Abstract
Background
In quantifying left ventricular (LV) diameter, which position for echocardiographic measurements, mitral valve tip level (MV-tip) or LV mid level (LV-mid), more accurately represents the LV volume is unclear. Furthermore, which factor affects the measurement error also has not been elucidated.
Methods
We enrolled 150 patients without myocardial infarction and local asynergy who underwent echocardiography and cardiac magnetic resonance imaging (CMRI). Echocardiographic LV diastolic diameter (LVDD) and LV systolic diameter (LVDS) were measured at both MV-tip and LV-mid, and the LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were quantified using CMRI. We quantified the degree of aortic wedging as the angle between the anterior wall of the aorta and the ventricular septal surface (ASA).
Results
The average LVDD was smaller and average LVDS larger when measured at the MV-tip than at the LV-mid. In regression analyses, the correlation coefficient between LVDD and LVEDV was larger at LV-mid (R = 0.89) than at MV-tip (R = 0.82), and the correlation coefficient between LVDS and LVESV also larger at LV-mid (R = 0.93) than MV-tip (R = 0.87). ASA, Valsalva diameter, left atrial diameter, patient height, and LV mass significantly affected the echocardiographic measurement error, but no factor affected the measurement error when quantifying LVDD at the LV-mid level.
Conclusions
The echocardiographic LV diameter measured at LV-mid has a stronger correlation with LV chamber size derived from CMRI than measurements at MV-tip. The LVDD measured at the LV-mid level is not affected by other factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) diastolic diameter (LVDD) and LV systolic diameter (LVDS) derived from transthoracic echocardiography are crucial information in the diagnosis of LV dysfunction, estimating the prognosis of patients with heart diseases [1,2,3,4,5,6,7], and the surgical indications in valvular heart disease [8]. LVDD and LVDS are used as simplified substitutions for LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV). The guidelines of the American Society of Echocardiography recommend measuring LVDD and LVDS at the level of the tip of the mitral valve (MV-tip) [9]. In the case of ventricular sigmoid septum, the guidelines recommend assessing the diameter at one point distal from the discrete upper septal thickening [10]. However, whether the measurement of LV diameter at the MV-tip level is inappropriate in sigmoid septum has not been confirmed, or the appropriate point to measure LV diameter in the sigmoid septum and the definition of sigmoid septum. Furthermore, we sometimes experience other inappropriate shapes for measuring the LV diameter at the MV-tip level, such as a round LV and hamstring of the posterior wall of the left atrium. Including these cases, the proper position for echocardiographic quantification of the LV diameter seems debatable, possibly suggesting that the middle level of the LV (LV-mid) is better than the MV-tip level [11]. Therefore, the purpose of the current study was to investigate which level, MV-tip or LV-mid, more accurately represents the LV volume in echocardiographic quantification of LVDD and LVDS and which factors affected the measurement error in quantifying LV diameter.
Materials and methods
Study population
We retrospectively screened 168 consecutive patients who underwent cardiac magnetic resonance imaging (CMRI) between January 2019 and October 2020 at Nagoya City University Hospital. Of these patients, we excluded 13 who had local asynergy with a history of old myocardial infarction, 3 who underwent new onset decompensated heart failure (1 patient) or cardiac-specific therapy (2 ablations for atrial fibrillation) between echocardiography and CMRI, and 2 who did not undergo echocardiographic examination at our institution. Finally, 150 patients were eligible for the current study (Fig. 1). This retrospective study was approved by the Institutional Review Board of Nagoya City University and carried out following the principles of the Helsinki Declaration. The institutional review board waived directly informed consent for this retrospective study. The information is available on the Nagoya City University website, and the patients had the opportunity to withdraw from the study.
Echocardiographic measurements
A cardiologist (T.N.) measured LVDD and LVDS using the parasternal long-axis view at both the MV-tip level and LV-mid level. The LV-mid level was defined as the largest LV diameter level within 10 mm of the papillary muscle level in the end-diastolic phase (Fig. 2). Valsalva diameter and left atrial diameter were measured according to the American Society of Echocardiography’s guidelines [9]. We quantified the degree of aortic wedging as an alternative for sigmoid septum as the angle between the anterior wall of the aorta and the ventricular septal surface (aorto-septal angle; ASA) (Fig. 2) [12, 13].
Representative images of the echocardiographic measurements of LV diameter at the MV-tip level (yellow arrows) and LV-mid level (blue arrows) (a), left atrial diameter (b), and valsalva diameter (c). Representatives of the measurements of each level of diameter in patients with normal geometry (d) and aortic wedging (e), and the angle between the anterior wall of the aorta and the ventricular septal surface in patients with normal geometry (f) and aortic wedging (g)
Measurements by CMRI
LVEDV and LVESV were measured by CMRI by a radiologist (K.O.) using the SYNAPSE VINCENT system (Fujifilm Co., Tokyo, Japan) by tracing all endocardial surfaces of the serial short axis sections from the level of the mitral valve to the apex (Fig. 3).
Factors possibly affecting LV measurements
As we quantified the LV measurements using a parasternal axis view, we analyzed the parameters that comprise the parasternal axis view to determine if they affect the measurement error. The frequency of conventionally defined sigmoid septum is too small (1.5–7%) [14, 15] to statistically assess it in the current study. Furthermore, the conventional definition “protruding toward the inside of the LV cavity” is the same as what we quantified for the measurement error. Therefore, we used ASA [12, 13], specifically aorta wedging, for the alternative parameter of the sigmoid septum.
Assessments between LV overload and each LV measurement
LV diameter is the alternative for LV volume, and increased LV volume means decreased net LV contractility [16,17,18] and results in greater wall stress [19,20,21]. As B-type natriuretic peptide (BNP) is one of the markers for LV overload [22], we compared the plasma BNP level and each level of LV diameter to assess whether the LV-mid level diameter, indicating the maximum LV volume, more accurately reflected the hemodynamic LV overload.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and were compared by paired t test. Categorical variables were expressed as numbers and (%) and compared using the Chi-square test.
In the current study, we evaluated the accuracy of each level of echocardiographic measurement using the following methods. First, regression analyses were performed between both levels of echocardiographic LV diameter and MRI-derived LV volume. Second, to assess the dispersion of each regression analysis, we calculated the distance between the MRI-derived volume and the equation curve of the regression analysis (Fig. 4). This distance was calculated as the absolute value of the difference (|MRI-derived volume \(-\) echocardiographically estimated volume|). We did not intend to estimate LV volume by echocardiography. The equation curve and echocardiographically estimated volume were only used to assess the dispersion of the association between MRI-derived LV volume and each level of the LV diameter. Therefore, the regression equations used in this study cannot be used to estimate LV volume in other cohorts. Third, the Bland–Altman plots were used to compare the differences in the two levels of measurement between MRI-derived volume and echocardiographic estimated volume.
The regression analyses were also used to identify the factors influencing the difference between MRI-derived volume and echocardiographic-estimated volume. We chose ASA, Valsalva diameter, and left atrial diameter as possible influencing factors because they contribute to the same surface of the parasternal long-axis view, and chose patient height, weight, BMI, and LV mass as possible factors having a physical impact.
In the regression analyses, the cubic formula was applied when analyzing one-dimensional variables (e.g., diameter and angle-degree) and three-dimensional variables (e.g., volume and weight), and the linear formula was applied when analyzing the same dimensional variables.
We compared the plasma BNP level (logarithmic conversion) and each level of LV diameter to assess which level of the diameter reflected the hemodynamic LV overload. T tests were used to compare between the two dependent correlations.
The inter-rater reliability of LVDD and LVDS at both levels was evaluated by T.N. (cardiologist of 13 years) and Y.S. (cardiologist of 9 years) using the last 32 patients’ data with intraclass correlation coefficients (ICCs). An ICC ≥ 0.80 was considered the preferred level of reliability [23].
Two-sided P < 0.05 was considered significant. All analyses were performed using SPSS ver. 26 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics and echocardiographic measurements at each level
The characteristics and measurements of the 150 patients enrolled in the present study are summarized in Table 1. The average age and body mass index were 60.5 years and 22.6 kg/m2. The average LVDD was smaller and average LVDS larger at the MV-tip level than at the LV-mid level. The mean date interval between echocardiographic examination and CMRI was 8 days (interquartile range, 2–45 days).
Comparison between each echocardiographic LV diameter and CMRI volume
The regression plots of echocardiographic LVDD and LVDS at each level and CMRI-derived LVEDV and LVESV are presented in Fig. 5. The correlation coefficient between LVDD and LVEDV was larger at the LV-mid level (R = 0.89, F = 184.00) than the MV-tip level (R = 0.82, F = 154.62), and the correlation coefficient between LVDS and LVESV was also larger at the LV-mid level (R = 0.93, F = 293.92) than at the MV-tip level (R = 0.87, F = 153.90).
The mean absolute values of the difference between the CMRI-derived volume and echocardiographic-estimated volume are presented in Table 2. For both LVEDV and LVESV, the absolute values of the difference were significantly smaller at the LV-mid level than the MV-tip level (LVEDV, 24.5 ± 19.0 vs. 29.2 ± 25.1, P = 0.014; LVESV, 19.6 ± 17.9 vs. 24.4 ± 24.5, P = 0.004). The Bland–Altman plots showed that both LVEDV and LVESV had a narrower 95% confidence interval at the LV-mid level than the MV-tip level (Fig. 6).
Factors influencing the difference between MRI-derived volume and echocardiographic-estimated volume
The correlations between each possibly influential factor (ASA, Valsalva diameter, left atrial diameter, patient height, body weight, body mass index, and LV mass) and each absolute value of the difference between MRI-derived volume and echocardiographic-estimated volume are summarized in Table 3 (regression plots are presented in Figs. 7, 8, 9, 10, 11, 12, 13). When LV diameter was measured at MV-tip level, four factors (ASA, left atrial diameter, patient height, and LV mass) significantly correlated with the absolute value of the difference between MRI-derived LVEDV and echocardiographic-estimated LVEDV, and four factors (ASA, Valsalva diameter, patient height, and LV mass) significantly correlated with the absolute value of the difference between MRI-derived LVESV and echocardiographic-estimated LVESV. However, when LV diameter was measured at LV-mid level, no factor was associated with the absolute value of the difference between MRI-derived LVEDV and echocardiographic-derived LVEDV, but four factors (ASA, Valsalva diameter, patient height, and LV mass) significantly correlated with the absolute value of the difference between MRI-derived LVESV and echocardiographic-estimated LVESV.
Each level of echocardiographic LV diameter and BNP level
The correlation coefficients between each level of LV diameter and Log BNP are presented in Table 4. Log BNP had greater R values in LVDD and LVDS at the LV-mid level than the MV-tip level (LVDD, 0.29 vs. 0.23; LVDS, 0.41 vs. 0.36). The differences between R values were not significant (LVDD-Log BNP, P = 0.12, T = 1.56; LVDS-Log BNP, P = 0.052, T = 1.96).
The inter-rater reliability for measuring LV diameter at each level
The ICCs for inter-rater reliability of LVDD and LVDS were 0.99 (0.98–1.00) and 0.98 (0.92–0.99) at the MV-tip level and 0.95 (0.91–0.98) and 0.99 (0.97–0.99) at the LV-mid level, respectively (Table 5).
Discussion
We demonstrated that the LV diameter measured at the LV-mid level represents the LV volume more accurately than when measured at the MV-tip level. We also verified that ASA, Valsalva diameter, left atrial diameter, patient height, and LV mass affected the measurement error; however, none of these factors affected the measurement error only when quantifying LV diameter at LV-mid level. We confirmed a high level of inter-rater reliability in measuring LVDD and LVDS at the LV-mid level, as well as the MV-tip level. As the LV diameter is easy to measure and is one of the most crucial parameters for a patient’s prognosis and therapeutic strategy, we should always consider whether the other method is better than the current one. However, LV measurement at the MV-tip level does not always reflect the maximum LV volume, but the MV-tip level was used in recent research and the current guidelines because of its clear definition and good reproducibility. Therefore, we should use the LV diameter at the MV-tip level in current clinical practice until the LV measurement method is updated.
Morphological reasons for misestimating LV diameter
The presence of the sigmoid septum also results in an underestimation of the LV diameter at the MV-tip level. We could share experiences with many physicians in which the quantification of LV diameter by transthoracic echocardiography at the MV-tip level often seems incorrect in patients with sigmoid septum. Sigmoid septum is considered to be associated with aortic atherosclerosis, hypertension, or thickening and calcification of the aortic or mitral valve [24,25,26], which are known changes with age that lead to worse prognosis. We quantified ASA as an alternative for sigmoid septum. ASA was an exactly representative parameter of aortic wedging, which is an age-related change with aortic calcification and elongation [27,28,29]. Valsalva dilatation, as well as aortic root dilatation, is associated with aortic atherosclerosis and contributes to aortic wedging and sigmoid septum. Furthermore, Valsalva dilatation is a risk factor for poor clinical prognosis [30, 31]. Although the clinical importance of sigmoid septum has not been fully elucidated, recent studies have reported some associations between a smaller ASA and poor clinical outcomes [12, 32]. Accordingly, the LV diameter should not be underestimated in patients with aortic wedging associated with cardiovascular risks.
We also tried to investigate the conventionally defined sigmoid septum. Previous studies used the ratio of basal to mid-interventricular septal diameter (B-M ratio) to diagnose sigmoid septum. However, the frequency of diagnosed sigmoid septum was small in those studies: 1.5% met the criteria of an upper septal thickness ≥ 14 mm and a B–M ratio ≥ 1.3 [14], or 7% met the criteria of an upper septal thickness ≥ 13 mm in men or ≥ 12 mm in women and a B–M ratio ≥ 1.5 [15]. In our cohort, only 2.7% (n = 4) and 1.3% (n = 2) of patients fulfilled the above criteria, and it was difficult to assess its impact statistically. When we compared the average absolute values of the difference (|MRI-derived volume – echocardiographically estimated volume|) with and without sigmoid septum, no significant differences were observed (P = 0.26 for the former criteria, P = 0.16 for the latter criteria).
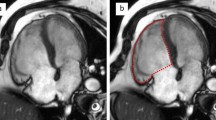
Furthermore, hamstring of the posterior wall of the left atrium, which is usually accompanied by chronic atrial fibrillation and giant left atrium, could cause underestimation of the LV diameter at the MreV-tip level because the basal LV posterior wall is placed inward to the inside of the LV by the extended left atrium [33, 34]. The presence of the left atrial dilatation [35, 36], as well as secondary atrial functional mitral regurgitation [37,38,39], has been recognized as an independent risk factor for poor cardiovascular outcomes. In addition, a round-shaped LV is sometimes observed in non-ischemic and ischemic cardiomyopathy, and LV diameter seems to be shorter at the MV-tip level than that at the LV-mid level in patients with a round LV. LV dilatation at the LV-mid level could also cause mitral valve tethering, leading to secondary mitral regurgitation. Thus, underestimating the LV diameter in these patients could also mislead the prognosis (Fig. 14).
The representative images of round-shaped LV (a) and hamstring of the posterior wall of the LA (b). LV diameter at the MV-tip level seems smaller than that at the LV-mid level in patients with round-shaped LV (a). The giant LA lifts up the LV basal-posterior wall, resulting in a smaller measurement of LV diameter at the MV-tip level in patients with hamstring of the posterior wall of the LA (b)
In our cohort, greater patient height was associated with more significant measurement errors at both levels of LVDD and MV-tip level of LVDS. Patient height was associated with LV size as typified by MRI-LVEDV (Fig. 15), and LVEDV significantly correlated with the difference between MRI-derived volume and echocardiographic-estimated volume (Fig. 16). We suggest that these correlations can explain the mechanisms by which the patient’s height influenced the measurement error.
We also investigated the difference between MRI-derived volume and Teichholz-calculated volume [40]. The mean absolute value of the difference in LVEDV was significantly smaller at the LV-mid level than the MV-tip level (LVEDV, 30.5 ± 22.7 vs. 39.5 ± 32.1, P < 0.001; LVESV, 24.0 ± 21.3 vs. 26.7 ± 29.0, P = 0.097) (Table 6). The correlations between each possibly influential factor and each absolute value of the difference between MRI-derived volume and Teichholz-calculated volume are presented in Table 7. ASA affected the measurement error when quantifying LV diameter at MV-tip level, and greater LV mass was associated with a larger measurement error in general.
Misestimation of the LV diameter may not be due to a single reason; however, we should be aware of these underlying morphological mechanisms because many of them unfavorably impact a patient’s prognosis. On the contrary, the measurement of LVDD at the LV-mid level does not seem to be affected by morphological changes such as those given above.
Hemodynamical meaning of quantifying LV size
LVEDV is the preload that works to produce the LV stroke volume. When the net LV contractility is reduced, the chamber size of the LV usually increases according to the Frank–Starling law to maintain the LV output enough for the systemic circulation [16,17,18]. On the other hand, increased preload can be mechanically forced by regurgitative valvular diseases and shunt diseases. Increased LVEDV should provoke increased LVESV and pressure associated with arterio-LV coupling, resulting in decreased energy efficiency [41]. Furthermore, the greater the LV chamber size, the more wall stress to which the LV is exposed according to Laplace’s law [19,20,21]. The primary reason for quantifying LV chamber size is to estimate the net LV contractility and/or load; however, LV diameter at the MV-tip level may be fixed by the aortic and mitral valvular annulus and pushed inward by an elongated ascending aorta with atherosclerosis, especially in cases with sigmoid septum. In the current study, though the differences were not significant (LVDD-Log BNP, P = 0.12, T = 1.56; LVDS-Log BNP, P = 0.052, T = 1.96), the correlation coefficients between Log-converted plasma BNP level and LVDD and LVDS were higher at the LV-mid level than the MV-tip level (Table 4), suggesting that the LV diameter at LV-mid level represented LV load more accurately. As the statistical difference was close to reaching significance, it is expected that a larger cohort will prove this.
Limitations
The current study has several limitations. The sample size was small, and this study was performed retrospectively at a single center. Although we used regression equations to assess the dispersion of each difference between MRI-derived volume and echocardiographic-estimated volume, we must note that the regression equations are not available in the other cohort due to the possibility of overfitting because the model was not evaluated in an external cohort. Myocardial infarction was an exclusion criterion, but all patients required CMRI, suggesting that the clinical presentations of enrolled patients were relatively suspicious of cardiomyopathy. Therefore, we could not apply the current results to the healthy population and patients with myocardial infarction. We also could not report the results in the normal population because this was a retrospective study and CMRI was performed in patients who needed CMRI clinically. The posture differences between CMRI and echocardiography may affect the results in some types of cardiac disease [42,43,44,45,46]. The impact of posture change on transthoracic echocardiographic LV measurements should be investigated by combining echocardiography with CMRI. The variable date interval between the date of echocardiographic examination and CMRI is another limitation. A prospective observational study with a large cohort undergoing echocardiography and CMRI on the same day in various cardiac diseases and normal populations at multiple centers is needed to confirm the theory this study proposed. Finally, the current research aimed to advocate a better LV measurement position for future research and guidelines; we should quantify LV diameter at the MV-tip level when referring to the latest guidelines in current clinical practice.
Conclusion
The echocardiographic LV diameter measured at the LV-mid level had a stronger correlation with LV chamber size derived from CMRI than measurements at the MV-tip level. ASA, Valsalva diameter, left atrial diameter, patient height, and LV mass affected the measurement error, whereas LVDD at LV-mid level was not influenced by these factors.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Nestico PF, Hakki AH, Iskandrian AS. Left ventricular dilatation. Prognostic value in severe left ventricular dysfunction secondary to coronary artery disease. Chest. 1985;88:215–20.
White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51.
Lauer MS, Evans JC, Levy D. Prognostic implications of subclinical left ventricular dilatation and systolic dysfunction in men free of overt cardiovascular disease (the Framingham Heart Study). Am J Cardiol. 1992;70:1180–4.
Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5.
Yeboah J, Bluemke DA, Hundley WG, Rodriguez CJ, Lima JA, Herrington DM. Left ventricular dilation and incident congestive heart failure in asymptomatic adults without cardiovascular disease: multi-ethnic study of atherosclerosis (MESA). J Card Fail. 2014;20:905–11.
Ito K, Li S, Homma S, Thompson JLP, Buchsbaum R, Matsumoto K, et al. Left ventricular dimensions and cardiovascular outcomes in systolic heart failure: the WARCEF trial. ESC Heart Fail. 2021;8:4997–5009.
Kato M, Kitada S, Kawada Y, Nakasuka K, Kikuchi S, Seo Y, et al. Left ventricular end-systolic volume is a reliable predictor of new-onset heart failure with preserved left ventricular ejection fraction. Cardiol Res Pract. 2020;2020:3106012.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–227.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64.
Chetrit M, Roujol S, Picard MH, Timmins L, Manning WJ, Rudski LG, et al. Optimal technique for measurement of linear left ventricular dimensions. J Am Soc Echocardiogr. 2019;32:476-83.e1.
Nakayama T, Oshima Y, Shintani Y, Yamamoto J, Yokoi M, Ito T, et al. Ventricular sigmoid septum as a risk factor for anthracycline-induced cancer therapeutics-related cardiac dysfunction in patients with malignant lymphoma. Circ Rep. 2022;4:173–82.
Nakayama T, Yamamoto J, Ozeki T, Tokoroyama S, Mori Y, Hori M, et al. Impact of an angulated aorto-septal relationship on cardio-cerebrovascular outcomes in patients undergoing hemodialysis. PLoS ONE. 2024;19: e0298637.
Diaz T, Pencina MJ, Benjamin EJ, Aragam J, Fuller DL, Pencina KM, et al. Prevalence, clinical correlates, and prognosis of discrete upper septal thickening on echocardiography: the Framingham Heart Study. Echocardiography. 2009;26:247–53.
Canepa M, Malti O, David M, AlGhatrif M, Strait JB, Ameri P, et al. Prevalence, clinical correlates, and functional impact of subaortic ventricular septal bulge (from the Baltimore Longitudinal Study of Aging). Am J Cardiol. 2014;114:796–802.
Chaui-Berlinck JG, Monteiro LHA. Frank-Starling mechanism and short-term adjustment of cardiac flow. J Exp Biol. 2017;220:4391–8.
Kuhtz-Buschbeck JP, Drake-Holland A, Noble MIM, Lohff B, Schaefer J. Rediscovery of Otto Frank’s contribution to science. J Mol Cell Cardiol. 2018;119:96–103.
Sequeira V, van der Velden J. Historical perspective on heart function: the Frank–Starling Law. Biophys Rev. 2015;7:421–47.
Fowler NO. Law of Laplace. N Engl J Med. 1971;285:1087–8.
Sandler H, Dodge HT. Left ventricular tension and stress in man. Circ Res. 1963;13:91–104.
Tsuda T. Clinical assessment of ventricular wall stress in understanding compensatory hypertrophic response and maladaptive ventricular remodeling. J Cardiovasc Dev Dis. 2021;8(10):122.
Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–9.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Ieki K, Imataka K, Sakurai S, Okamoto E, Ashida T, Fujii J. Differentiation of hypertrophic cardiomyopathy and hypertensive cardiac hypertrophy using the patterns of interventricular septum hypertrophy. J Cardiol. 1996;27:309–14.
Chen-Tournoux A, Fifer MA, Picard MH, Hung J. Use of tissue Doppler to distinguish discrete upper ventricular septal hypertrophy from obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:1498–503.
Belenkie I, MacDonald RP, Smith ER. Localized septal hypertrophy: part of the spectrum of hypertrophic cardiomyopathy or an incidental echocardiographic finding? Am Heart J. 1988;115:385–90.
Adriaans BP, Heuts S, Gerretsen S, Cheriex EC, Vos R, Natour E, et al. Aortic elongation part I: the normal aortic ageing process. Heart. 2018;104:1772–7.
Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 1985;2003(95):2591–7.
Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–48.
Covella M, Milan A, Totaro S, Cuspidi C, Re A, Rabbia F, et al. Echocardiographic aortic root dilatation in hypertensive patients: a systematic review and meta-analysis. J Hypertens. 2014;32:1928–35.
Mule G, Nardi E, Morreale M, Castiglia A, Geraci G, Altieri D, et al. The relationship between aortic root size and hypertension: an unsolved conundrum. Adv Exp Med Biol. 2017;956:427–45.
Manea P, Ghiuru R. Correlations between the presence of sigmoid interventricular septum and increased relapse risk of stroke in hypertensive patients. Rev Med Chir Soc Med Nat Iasi. 2013;117:857–62.
Ito K, Abe Y, Takahashi Y, Shimada Y, Fukumoto H, Matsumura Y, et al. Mechanism of atrial functional mitral regurgitation in patients with atrial fibrillation: a study using three-dimensional transesophageal echocardiography. J Cardiol. 2017;70:584–90.
Yamano T, Furukawa K, Matoba S. “Hamstring Leaflet” now demonstrated to be a determinant of functional mitral regurgitation in patients with atrial fibrillation. Circ J. 2016;80:2098–9.
Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006;151:412–8.
Losi MA, Izzo R, Canciello G, Giamundo A, Manzi MV, Strisciuglio T, et al. Atrial dilatation development in hypertensive treated patients: the Campania-Salute network. Am J Hypertens. 2016;29:1077–84.
Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, et al. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J. 2018;82:1451–8.
Saito C, Minami Y, Arai K, Haruki S, Yagishita Y, Jujo K, et al. Prevalence, clinical characteristics, and outcome of atrial functional mitral regurgitation in hospitalized heart failure patients with atrial fibrillation. J Cardiol. 2018;72:292–9.
Ito K, Abe Y, Watanabe H, Shimada Y, Shibayama K, Oe H, et al. Prognostic significance of residual functional mitral regurgitation in hospitalized heart failure patients with chronic atrial fibrillation and preserved ejection fraction after medical therapies. J Echocardiogr. 2019;17:197–205.
Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11.
Sunagawa K, Sugimachi M, Todaka K, Kobota T, Hayashida K, Itaya R, et al. Optimal coupling of the left ventricle with the arterial system. Basic Res Cardiol. 1993;88(Suppl 2):75–90.
Nakao S, Come PC, Miller MJ, Momomura S, Sahagian P, Ransil BJ, et al. Effects of supine and lateral positions on cardiac output and intracardiac pressures: an experimental study. Circulation. 1986;73:579–85.
Nakao S, Come PC, McKay RG, Ransil BJ. Effects of positional changes on inferior vena caval size and dynamics and correlations with right-sided cardiac pressure. Am J Cardiol. 1987;59:125–32.
Kandels J, Metze M, Hagendorff A, Marshall RP, Hepp P, Laufs U, et al. The impact of upright posture on left ventricular deformation in athletes. Int J Cardiovasc Imaging. 2023;39:1123–31.
Giustiniano E, Palma S, Meco M, Ripani U, Nisi F. Echocardiography in prone positioned critically ill patients: a wealth of information from a single view. Diagnostics (Basel). 2022;12(6):1460.
Taha HS, Mohamed AM, Mahrous HA, Shaker MM, Alsayed OS, Sayed HG, et al. Correlation of echocardiographic parameters in prone and supine positions in normal adults using a novel approach. Echocardiography. 2021;38:892–900.
Author information
Authors and Affiliations
Contributions
Conceptualization: Takafumi Nakayama and Yoshihiro Seo; methodology: Takafumi Nakayama, Masashi Yokoi, Toshihiko Goto, Hiroya Hashimoto, and Yoshihiro Seo; data collection: Takafumi Nakayama, Yasuhiro Shintani, Yukako Oishi, and Kengo Ohta; formal analysis and investigation: Takafumi Nakayama, Hiroya Hashimoto, and Yoshihiro Seo; writing—original draft preparation: Takafumi Nakayama; writing—review and editing: Takafumi Nakayama, Yukako Oishi, Noriyuki Ikehara, Masato Sugiura, Hiroya Hashimoto, Kazuhiro Yajima, and Yoshihiro Seo; supervision: Hiroya Hashimoto, Kazuhiro Yajima, and Yoshihiro Seo.
Corresponding author
Ethics declarations
Conflict of interest
Takafumi Nakayama, Yasuhiro Shintani, Masashi Yokoi, Toshihiko Goto, Yukako Oishi, Noriyuki Ikehara, Masato Sugiura, Kengo Ohta, Hiroya Hashimoto, Kazuhiro Yajima, and Yoshihiro Seo declare that they have no conflict of interest. This study was also not supported by any grant, individual, firm, or organization. Yoshihiro Seo is a vice-chairman of the Japanese Society of Echocardiography.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was done at Department of Cardiology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakayama, T., Shintani, Y., Yokoi, M. et al. The preferable position for quantifying left ventricular diameter by transthoracic echocardiography. J Echocardiogr (2024). https://doi.org/10.1007/s12574-024-00658-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12574-024-00658-8