Abstract
In the present study, impulse pressuring diffusion bonding technology (IPDB) was utilized between commercially pure titanium and 304 stainless steel (SS) using pure nickel (Ni) as interlayer metal. The interfacial microstructures of the bonded joints were investigated by scanning electron microscopy (SEM) and energy dispersive spectroscope (EDS) analyses. It is found that with the aid of the Ni interlayer, the interdiffusion and reaction between Ti and SS can be effectively restricted and robust joints can be obtained. Intermetallic compounds (IMCs) including Ti2Ni, TiNi, and TiNi3 are detected at the Ti/Ni interface; however, only Ni–Fe solid solution is found at the Ni/SS interface. The maximum tensile strength of 358 MPa is obtained by IPDB for 90 s and the fracture takes place along the Ti2Ni and TiNi phase upon tensile loading. The existence of cleavage pattern on the fracture surface indicates the brittle nature of the joints.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In resent years, the transition joints between titanium (Ti) and stainless steel (SS) are widely used in aerospace, vessels, nuclear industry, etc. [1, 2]. Titanium and its alloys are characterized by the extraordinary combination of excellent mechanical properties and corrosion resistance. However, the widespread usage of Ti or Ti alloys is limited primarily because of its expensive cost. As a result, it is frequently required to join Ti to other more commonly used structural materials, such as stainless steel, to exploit the individual properties of both variants but concurrently minimize the cost of the components. Previous experiments proved that fusion welding was not applicable in joining Ti to SS due to the formation of brittle intermetallics compounds (IMCs) and chemical segregation and accumulation of residual stress at the joint [3–5]. As a consequence, solid-state joining techniques, such as diffusion bonding and brazing, are generally preferred for the dissimilar joining of Ti to stainless steel, as most of the problems encountered in fusion welding can be effectively avoided, attributing to the absence of melting and re-solidification of both substrates [3, 5, 6].
Previously, diffusion bonding was sufficiently demonstrated to be a feasible approach to join Ti to stainless steel. Literatures report that the joints produced by direct diffusion bonding of Ti and SS reveal poor performance due to the formation of various brittle IMCs, such as σ, Fe2Ti, FeTi, Cr2Ti, λ, Fe2Ti4O, and TiC, in the diffusion bonded joints owing to the poor metallurgical compatibility between Ti and SS [3, 5–7]. It is reported that the maximum tensile strength of only 242.4 MPa, which is rather low compared with that of the base metal, can be obtained at 850 °C for 90 min by the direct diffusion bonding of Ti and SS [8]. Therefore, it is of great interest to further improve the properties of diffusion bonding joints between Ti and SS.
Previous attempts showed that the use of suitable interlayer materials could minimize the formation of brittle IMCs between Ti and SS, and enhance the bonding strength observably [3, 7, 9, 10]. Looking up the phase diagram data, Ni is found to be a desirable intermediate material for the diffusion bonding between Ti and SS. The Ni–Fe binary phase diagram shows that Ni/SS interface is free of any IMCs; however, Ti–Ni binary phase diagram shows that there are some IMCs forming in the Ti/Ni interface. Although Ti–Ni IMCs would remain at the interface when Ni is used as interlayer, it was experimentally demonstrated by He et al. [11] that the Ti–Ni IMCs exhibit better plasticity than the Ti–Fe IMCs. Accordingly, Ni can be considered to be a preferred interlayer to alleviate the detrimental effect of Ti–Fe IMCs on the mechanical performance of diffusion bonded Ti/SS joints.
In spite of the success of diffusion bonding, it is worthwhile to mention that the diffusion bonding consuming 60–180 min is generally required to complete the bonding process [3, 12, 13]. Furthermore, owing to the reactive nature of Ti, sophisticated vacuum condition which is expensive to maintain is mandatory for the bonding. In this regard, it is of great interest to further optimize the bonding circle to shorten the bonding time for the purpose of both productive efficiency and cost saving. Additionally, it is reported that the excessive growth of the interfacial IMCs would significantly deteriorate the joint strength [12]. Thus thermodynamically, a reduction in bonding time which retards the growth of interfacial IMCs would in turn potentially contributes to the bonding strength.
Impulse diffusion bonding (IPDB), firstly developed by the Ukraine Barton Welding Institute, is a novel and well established diffusion bonding technology to achieve successful bonding within a dramatically reduced duration [14]. Moreover, it can restrict and break the oxide film effectively. So, the bonding strength can be possibly improved using this technique. Hence, the IPDB technology was employed in the diffusion bonding between Ti and SS with a pure Ni interlayer in this study in an attempt to obtain diffusion bonded Ti/SS joint with improved strength in a shortened processing time. Detailed microstructural characterization and mechanical assessment were carried out to manifest the feasibility of this technology.
2 Experimental
2.1 Preparation of specimens
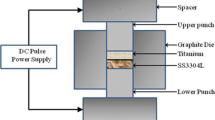
Commercial pure Ti and 304 SS used in this experiment were machined to small cylindrical samples by wire-electrode cutting technique with the dimension of Φ12 mm × 35 mm. Both surfaces of cylindrical samples were grinded by metallographic sandpaper and the mating surfaces were further polished by polishing powder of Al2O3. About 200 μm thick pure nickel foil was used as an intermediate material in this experiment as well. The foil was cut into circle with the same size as the cross section of cylindrical samples, and both surfaces of the foil were grinded by fine SiC sandpaper. Before diffusion bonding, mating surfaces of the samples and both surfaces of the nickel foil were cleaned with acetone in order to eliminate surface contamination.
2.2 Impulse pressuring diffusion bonding process
Diffusion bonding test was conducted on the Gleeble 1500D thermal-simulation experiment machine. The IPDB technology was adopted and bonding pressure was 8–20 MPa along the longitudinal direction of the sample. The experiment was carried out between commercial pure Ti and 304 SS at 850 °C for 60–150 s in vacuum. During the bonding process, the heating rate was 5 °C·s−1, and after bonding, all of the samples were cooled in vacuum.
2.3 Microscopic observation and performance testing
Selected samples were cut along the longitudinal direction and polished by conventional polishing technology. An analysis of detailed microstructure of the interfaces was performed by scanning electron microscope (SEM, TESCAN VEGA II) using back-scattered mode (SEM-BSE). Quantitative chemical analyses of the interfaces were conducted by energy dispersive spectroscope (EDS). The mechanical performance tests of the bonded joints with a gauge diameter of 6 mm and a gauge length of 30 mm were carried out in a tensile testing machine (Instron 1342) at a crosshead speed of 0.5 mm·min−1, at room temperature. Fracture morphology observations were assessed by SEM to analyze the fracture failure form and fracture location.
3 Results and discussion
3.1 Microstructure observation
The SEM-BSE images of the joints with impulse pressuring diffusion bonded at 850 °C for 60, 90, 120, and 150 s under the bonding pressure of 8–20 MPa are presented in Fig. 1. It is observed that the joints bonded for different time are similar to each other. Each interface of the joint is planar in characteristic and many residual Ni foils are observed. Accordingly, the Ni foil with 200 μm in thickness is able to restrict the interdiffusion and reaction between Ti and SS completely. Some reaction layers come up on the Ti/Ni interface; however, only Ni–Fe solid solution is found at the Ni/SS interface. Figure 2 shows the microstructure and linear EDS results of the Ni/SS interface of the bonded joints for 120 s. Gradual changes of the element content near the interface demonstrate that solid solution forms, which indicates that a good metallurgical bonding forms on the interface of Ni interlayer and SS substrate. Further study of Ti/Ni interface at higher magnification and EDS results of the marked areas in the joints are presented in Fig. 3 and Tables 1, 2, 3 and 4, respectively. The micrographs exhibit some different regions on Ti/Ni interface. In the Ti side, Area 1 shows the feather-like structures of Widmanstatten α + β Ti. Ni is a β-stabilizing element, so the diffusion of Ni toward Ti lowers the eutectoid transformation temperature of Ti, leading to the formation of β Ti phase at 850 °C under the phase transformation point (~882 °C) of pure Ti. Therefore, Widmanstatten α + β Ti structure appears from the decomposition of β Ti during cooling [12]. It is found that Area 2, which is adjacent to the interface, is still filled with β Ti, attributing to the higher Ni content.
Several distinct reaction layers are observed in the middle of the interface, and the corresponding EDS results of these areas demonstrate that some IMCs form. For 60 s (Fig. 3a), Area 3 consists of Ti (~67.9 at%) and Ni (bal.). Then, the intermetallic compound of Ti2Ni is proved to form according to the Ti/Ni binary-phase diagram. Similarly, Area 4 consists of Ti (~51.0 at%) and Ni (bal.), and the composition of the two elements indicates the formation of TiNi intermetallic compound. For Area 5, no element other than Ni is detected, so this is the remnant Ni interlayer. Likewise, for 90 s (Fig. 3b), 120 s (Fig. 3c) and 150 s (Fig. 3d), intermetallic compounds from Areas 3 and 4 are also Ti2Ni and TiNi, respectively. The difference lies in the area between TiNi layer and remnant Ni interlayer. It can be seen that one more layer forms. For 90 s, the area contains Ti (~30.2 at%) and Ni (bal.). According to the Ti/Ni binary phase diagram, this is the intermetallic compound of TiNi3. Owing to the insufficient bonding time, the TiNi3 layer does not occur for 60 s.
The thicknesses of all Ti/Ni interfacial reaction products are shown in Table 5. It reveals that the thickness of the layers increases gradually with the increase of bonding time. Among the intermetallic compound layers, the thickness of Ti2Ni changes inconspicuously, while the thickness of TiNi is on the contrary.
3.2 Mechanical properties
The tensile strength of the IPDB joints at room temperature with the change of bonding time is given in Fig. 4. The lowest tensile strength value of ~330 MPa is got for 60 s, precisely because of the inadequate bonding time. Optimized tensile strength value of ~358 MPa is obtained for the joints bonded for 90 s. In that case, with the increase of bonding time, the strength value begins to increase and finally remains stable due to the increase of thickness of the IMCs. In general, it is not evident for the changes of the strength data, owing to the inconspicuous variation of the microstructures.
3.3 Fracture morphology observation
The fracture surface morphologies of the IPDB joints bonded for different time are shown in Fig. 5. It can be seen that all of the three images are much alike in characteristic. The fracture morphologies are composed of stream like cleavage patterns, which indicates that the brittle fracture takes place upon tensile loading. Figure 6 shows the XRD patterns from the fracture surface of the joints. IMCs of Ti2Ni and TiNi are detected on the fracture surface. In other words, fracture occurs along the Ti2Ni and TiNi phases in a brittle manner. Just because of the formation of these brittle IMCs, the tensile strength of the joints is affected seriously.
3.4 Discussion
The primary purpose of the current investigation is to achieve successful Ti/SS dissimilar diffusion bonding in a significantly reduced duration. It is well known that for diffusion bonding, the most important parameters, i.e., bonding temperature, pressure, and holding time, are not independent with each other. At a given temperature, the holding time required to complete the bonding is a function of the applied pressure. A higher pressure would preferentially result in a reduced time required.
In one of the preliminary study, the impulse pressuring diffusion bonding is validated to be a feasible approach to join TA17 alloy to stainless steel within the duration of only 180 s, which is dramatically shorter than the conventional ones (60–180 min) [3, 12–15]. The reduction in bonding time is ascribed to the higher pressure applied which promotes the intimate contact of the faying surfaces. Integrated joints are obtained and the relative deformation of the substrate is acceptable despite the much higher pressure applied. The limited macroscopic deformation of the joint can be attributed to the short loading cycle that the substrate experiences. However, owing to the presence of Ti–Fe IMCs at the interface, the joint strength is rather low compared with the base metal. In the present study, a Ni interlayer is intended to eliminate the Ti–Fe IMCs. In addition, the application of Ni with relative low flow stress might further reduce the pressuring required to achieve intimate contact of the mating surfaces. It is experimentally verified that the Ni interlayer can effectively eradicate the formation of Ti–Fe IMCs and the improved strength can be achieved.
In addition, it is found that the impulse pressuring diffusion bonded joint exhibits higher tensile strength than the counterparts bonded by conventional diffusion bonding technique. It was previously reported that the maximum tensile strength of diffusion bonded commercially pure Ti and 304SS with Ni interlayer was 311 MPa which is ~40 MPa lower than that of the present study [3]. The improvement in bonding strength can be attributed to the retardation of the excessive growth of the IMCs formed at the Ti/Ni interface. However, it should be mentioned that although the bonding strength can be improved in certain extent by alleviating the effect of IMCs via impulse pressuring diffusion bonding, the bonding strength is still limited by the brittle IMCs. For further improving the bonding characteristics, completely eliminating the brittle interfacial metallic compounds may be required.
4 Conclusion
Impulse pressuring diffusion bonding between commercial pure Ti and 304 SS using 200 μm thick pure Ni foil as an interlayer was conducted at 850 °C for 60, 90, 120, and 150 s under the bonding pressure of 8–20 MPa in vacuum. Successful bonding of Ti to SS can be achieved within a greatly shortened duration of 90 s by IPDB in combination with Ni interlayer. Thus, the formed joints are composed of Ti–Ni IMCs at the Ti–Ni interface and the remnant Ni interlayer, and Ni–Fe solid solution at the Ni/SS interface. Cleavage patterns are found in the fracture surfaces, indicating that the joints are brittle in nature.
References
Boyer R, Welsch G, Collings EW. Materials Properties Handbook: Titanium Alloy. 4th ed. Ohio: Materials Park; 2007. 1.
Boyer RR. An overview on the use of titanium in the aerospace industry. Mater Sci Eng A. 1996;213(1–2):103.
Kundu S, Chatterjee S, Olson D, Mishra B. Effects of intermetallic phases on the bond strength of diffusion-bonded joints between titanium and 304 stainless steel using nickel interlayer. Metall Mater Trans A. 2007;38(9):2053.
Lee MK, Lee JG, Choi YH, Kim DW, Rhee CK, Lee YB, Hong SJ. Interlayer engineering for dissimilar bonding of titanium to stainless steel. Mater Lett. 2010;64(9):1105.
Ghosh M, Chatterjee S, Ghosh M, Chatterjee S. Diffusion bonded transition joints of titanium to stainless steel with improved properties. Mater Sci Eng A. 2003;358(1–2):152.
Gu Y, Chao YS, Zhang YH. Soft magnetic properties of amorphous Fe52Co3 4Hf7B6Cu1 alloy treated by pulsed magnetic field and annealing. Chin Phys B. 2012;21(12):127805.
Kundu S, Chatterjee S, Olson D, Mishra B. Interface microstructure and strength properties of the diffusion-bonded joints of titanium/Cu interlayer/stainless steel. Metall Mater Trans A. 2008;39(9):2106.
Ghosh M, Das S, Banarjee PS, Chatterjee S. Variation in the reaction zone and its effects on the strength of diffusion bonded titanium-stainless steel couple. Mater Sci Eng A. 2005;390(1–2):217.
Li P, Li JL, Xiong JT, Zhang FS, Raza SH. Diffusion bonding titanium to stainless steel using Nb/Cu/Ni multi-interlayer. Mater Charact. 2012;68(6):82.
Kundu S, Chatterjee S. Interface microstructure and strength properties of the diffusion bonded joints of titanium-Al interlayer-18Cr-8Ni stainless steel. Mater Sci Eng A. 2010;527(10–11):2714.
He P, Zhang JH, Zhou RL, Li XQ. Diffusion bonding technology of a titanium alloy to a stainless steel web with a Ni interlayer. Mater Charact. 1999;43(5):287.
Kundu S, Chatterjee S. Interfacial microstructure and mechanical properties of diffusion-bonded titanium-stainless steel joints using a nickel interlayer. Mater Sci Eng A. 2006;425(1–2):107.
Kundu S, Chatterjee S. Characterization of diffusion bonded joint between titanium and 304 stainless steel using a Ni interlayer. Mater Charact. 2008;59(5):631.
Dai GQ, Qu WQ, Zhuang HS. Structure performance and diffusion mechanism of aluminum alloy heat pipe low-temperature diffusion brazing joints. Chin J Rare Metals. 2013;37(6):851.
Yuan XJ, Sheng GM, Qin B, Huang WZ, Zhou B. Impulse pressuring diffusion bonding of titanium alloy to stainless steel. Mater Charact. 2008;59(7):930.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 50675234).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, FL., Sheng, GM. & Deng, YQ. Impulse pressuring diffusion bonding of titanium to 304 stainless steel using pure Ni interlayer. Rare Met. 35, 331–336 (2016). https://doi.org/10.1007/s12598-014-0368-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0368-2