Abstract
Heavy metal containing pickling sludge (PS) is one of the by-products of the stainless-steel-making industry, which has been considered hazardous due to contained chromium and nickel. Traditional methods of PS disposing are landfill and cement solidification. This research is aimed at disposing PS by solidification/stabilization and reusing it as a nucleation agent of glass–ceramics. The crystallization behavior and the properties of a glass in the CaO–MgO–SiO2–Al2O3 system were studied by considering PS as the nucleation agent. Experimental results confirm that introducing 14 wt% PS as the nucleation agent of glass–ceramics can decrease crystallization temperature by 110.8 °C, refine the grain size by forming isometric crystals with size of 2 μm, enhance Vickers hardness by 2690 MPa and decrease water absorption from (1.21 ± 0.10) wt% to (0.04 ± 0.01) wt%. Therefore, it is reasonable to conclude that PS can be utilized as a nucleation agent to improve the crystallization and mechanical properties of the glass–ceramics. The testing results of US EPA toxicity characteristic leaching procedure (TCLP) confirm the safety of this reusing method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Stainless steel pickling has been classified as an environmental hazard due to the potential hazardous nature of its sludge, which often contains substantial amounts of Cr and Ni [1]. The leaching concentration of some heavy metals in the pickling sludge (PS) is relatively high [2], making disposal an urgent issue from the environmental and social standpoints. Traditional methods of PS disposing include landfill and cement solidification [3], which have been utilized in some areas for many years, and are still adopted by many plants in China [4]. The negative environmental effects of these disposing methods have gradually been reflected in water safety and health issues caused by the heavy metal pollution [5]. Thus, alternative options need to be explored to solve the PS handling in a more environmentally friendly manner [1].
Glass–ceramics, composed of the glass phase and the microcrystalline phase with fine microstructure, is produced by controlled crystallization of glass [6, 7]. As a high value-added products, glass–ceramics attracted significant attention due to excellent solidification capacity of heavy metals, mechanical properties, along with the possibility of using solid waste as raw materials [8–10]. In recent years, many types of solid wastes, such as steelwork slag [11, 12], bottom ash [13], fly ash [14, 15], soda lime waste glass [16] and copper slag [17], have been widely tested as alternatives to produce glass–ceramics. Generally, the properties of glass–ceramics are defined by the chemical composition, microstructure and crystal [18]. Therefore, it is necessary to add some nucleation agents, such as pure CaF2, Fe2O3 and Cr2O3 into the formulations to improve the crystallization and mechanical properties of glass–ceramics in most cases [19–23].
Stainless steel pickling is mainly carried out with an aggressive solution (nitric acid and hydrofluoric acid) [2, 24]. Precipitation with lime is one of the oldest methods applied to spent pickling liquor disposing, still used in many plants [25]. Owing to the chemical reaction of the spent pickling liquor with lime, CaF2, Fe(OH)3 and Cr(OH)3 are formed and become the main compositions of PS. Furthermore, these hydroxides can transform into oxides by heating the PS. Thus, the dried PS (the main components are CaF2, Fe2O3 and Cr2O3) can be obtained. In the glass–ceramics industry, CaF2 is widely used to decrease the crystallization temperature of the parent glass by replacing the strong Si–O linkage with a weak Si–F pair [22, 26, 27]. The presence of Cr2O3 benefits the formation of fine-grained bulk crystallization in the glass matrix [28–30]. Besides, Wang [31] suggested that Fe2O3 has a significant effect on the crystallization of fly ash glass–ceramics, because it leads to phase separation of the glass and decreases the crystallization activation energy. Thus, CaF2, Fe2O3, and Cr2O3 are widely used in the glass–ceramics industry in the form of effective nucleation agents. Furthermore, composite nucleating agents were more effective in some industrial waste glass–ceramics [22]. From the above viewpoint, it is clear that PS is a potential nucleation agent of glass–ceramics. Thus, utilization of PS as a nucleation agent of glass–ceramics is of great interest and significance. However, to the best of our knowledge, there are few researches aiming to reuse PS as a glass–ceramic nucleation agent.
Therefore, the objective of this paper was to study the use of stainless steel PS as a nucleation agent in CaO–MgO–SiO2–Al2O3 system glass–ceramics, based on stainless steel slag (SSS) and cullet, in order to improve the crystallization characteristics and properties. The PS was added in a glass–ceramics formulation maintaining its processing characteristics. The performance of different PS content glass–ceramics was measured in order to estimate the effect of the PS addition in the crystallization behavior and microstructure transition of the glass–ceramics samples. Moreover, to assess the safety and reliability of this reusing method, the obtained glass–ceramics were tested for heavy metals leaching.
2 Experimental
2.1 Raw materials
Pickling sludge (obtained from Baosteel Group Co., Ltd., Shanghai, China) was dried at 300 °C for 3 h to complete dehydration. Then it was ball-milled for 1 h and passed through 40-mesh sieve for complete mixing. Cullet was obtained by grinding down the waste glass from common soda glass and passing through 40-mesh sieves. Typical SSS (obtained from Qingshan Jinhui Stainless Steel Industry Co., Ltd., Henan, China) was completely mixed by ball-milling for 1 h and passed through 40-mesh sieves. Table 1 shows the details of PS, SSS and the cullet chemical composition, obtained by X-ray fluorescence (XRF, XRF-1800, shimadzu).
2.2 Glass–ceramics preparation
Glass–ceramics were prepared from SSS, cullet and PS without any additives. Four mixtures of SSS and cullet (SSS/cullet mass ratio of 0.78:1.00) were obtained with PS addition of 0 wt% (GC-0, standard), 7 wt% (GC-1), 14 wt% (GC-2) and 21 wt% (GC-3). These mixtures were mixed by dry ball-milling for 2 h. Then, the mixed powders were melted in an alumina crucible at 1460 °C for 2 h in a muffle furnace. After melting, one drop of the melt was poured in water and rapidly cooled down to room temperature. As-quenched products were pulverized and characterized by differential scanning calorimetry (DSC) to obtain the glass transition temperature (T g) and the position of the exothermic peaks to guide the following heat treatment process.
The rest melt was poured on a piece of steel plate preheated to 600 °C and held for 0.5 h for residual stress relaxation. Then, the “single-stage” isothermal heat treatment process was applied to all parent glass. In this study, exothermal peak temperature (T p) was chosen as the heat treatment temperature for each sample, held for 1 h. Finally, the samples were cooled slowly to room temperature to obtain glass–ceramics.
2.3 Characterization techniques
Differential scanning calorimetry (DSC) scans were obtained with a NETZSCH STA 409 C/CD thermal analyzer in argon. The reference material was α-Al2O3 powder, and the parent glass powder samples were heated from room temperature to 1000 °C at a heating rate of 10 °C·min−1. The crystalline phases in the glass–ceramics samples were identified from X-ray diffractometer (XRD, Philips APD-10, 40-kV and 150-mA, monochromatic Cu Kα radiation) at a scan rate of 10 (°)·min−1.
The fracture surfaces of glass–ceramics samples were examined by scanning electron microscopy (SEM, Carl Zeiss EVO 18) working in the secondary electron detector mode, at 10-kV acceleration voltage. The relative density was tested using conventional liquid displacement method, according to the Archimedes principle. Water absorption rate was measured as a percentage of weight increment. The Vickers hardness was measured according to the ASTM standard (ASTM E384-11, 2011). Samples were ground and polished with diamond paste. The maximum load of 2 N was applied, and the time of indentation was fixed at 10 s.
The leaching amounts of heavy metals (Cr, Ni, Cu, Mn) were tested by means of leaching experiment, according to toxicity characteristic leaching procedure (TCLP) test (EPA1311, 1992), with a pH-2.9 acid solution (extraction fluid 2) as the leaching fluid. Each leaching vial was filled with 100 ml TCLP extraction fluid and 5 g powder. The leaching vials were rotated end-over-end at 30 r·min−1 for 18 h. To simulate long-term leaching potential of toxic metals regulated by USA government, the PS was subjected to multiple TCLP extractions four times. The concentrations of leached heavy metals were measured using inductive coupled plasma analyzer (iCPA 6300, Thermo Fisher Scientific America, USA).
3 Results and discussion
3.1 Extraction toxicity of heavy metals in PS
The PS was obtained from the open-air depot (Fig. 1a), and the color of the PS is tawny (Fig. 1b). The major constituents of PS are nucleation agent compounds (CaF2, Fe2O3 and Cr2O3) of glass–ceramics. The total content of these compounds reaches 76.23 wt%, demonstrating the potential application in glass–ceramics preparation. However, the Cr2O3 and NiO contents detected in PS reach up to 5.01 wt% and 1.84 wt%, respectively, as shown in Table 1. Four sequential TCLP extractions were carried on the PS to evaluate its leaching characteristics for heavy metals. The leaching results are collected in Fig. 1c. The leaching concentration of Cr (117 mg·L−1) in the first TCLP test is more than 23 times US EPA limitation of 5 mg·L−1. The 101 mg·L−1 Ni leachate concentration of the first leaching is 20 times the limitation of 5 mg·L−1. The leaching concentration of Cr, after four sequential TCLP extractions, of 19 mg·L−1 still exceeds the limits of the TCLP standards by a factor of four. Thus, it is urgent to dispose this tawny PS properly, rather than open-air storage, like in Fig. 1a.
3.2 Crystallization behavior of PS-doped glass–ceramics
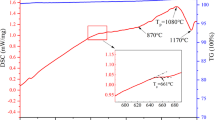
DSC curves for the four glass samples with particle sizes of 75 μm obtained at a heating rate of 10 °C·min−1 are shown in Fig. 2. It can be seen from Fig. 2 that there is only one exothermal peak detected in GC-0 through GC-2. The crystallization peak temperature decreases from 889.8 to 779 °C with more PS additions. The PS is abundant in CaF2, as seen in Table 1. The fluorine from PS may act as a network breaker in glass network by replacing strong Si–O bonds with Si–F bonds, resulting in the decrease of crystallization temperature in the glass [32]. Banijarmali et al. [26] suggested that increasing fluorine content reduced the crystallization peak temperature via acceleration of the diffusion rate of the crystalline phase constituent. The crystallization peak temperature increases by 2 °C from GC-2 to GC-3, indicating that adding 14 wt% PS is enough to reduce the crystallization temperature. Additionally, there is another exothermal peak in GC-3, located at 792.7 °C, which may be corresponding to the grain ripening.
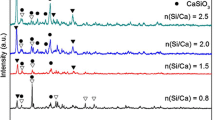
In this study, XRD analysis was utilized to obtain crystal composition of powder glass–ceramics specimens. It can be seen from Fig. 3 that the main crystalline phases change from akermanite to augite by introducing PS into the formula of glass–ceramics. Unlike GC-0, there are no akermanite and sodium aluminum silicate phases detected in GC-1. Nevertheless, cuspidine and nepheline phases appear in XRD patterns with PS content increasing. These results suggest that PS plays an important role in the crystals formation process.
Khater [22] suggested that the glass network structure can be weakened by replacing Si–O band with Si–F. Thus, the solid solubility of the impurity ions, such as sodium, potassium and calcium, is improved in the residual glass phase, confirmed by the disappearance of akermanite and sodium aluminum silicate phases in XRD patterns of GC-1 and GC-2 samples. Besides, fluorine does not enter into the crystalline structure, but remains dissolved in the residual glass [32]. The fluorine content in the residual glass increases with higher PS amount. Thus, it aids the crystallization in the residual glass phase, obtaining cuspidine and nepheline phases in return, confirmed by XRD patterns of GC-2 and GC-3 samples.
SEM analysis was conducted to understand the microstructure of glass–ceramics, as shown in Fig. 4. With PS content increasing, the microstructure of the glass–ceramics changes from columnar-shaped crystals (GC-0 with typical crystal microstructure of surface crystallization) to sheet-shaped crystals (GC-1), and afterward to granular crystals (GC-2 with typical crystal morphology of bulk crystallization, 1–2 μm in size, distributed homogeneously in the glass matrix), and finally to non-uniform coarse grains (GC-3 with typical morphology of the abnormal grain growth).
Wang [31] suggested that Fe2O3 doped in glass–ceramics can induce the formation of small amorphous droplets distributed homogeneously in the glass matrix before nucleation. The interfaces between the amorphous droplets and glass matrix phases serve as the heterogeneous nucleation sites for the final crystallization. In terms of Cr2O3, Khater [22] pointed out that it has a similar mechanism with Fe2O3 for inducing nucleation. Besides, increasing fluorine content is responsible for more effective nucleation, probably via weakening the glass network structure and accelerating the diffusion of the crystalline phase constituents [26]. Based on the discussion above, adding more CaF2, Cr2O3 and Fe2O3 (in the PS form) would bring more nucleation sites and promote grain refinement. This is corresponding to the changing trend of the crystalline microstructure in Fig. 4. However, there is excessive growth of the crystalline particles in GC-3 sample. This would be caused by the F− overdose [22]. In summary, PS effectively promotes the nucleation and crystallization processes of the glass–ceramics. Granular crystals with the size of 1–2 μm are formed and distributed uniformly in glass–ceramics with 14 wt% PS.
3.3 Physical and mechanical properties evolution
The relative density, water absorption and Vickers hardness of all specimens were measured to evaluate the physical and mechanical properties of the PS-doped glass–ceramics. The results, summarized in Table 2, indicate that the water adsorption of the glass–ceramics decreases first and then increases with the increase in PS content. The varied tendencies of the density and Vickers hardness of these samples are on the contrary. GC-2 sample shows the highest relative density of 3.07 g·cm−3 and the lowest water absorption of 0.04 wt% with peak value of Vickers hardness of 7580 MPa. Compared with GC-0, adding 14 wt% PS as the nucleation agent of SSS glass–ceramics enhances Vickers hardness by 2690 MPa, decreases water absorption by 1.17 wt% and increases the density by 0.4 g·cm−3. Thus, it is clear that adding PS as the nucleation agent improves the physical and mechanical properties to a great degree. Based on the above results, the GC-2 sample doped with 14 wt% PS is obviously the optimal glass–ceramic product.
3.4 TCLP tests of glass–ceramics
The leaching concentrations of heavy metals, based on the TCLP test, are shown in Table 3. These results indicate that the leaching concentration of heavy metals from the final glass–ceramic products is much lower than the US EPA regulatory standards. Vu et al. [13] suggested that the glass–ceramics have excellent stabilization capacity, because the heavy metals are bonded inside the lattice of the crystalline phase. Generally, the leaching concentrations of heavy metals in the glass–ceramics are all far below the US EPA regulatory limits, meaning that the final products are safe if they are used as construction materials.
4 Conclusion
This study confirms that introducing 14 wt% PS as the nucleation agent decreases the crystallization temperature by 110.8 °C, refines the grain size by forming isometric crystal with 2 μm in size, enhances Vickers hardness by 2690 MPa and decreases water absorption from (1.21 ± 0.10) wt% to (0.04 ± 0.01) wt%. These results indicate that PS can be utilized as a nucleation agent to improve the crystallization and mechanical properties of glass–ceramics. The TCLP test results confirm the safety of this reusing method. Therefore, the described technique of the PS disposal, reusing PS as a nucleation agent of glass–ceramics, is promising and reliable.
References
Singhal A, Tewari VK, Prakash S. Characterization of stainless steel pickling bath sludge and its solidification/stabilization. Build Environ. 2008;43(6):1010.
Rögener F, Sartor M, Bán A, Buchloh D, Reichardt T. Metal recovery from spent stainless steel pickling solutions. Resour Conserv Recl. 2012;60:72.
Singhal A, Prakash S, Tewari VK. Trials on sludge of lime treated spent liquor of pickling unit for use in the cement concrete and its leaching characteristics. Build Environ. 2007;42(1):196.
Regel-Rosocka M. A review on methods of regeneration of spent pickling solutions from steel processing. J Hazard Mater. 2010;177(1–3):57.
Nie ZR, Ma LW, Xi XL. “Complexation-precipitation” metal separation method system and its application in secondary resources. Rare Met. 2014;33(4):369.
Zhou Y, Zhang QM, Luo J, Tang Q, Du J. Crystallization and dielectric properties of lead-free glass-ceramic composites with Gd2O3 addition. Rare Met. 2012;31(3):281.
Tunali A, Ozel E, Turan S. Production and characterisation of granulated frit to achieve anorthite based glass-ceramic glaze. J Eur Ceram Soc. 2015;35(3):1089.
Garcia-Valles M, Avila G, Martinez S, Terradas R, Nogues JM. Heavy metal-rich wastes sequester in mineral phases through a glass-ceramic process. Chemosphere. 2007;68(10):1946.
Yuan SQ, Dong J, Wang C, Wang ZJ. Comprehensive treating copper tailing and nickel residue. Chin J Rare Met. 2014;38(1):108.
Ghosh S, Pal KS, Dandapat N, Ghosh J, Datta S. Glass-ceramic glazes for future generation floor tiles. J Eur Ceram Soc. 2013;33(5):935.
Zhang K, Liu J, Liu W, Yang J. Preparation of glass–ceramics from molten steel slag using liquid–liquid mixing method. Chemosphere. 2011;85(4):689.
Wang ZJ, Ni W, Jia Y, Zhu LP, Huang XY. Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand. J Non-Cryst Solids. 2010;356(31):1554.
Vu DH, Wang KS, Chen JH, Nam BX, Bac BH. Glass–ceramic from mixtures of bottom ash and fly ash. Waste Manag. 2012;32(12):2306.
Cheng TW, Chen YS. On formation of CaO–Al2O3–SiO2 glass-ceramics by vitrification of incinerator fly ash. Chemosphere. 2003;51(9):817.
Kim JM, Kim HS. Temperature-time-mechanical properties of glass-ceramics produced from coal fly ash. J Am Ceram Soc. 2005;88(5):1227.
Zhang WY, Gao H, Xu Y. Sintering and reactive crystal growth of diopside–albite glass–ceramics from waste glass. J Eur Ceram Soc. 2011;31(9):1669.
Yang Z, Lin Q, Xia J, He Y, Liao G, Ke Y. Preparation and crystallization of glass–ceramics derived from iron-rich copper slag. J Alloy Compd. 2013;574:354.
Karpukhina N, Hill RG, Law RV. Crystallisation in oxide glasses—a tutorial review. Chem Soc Rev. 2014;43(7):2174.
Alizadeh P, Yekta BE, Gervei A. Effect of Fe2O3 addition on the sinterability and machinability of glass-ceramics in the system MgO–CaO–SiO2–P2O5. J Eur Ceram Soc. 2004;24(13):3529.
Abdel-Hameed Salwa AM, Elwan RL. Effect of La2O3, CoO, Cr2O3 and MoO3 nucleating agents on crystallization behavior and magnetic properties of ferromagnetic glass-ceramic in the system Fe2O3·CaO·ZnO·SiO2. Mater Res Bull. 2012;47(5):1233.
Mirsaneh M, Reaney IM, James PF, Hatton PV. Effect of CaF2 and CaO substituted for MgO on the phase evolution and mechanical properties of K-fluorrichterite glass ceramics. J Am Ceram Soc. 2006;89(2):587.
Khater GA. Influence of Cr2O3, LiF, CaF2, TiO2 nucleants on the crystallization behavior and microstructure of glass–ceramics based on blast-furnace slag. Ceram Int. 2011;37(7):2193.
Huang SF, Cao P, Li Y, Huang ZH, Gao W. Nucleation and crystallization kinetics of a multicomponent lithium disilicate glass by in situ and real-time synchrotron X-ray diffraction. Cryst Growth Des. 2013;13(9):4031.
Schmidt B, Wolters R, Kaplin J, Schneiker T, Lobo-Recio MA, López F, López-Delgado A, Alguacil FJ. Rinse water regeneration in stainless steel pickling. Desalination. 2007;211(1):64.
Tang B, Yuan LJ, Shi TH, Yu LF, Zhu YC. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical co-precipitation. J Hazard Mater. 2009;63(2):1173.
Banijamali S, Eftekhari Yekta B, Rezaie HR, Marghussian VK. Crystallization and sintering characteristics of CaO–Al2O3–SiO2 glasses in the presence of TiO2, CaF2 and ZrO2. Thermochim Acta. 2009;488(1):60.
Mukherjee DP, Das SK. SiO2–Al2O3–CaO glass-ceramics: effects of CaF2 on crystallization, microstructure and properties. Ceram Int. 2013;39(1):571.
Rezvani M, Eftekhari-Yekta B, Solati-Hashjin M, Marghussian VK. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2–Al2O3–CaO–MgO(R2O) glass–ceramics. Ceram Int. 2005;31(1):75.
Niyompan A, Phumas S, Tipakontitikul R, Tunkasiri T. Phase formation, microstructure and electrical properties of mica glass-ceramics containing Cr2O3 produced by heat treatment. Ceram Int. 2013;39(S1):S427.
Mirhadi B, Mehdikhani B. Crystallization behavior and microstructure of (CaO·ZrO2·SiO2)–Cr2O3 based glasses. J Non-Cryst Solids. 2011;357(22–23):3711.
Wang SM. Effects of Fe on crystallization and properties of a new high infrared radiance glass–ceramics. Environ Sci Technol. 2010;44(12):4816.
Fan CS, Li KC. Production of insulating glass ceramics from thin film transistor-liquid crystal display (TFT-LCD) waste glass and calcium fluoride sludge. J Clean Prod. 2013;57:335.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Nos. 51502014,51472030 and U1360202), the National Key Project of the Scientific and Technical Support Program of China (No. 2012BAC02B01), the National Hi-Tech R&D Program of China (No. 2012AA063202), the Fundamental Research Funds for the Central Universities (No. FRF-TP-15-050A2), and the China Postdoctoral Science Foundation Funded Project (No. 2014M560885).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Zhang, SG., Pan, DA. et al. Treatment method of hazardous pickling sludge by reusing as glass–ceramics nucleation agent. Rare Met. 35, 269–274 (2016). https://doi.org/10.1007/s12598-015-0673-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0673-4