (0.40 eV) and higher than the activation of the 5@7311 electrolyte (0.27 eV).
To validate the effect of introducing Li3InCl6 electrolyte to replace sulfide electrolyte in the cathode mixture, LiNi0.6Mn0.2Co0.2O2/Li3InCl6/5@7311/Li-In solid-state battery was fabricated and charged/discharged with the same rate (0.1C) and voltage window (2.4–3.7 V (vs. Li-In)) at room temperature. As illustrated in Fig. 6a, it delivered an initial charge capacity of 204 mAh·g−1 and discharge capacity of 175 mAh·g−1 at room temperature with a Coulombic efficiency of 86%. After introducing the Li3InCl6 electrolyte additive in the cathode mixture and the interface layer between the cathode mixture and 5@7311 electrolyte, the new solid-state battery showed much higher charge/discharge capacities and Coulombic efficiency due to the improvement of compatibility between LiNi0.6Mn0.2Co0.2O2 active material and the 5@7311 electrolyte. This also confirms that Li3InCl6 electrolyte has excellent stability with high-voltage cathode material. Further, 86% of the discharge capacity was retained after the 10th cycle (Fig. 6b). EIS before and after 10 cycles of the assembled solid-state battery showed that the 5@7311 electrolyte layer slightly changed, while the interfacial resistance from the LiNi0.6Mn0.2Co0.2O2/Li3InCl6 interface and the Li3InCl6/5@7311 was the major bottleneck for this battery (Fig. 6c). This also can be proved as the resistance change depicted in Figure S5. The resistance of Li3InCl6/5@7311/Li3InCl6 battery slightly increased as a function of the storage time, suggesting that the stability between Li3InCl6 and 5@7311 electrolyte still needs to be enhanced. For comparison, the battery of LiNi0.6Mn0.2Co0.2O2/Li3InCl6/Li-In with Li3InCl6 electrolyte as ionic conductor was assembled with the same rate (0.1C) and voltage window (2.4–3.7 V (vs. Li-In)) at room temperature (Figure S6). It delivered an initial charge capacity of 167 mAh·g−1 and discharge capacity of 132 mAh·g−1 at room temperature with a Coulombic efficiency of 79%, and a discharge capacity retention rate of 47% after 10 cycles. It also demonstrated the design rationality of double-layer electrolyte structure. Finally, the low-temperature application of this solid-state battery was also investigated by charging/discharging the battery at 0.05C between 2.4 and 3.7 V under − 20 °C. The assembled LiNi0.6Mn0.2Co0.2O2/Li3InCl6/5@7311/Li-In battery exhibited much lower initial charge/discharge capacities, 122 and 79 mAh·g−1, respectively, with a much lower Coulombic efficiency (64%), as shown in Fig. 6d. It showed stable cyclability in the subsequent 9 cycles at − 20 °C (Fig. 6e), delivered a discharge capacity of 80 mAh·g−1 with almost undecayed capacity. The low charge/discharge capacities depicted here were associated with the low ionic conductivity of the Li3InCl6 electrolyte and the Li3InCl6/5@7311 solid electrolyte bilayer (5.5 × 10–5 S·cm−1, − 20 °C) at low temperature. EIS results showed that two half circles belonged to the LiNi0.6Mn0.2Co0.2O2/Li3InCl6 interface and the Li3InCl6/5@7311 interface was observed when the temperature was lowered to − 20 °C, respectively. The resistance associated with the LiNi0.6Mn0.2Co0.2O2/Li3InCl6 interface was almost unchanged after 10 cycles, while the resistance attributed to the Li3InCl6/5@7311 interface slightly increased and the resistance due to the Li-In/5@7311 electrolyte was also observed (Fig. 6f).
a Initial charge/discharge curve and b corresponding cycling performances and Coulombic efficiency changes of LiNi0.6Mn0.2Co0.2O2/Li3InCl6/5@7311/In-Li solid-state battery using Li3InCl6 as solid electrolyte additive in cathode mixture cycled at 0.1C between 2.4 and 3.7 V (vs. Li-In) at room temperature; c corresponding EIS of solid-state battery before and after 10 cycles; d initial charge/discharge curve of above solid-state battery cycled at 0.1C between 2.4 and 3.7 V versus Li-In at − 20 °C; e corresponding cycling performances and Coulombic efficiency changes; f corresponding EIS of solid-state battery before and after 10 cycles
4 Conclusion
In summary, the stability of 70Li2S–30P2S5 was improved by the introduction of Li2O additives via mechanical milling followed by an annealing process. A small amount of Li2O additives was introduced in the structure to enhance the ionic conductivity of 70Li2S–30P2S5 electrolyte, yielding the highest lithium-ion conductivity (1.2 × 10–3 S·cm−1) at room temperature for 69Li2S–30P2S5–1%Li2O, while the ionic conductivities decreased with a higher amount of Li2O additives. Moisture stability of the electrolyte was significantly enhanced by the replacement of S with O in the structure. The tailored 65Li2S–30P2S5–5%Li2O electrolyte showed the highest moisture stability and improved compatibility toward lithium metal. LiNi0.6Mn0.2Co0.2O2/65Li2S–30P2S5–5%Li2O/Li-In solid-state battery delivered enhanced charge/discharge capacities (144 and 100 mAh·g−1) and initial Coulombic efficiency (69%) in comparison with LiNi0.6Mn0.2Co0.2O2/Li7P3S11/Li–In battery (88 and 54 mAh·g−1), which are still insufficient due to the redox reactivity of sulfide electrolyte in the cathode mixture toward high-voltage active material. Li3InCl6 electrolyte was successfully applied as the additive in the cathode mixture and the interface layer to avoid the direct contact of LiNi0.6Mn0.2Co0.2O2 and solid electrolyte. A solid-state battery with the structure of LiNi0.6Mn0.2Co0.2O2/Li3InCl6/65Li2S-30P2S5–5%Li2O/Li-In was constructed and showed enhanced charge/discharge capacities (204 and 175 mAh·g−1) and higher initial Coulombic efficiency (86%). The improvement of solid-state battery performance was due to the much lower interfacial resistance (~ 200 Ω vs. 1000 Ω) after introducing Li3InCl6 additive. Furthermore, this solid-state battery even exhibited good electrochemical performance at an extremely low temperature (− 20 °C).
References
Lee H, Oh P, Kim J, Cha H, Chae S, Lee S, Cho J. Advances and prospects of sulfide all-solid-state lithium batteries via one-to-one comparison with conventional liquid lithium ion batteries. Adv Mater. 2019;31(29):1900376.
Randau S, Weber DA, Kötz O, Koerver R, Braun P, Weber A, Ivers-Tiffée E, Adermann T, Kulisch J, Zeier WG, Richter FH, Janek J. Benchmarking the performance of all-solid-state lithium batteries. Nat Energy. 2020;5(3):259.
Lee YG, Fujiki S, Jung C, Suzuki N, Yashiro N, Omoda R, Ko DS, Shiratsuchi T, Sugimoto T, Ryu S, Ku JH, Watanabe T, Park Y, Aihara Y, Im D, Han IT. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat Energy. 2020;5(4):299.
Tu Z, Choudhury S, Zachman MJ, Wei S, Zhang K, Kourkoutis LF, Archer LA. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat Energy. 2018;3(4):310.
Liu XZ, Ding L, Liu YZ. Room-temperature ionic conductivity of Ba, Y, Al co-doped Li7La3Zr2O12 solid electrolyte after sintering. Rare Met. 2021;40(8):2301.
Xiao Y, Wang Y, Bo SH, Kim JC, Miara LJ, Ceder G. Understanding interface stability in solid-state batteries. Nat Rev Mater. 2019;5(2):105.
Banerjee A, Wang X, Fang C, Wu EA, Meng YS. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem Rev. 2020;120(14):6878.
Xin Y, Gong JJ. Solid-state batteries: from fundamental interface characterization to realize sustainable promise. Rare Met. 2020;39(7):743.
Jiang BX, Li YM, Wang ZM, Li ZK, Shen ZY. Electrical properties of Na-β″-Al2O3 solid electrolyte with Ti4+ doping. Chin J Rare Met. 2020;44(8):870.
Wu J, Shen L, Zhang Z, Liu G, Wang Z, Zhou D, Wan H, Xu X, Yao X. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electrochem Energy Rev. 2020;4(1):101.
Zhang Q, Cao D, Ma Y, Natan A, Aurora P, Zhu H. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv Mater. 2019;31(44):1901131.
Yu C, Ganapathy S, Eck ER, Wang H, Basak S, Li Z, Wagemaker M. Accessing the bottleneck in all-solid state batteries, lithium-ion transport over the solid-electrolyte-electrode interface. Nat Commun. 2017;8(1):1086.
Wu J, Shen L, Zhang Z, Liu G, Wang Z, Zhou D. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electrochem Energy Rev. 2020;4(1):101.
Zhang Z, Sun Y, Duan X, Peng L, Jia H, Zhang Y. Design and synthesis of room temperature stable Li-argyrodite superionic conductors via cation doping. J Mater Chem A. 2019;7(6):2717–22.
Zhang Z, Zhang J, Jia H, Peng L, An T, Xie J. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-argyrodite. J Power Sources. 2020;450(29):227601.
Yu C, Ganapathy S, de Klerk NJ, Roslon I, van Eck ER, Kentgens AP, Wagemaker M. Unravelling Li-ion transport from picoseconds to seconds: bulk versus interfaces in an argyrodite Li6PS5Cl–Li2S all-solid-state Li-ion battery. J Am Chem Soc. 2016;138(35):11192.
Zhang Z, Zhang L, Liu Y, Yu C, Yan X, Xu B, Wang LM. Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries. J Alloys Compd. 2018;747(30):227.
Minami K, Mizuno F, Hayashi A, Tatsumisago M. Lithium ion conductivity of the Li2S–P2S5 glass-based electrolytes prepared by the melt quenching method. Solid State Ionics. 2007;178(11–12):837.
Liu Z, Fu W, Payzant EA, Yu X, Wu Z, Dudney NJ, Kiggans J, Hong K, Rondinone AJ, Liang C. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J Am Ceram Soc. 2013;135(3):975.
Yamane H, Shibata M, Shimane Y, Junke T, Seino Y, Adams S, Minami K, Hayashi A, Tatsumisago M. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ionics. 2007;178(15–18):1163.
Minami K, Hayashi A, Tatsumisago M. Crystallization process for superionic Li7P3S11 glass-ceramic electrolytes. J Am Ceram Soc. 2011;94(6):1779.
Hayashi A, Minami K, Ujiie S, Tatsumisago M. Preparation and ionic conductivity of Li7P3S11−z glass-ceramic electrolytes. J Non-Cryst Solids. 2010;356(44–49):2670.
Seino Y, Ota T, Takada K, Hayashi A, Tatsumisago M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ Sci. 2014;7(2):627.
Prasada Rao R, Yuen JM, Adams S. Rechargeable lithium semi-flow battery using Li7P3S11. Solid State Ionics. 2016. https://doi.org/10.1016/j.ssi.2016.01.015.
Zhu Y, Mo Y. Materials design principles for air-stable lithium/sodium solid electrolytes. Angew Chem Int Ed Engl. 2020;59(40):17472.
Liu H, Yang Z, Wang Q, Wang X, Shi X. Atomistic insights into the screening and role of oxygen in enhancing the Li+ conductivity of Li7P3S11−Ox solid-state electrolytes. Phys Chem Chem Phys. 2019;21(48):26358.
Guo Y, Guan H, Peng W, Li X, Ma Y, Song D, Zhang H, Li C, Zhang L. Enhancing the electrochemical performances of Li7P3S11 electrolyte through P2O5 substitution for all-solid-state lithium battery. Solid State Ionics. 2020;358(15):115506.
Ahmad N, Zhou L, Faheem M, Tufail MK, Yang L, Chen R, Zhou Y, Yang W. Enhanced air stability and high Li-ion conductivity of Li6.988P2.994Nb0.2S10.934O0.6 glass-ceramic electrolyte for all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces. 2020;12(19):21548.
Zhao F, Alahakoon SH, Adair K, Zhang S, Xia W, Li W. An air-stable and Li-metal-compatible glass-ceramic electrolyte enabling high-performance all-solid-state Li metal batteries. Adv Mater. 2021;33(8):2006577.
Liang J, Chen N, Li X, Li X, Adair KR, Li J. Li10Ge(P1–xSbx)2S12 lithium-ion conductors with enhanced atmospheric stability. Chem Mater. 2020;32(6):2664.
Zhang Z, Zhang J, Sun Y, Jia H, Peng L, Zhang Y. Li4-xSbxSn1-xS4 solid solutions for air-stable solid electrolytes. J Energy Chem. 2020. https://doi.org/10.1016/j.jechem.2019.05.015.
Du M, Liao K, Lu Q, Shao Z. Recent advances in the interface engineering of solid-state Li-ion batteries with artificial buffer layers: challenges, materials, construction, and characterization. Energy Environ Sci. 2019;12(6):1780.
Xu R, Han F, Ji X, Fan X, Tu J, Wang C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy. 2018. https://doi.org/10.1016/j.nanoen.2018.09.061.
Wang S, Bai Q, Nolan AM, Liu Y, Gong S, Sun Q, Mo Y. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew Chem Int Ed Engl. 2019;58(24):8039.
Zhao X, Zhao-Karger Z, Fichtner M, Shen X. Halide-based materials and chemistry for rechargeable batteries. Angew Chem Int Ed Engl. 2020;59(15):5902.
Li X, Liang J, Yang X, Adair KR, Wang C, Zhao F, Sun X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ Sci. 2020;13(5):1429.
Park K-H, Kaup K, Assoud A, Zhang Q, Wu X, Nazar LF. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 2020;5(2):533.
Riegger LM, Schlem R, Sann J, Zeier WG, Janek J. Lithium-metal anode instability of the superionic halide solid electrolytes and the implications for solid-state batteries. Angew Chem Int Ed Engl. 2021;60(12):6792.
Yin J, Yao X, Peng G, Yang J, Huang Z, Liu D, Tao Y, Xu X. Influence of the Li–Ge–P–S based solid electrolytes on NCA electrochemical performances in all-solid-state lithium batteries. Solid State Ionics. 2015. https://doi.org/10.1016/j.ssi.2015.02.014.
Qu H, Kafle J, Harris J, Zheng D, Koshina J, Boone D, Drake AM, Abegglen CJ, Qu D. Application of ac impedance as diagnostic tool—low temperature electrolyte for a Li-ion battery. Electrochim Acta. 2019;322(1):134755.
Choi S, Jeon M, Kim BK, Sang BI, Kim H. Electrochemical behaviors of Li-argyrodite-based all-solid-state batteries under deep-freezing conditions. Chem Commun. 2018;54(100):14116.
Seino Y, Nakagawa M, Senga M, Higuchi H, Takada K, Sasaki T. Analysis of the structure and degree of crystallisation of 70Li2S–30P2S5 glass ceramic. J Mater Chem A. 2015;3(6):2756.
Busche MR, Weber DA, Schneider Y, Dietrich C, Wenzel S, Leichtweiss T, Schröder D, Zhang W, Weigand H, Walter D, Sedlmaier SJ, Houtarde D, Nazar LF, Janek J. In situ monitoring of fast Li-ion conductor Li7P3S11 crystallization inside a hot-press setup. Chem Mater. 2016;28(17):6152.
Mori K, Ichida T, Iwase K, Otomo T, Kohara S, Arai H, Uchimoto Y, Ogumi Z, Onodera Y, Fukunaga T. Visualization of conduction pathways in lithium superionic conductors: Li2S-P2S5 glasses and Li7P3S11 glass–ceramic. Chem Phys Lett. 2013;584(1):113.
Zhang Z, Zhang L, Yan X, Wang H, Liu Y, Yu C, Cao X, van Eijck L, Wen B. All-in-one improvement toward Li6PS5Br-based solid electrolytes triggered by compositional tune. J Power Sources. 2019;410–411(15–31):162.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51821005, 21975087, U1966214 and 51902116), the Certificate of China Postdoctoral Science Foundation Grant (No. 2019M652634). We gratefully acknowledge the Analytical and Testing Center of HUST for allowing us to use its facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, HT., Zhang, ZQ., Zhang, JZ. et al. Improvement of stability and solid-state battery performances of annealed 70Li2S–30P2S5 electrolytes by additives. Rare Met. 41, 106–114 (2022). https://doi.org/10.1007/s12598-021-01804-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01804-2