Abstract
Four different sizes of Indian high ash coal and char are investigated. A simultaneous thermal analyzer and mass spectrometer is used for the characterization of the coal and char samples and the identification of the volatiles evolved during the heating of the sample upto 1,050 °C under combustion cum gasification related conditions. The TG and DTA results are discussed for the investigations under air, oxygen, steam and blended gas atmospheres. The thermogravimetry—mass spectrum profile of the coal provides information on combustion performance (ignition, peak combustion and burnout temperatures) and on chemical changes to the volatile matter (H2, O, CO and CO2), char and minerals. The size effects of the coal and char during pyrolysis, combustion and gasification are discussed. The appropriate temperature ranges to the high ash coal gasification in the steam and steam blended gases are evaluated. The Arrhenius model is applied to determine the kinetic parameters from TG/DTG curves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coal gasification is considered as an effective way for clean use of coal. Coal conversion via gasification is significantly considered to the world’s primary energy supply and global electricity production [1]. Coal is also playing an increasingly important role in the production of liquid fuels and chemicals [2], and is the fastest growing energy resource in the world with the 826 billion tons of proven reserves [3] expected to last for upto 200 years compared to about 65 and 40 years for natural gas and crude oil respectively [4]. However, the increase in coal utilization results to the growing environmental concern due to greenhouse gas (GHG) emissions. Carbon dioxide is the major GHG and its emissions from coal-fired power plants are very high, accounting for 40 % of total global emissions [5]. The GHG emissions from fossil fuel-fired power generation can be reduced by achieving the efficiency improvements, gasification process, switching to lower carbon fuels, and CO2 capture and storage [6]. There are several options for capture and storage of CO2 from coal combustion and gasification.
The thermogravimetric analysis (TG) is an effective method applied to research the physical and chemical reaction rate of material, which could be used in isothermal and non-isothermal conditions. Coal is a heterogeneous material and the temperature ranges of the various stages of decomposition overlap each other. These phenomena have led to the coupling of TG with other analytical techniques, which include chemical features. Consequently, the gases evolved can be assigned to the measured weight losses so that the chemical information is correlated with thermal event [7, 8].
Thermogravimetry coupled with mass spectrometry (TG–MS) is a well-recognized and common technique in the pyrolysis research of solid fuels, since it provides simultaneous and elaborate information about the weight loss and gas formation behaviors as a function of temperature [9–11]. Mass spectrometry is used to identify the gaseous species emitted by a sample, according to their molar mass. To detect and analyze the evolved volatiles, a variety of thermal-analysis methods and a combination of instruments are used. These should include the choice of a suitable coupling method and gas flow conditions for the combined instruments, together with an adequate response time. In this way, simultaneous analysis and measurement repeatability can be achieved. Some phenomena such as loss of gas by condensation at cold spots, low detection sensitivity because of heavy dilution with the purge gas, low-time-and-temperature resolution because of long transfer times, mixing with the purge gas by diffusion and by uncontrolled flow conditions, and variation in gas composition in the coupling interface, should be avoided [12]. The use of non-isothermal pyrolysis with associated evolved gas analysis provides a qualitative explanation of the pyrolysis behaviour of coal [13]. Pyrolysis is an important intermediate stage of all major coal utilization processes, such as combustion, gasification, carbonization and liquefaction. It is also a simple and effective method to remove sulfur from coal [14–18]. Campoy et al. [19] have demonstrated that the addition of steam in the gasification process can lead to higher H2 yield and the appropriate combination of temperature and steam leads to higher CO and H2 yields, heating value, carbon conversion and gasification efficiency.
Mahajan et al. [20] have reported the DSC results for 12 coals using various ranks in the helium ambience at 5.6 MPa and temperature upto 580 °C using ~20 mg of sample mass at a heating rate of 10 °C min−1. They have concluded that the thermal effects during pyrolysis of different rank coals from anthracite to bituminous are endothermic. Exothermic heats are observed only in the case of sub-bituminous coals and lignites. The net thermal effect is found to be strongly dependent on coal rank. Morris [21] has carried out pyrolysis in the temperature range from ambient to 900 °C for different particle sizes, and established the empirical correlation’s for the evolution rates of hydrogen, carbon monoxide, and methane as a function of particle size and instantaneous temperature. Jayaweera et al. [22] have examined the effect of particle size on the percentage weight loss of a low quality bituminous coal during combustion in air using thermal analysis. It was found that the method of sieving used to prepare the samples of different particle size have significant effect on the results. Ciuryla e al. [23] have analysed the thermal behavior of four different coals and their chars to obtain fundamental information on pyrolysis. The temperature of maximum rate of devolatilization increases with increasing heating rate for all the coals studied [23].
Generally, laboratory experiments of the coal reactivity studies are done in such a way that diffusive restrictions can be avoided. The analysis is done by plotting char conversion against time for different particle sizes. Diffusion restrictions should be considered when burn-off curves begin to level off for larger particles. This implies that particle size should be small enough so that no difference can be found in reactivity if smaller particles are used in the tests. This characteristics is mainly depends on coal type. Kok et al. [24] have examined the effect of particle size on coal pyrolysis by thermogravimetry (TG/DTG) and estimated the kinetic parameters. Hanson et al. [25] have done the experiments on the effect of coal particle size in the range of 0.5–2.8 mm on pyrolysis and steam gasification. They have found that the size effect is not significant for high rank coals.
Arenillas et al. [26, 27] have investigated the optimisation of TG–MS coupling and the development of a normalisation procedure allowed for semi quantitative comparisons between different pyrolysis species from various rank of coals. Nali et al. [7] have done the pyrolysis, gas chromatography and mass spectrometry studies on lignite originated from Poland and USA. Seo et al. [28] have studied the coal pyrolysis by thermo-gravimetric analysis (TG) and concentration measurements of the evolved species for Chinese coals under non-isothermal conditions at various heating rates. They have found that the kinetic parameters (activation energy and pre-exponential factor) of devolatilization during weight loss are represented as 130 kJ/mol and 3.19E+07 min−1, respectively.
In the present research, high ash Indian coals and chars are used to investigate the thermal and gasification characteristics. The gasification of high ash coals needs the systematic identification about the formation and evolution of H2, CO, and CO2 during reaction. Also, it is necessary to understand pyrolysis mechanism of coals in order to increase the conversion of coal into oils and gases, especially to increase H2 during coal gasification. Two particle size ranges are selected in such a way to investigate the diffusion effect in larger sized particles. The bigger size (925 µm) particle also indicates for comparing with fluidized bed particles sizes. The use of non-isothermal technique with associated evolved gas analysis can provide a qualitative explanation of the pyrolysis and gasification behaviour of coal and coal derived char samples. Hence, this method can be used to deeply understand thermochemical reaction during the pyrolysis of coal, and coal and char combustion and gasification process.
2 Experimental
2.1 Coal and char samples
High ash Indian coals and chars are used for the experiments. These coal samples are unwashed, air dried at room temperature and milled to air dry basis with the mean particle size of 98 and 925 µm. About 15 mg of samples are used in the experiments. For the char preparation, the coal is heated at inert ambience upto 800 °C, volatiles are evaporated, and samples are cooled and preserved for the experiments. The particle sizes of the chars are 98 and 925 µm. The ultimate and proximate analysis of the coal is given in Table 1.
2.2 Apparatus
A NETZSCH STA 429 thermal analyser combined with a quadrupole QMG 511 mass spectrometer is used to perform investigations on coal and char at various ambiences. The thermogravimetry—mass spectrometry runs are carried out in a dynamic gas atmosphere. A separate water vapour generator is connected with the STA, water vapour generator and transfer line are maintained at 180 and 150 °C respectively. During the experiment, about 15 mg coal sample is placed in a ceramic crucible and heated upto 1,150 °C with the heating rate of 40 °C/min. In gasification tests, two isothermal sections are maintained at 950 and 1,050 °C to confirm the complete fuel conversion in these temperature ranges. Argon is used as protective gas with the flow rate of 20 ml/min. In combustion tests, the oxygen flow rate is maintained at 50 ml/min. In case of gasification, argon is used as carrier gas for the steam, whereas oxygen with the flow rate of 2 ml/min, air with the flow rate of 2 ml/min and steam with the flow rate of 20 g/h are supplied to react with the coal. The output of the TG system is connected to the mass spectrometer through a heated line with quartz capillary tube. Mass spectrometric studies are used to detect and identify the gas evolution during the thermogravimetric studies. The excitation energy in the mass spectrometer is 1,100 eV. The balance adapter, transfer line, and MS gas cell are maintained at 250 °C, thus avoiding the condensation of the less volatile compounds. On the other hand, the low volumes in the thermo balance microfurnace, transfer line, and gas measurement cell permit low carrier gas flow rates allow for good detection of the gases evolved in the pyrolysis, combustion and gasification process. Online gas analyses are performed for the detection of product gases fed to mass spectrometer, and experimental data are stored as a function of time.
2.3 Kinetic analysis
2.3.1 Arrhenius method
In this non-isothermal kinetics estimation, the reaction process has been done at a low linear heating rate, so that temperature resolved measurements can easily be achieved over a long time period. Many authors have approximated the overall process as a first-order reaction occurring uniformly throughout the particle [29]. In Arrhenius method [30], the measured rate of mass loss accounts for gross changes in the system, the reaction model assumes that the oxidation rate of mass loss of the total sample is dependent only on the rate constant, the mass of sample remaining (W) and the temperature.
The temperature dependence of k was expressed by the following Arrhenius equation:
Assuming first-order kinetics,
taking the logarithm of both sides,
where \( \frac{dW}{dt} \) is the rate of mass change, E is the activation energy, T is the temperature, Ar is Arrhenius constant and n is the reaction order. When \( log\left[ {\left( \frac{dW}{dt} \right)\frac{1}{W}} \right] \) is plotted vs. \( \frac{1}{T} \), a straight line is obtained which have a slope equal to \( \frac{ - E}{2.303 R} \). The magnitude of the slope is used to calculate the activation energy (E).
3 Results and discussions
3.1 Combustion tests
Combustion tests of the high ash coal and char are carried out in oxygen-enriched (75 % O2 and 25 % argon) atmosphere. The characteristic temperatures and parameters of Tig(onset, ignition temperature), Tpmax,(the temperature corresponding to the peak of the derivative thermogravimetric—DTG curve), Tb (burn out temperature) and |(dm/dt)2max| are investigated to explore the size effects of the coal and char samples. Figure 1 shows the temporal weight losses and DTG of coal and char particle samples of two different sizes. In these tests, the TG/DTG plots clearly suggested that there are two stages of weight loss for 98 µm coal as shown in Fig. 1. The first region on the TG and DTG curve is associated the heating up, pyrolysis of coal particles and includes the release, ignition, and combustion of volatile matter which is started above 250 °C for 98 µm coal. The second region is due to the oxidation of the remaining char after the volatiles are removed from the samples and the gradual diffusion of oxygen to the surface of the fixed carbon and the subsequent combustion of coal which is illustrated in the Fig. 4b. A reduction in oxygen concentration reveals that the oxidation process has occurred in both the stages, but it is significant in the later stage. In the case of 925 µm coal, the weight loss starts above 320 °C, and the ignition temperature is 360 °C which is slightly higher with the reported values of Wang et al. [31] of similar sized high ash coals. This may be due to the thermal and size effect of the particle. Devolatilization and oxidation is followed by the fixed carbon combustion occurred in a continuous manner when compare the Fig. 4a, b. The order of reactivity of coal is assessed primarily on the peak temperature Tpmax [32], higher this temperature, the less reactive of the coal. Coals with higher weight loss rates at lower temperatures are easier to ignite and burn. Profiles which extend into very high temperature ranges indicate slow burning coals, for which longer combustion times are required for complete combustion. The burn out temperature of both sized coal is around 480 °C, but the Tpmax and |(dm/dt)2max| is varied. There is a slight variation in the residual mass of the burn out char samples which may be due to the ash content variation in the 98 and 925 µm samples. More amounts of hydrogen and water vapour is released during devolatilisation stage but declined in the later combustion stage as shown in Fig. 2a, b. During devolatilisation stage, a small reduction in oxygen concentration in the flow describes that the evolved gaseous species contribution. The detection of the CH4 is not significant which reveals the possibility of low temperature oxidation.
Significant differences are observed in the onset temperature of the derived chars and the original coal samples as shown in Fig. 1. The ignition temperature of the char particles occurred around 520 °C which is in accordance and slightly lower with the reported values of Jimenez et al. [33] for low ash coals. The increase in the ignition temperature of the chars has been examined by Quian et al. [34]. Except for the decrease of volatile content and increase of ash content, the decrease of combustion performance of the chars pyrolyzed at higher temperature may be attributed to the improvement of the degree of structural ordering and the increase of aromaticity and average crystallite size of char. Comprehensively, there may be three following reasons: (1) the compact macromolecule structure of the char can improve its thermostability and result in lower reactivity; (2) the ignition and combustion process become more difficult because of the less volatile content; (3) the relatively increasing proportion of combustible substances in the char sample contributes to the diffusion resistance of reactants and products. As the size of the char particle is increased, burn out temperature is also increased which is expected due to the higher reactivity of smaller sized char particles. During coal pyrolysis, the particle size affects the swelling and agglomeration process [25]. The residual mass difference of the burn out chars will be caused by the ash and mineral content of the samples. |(dm/dt)2max| is shifted to lower temperature regions for smaller sized char samples with higher value which is caused due to the higher reactivity of the char. During char combustion, the CO2 gas is substantially released, small amount of H2O, CH4 and H2 is released as shown in Fig. 3.
3.2 Pyrolysis and gasification tests
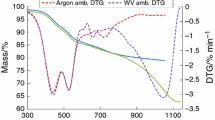
The reactivity of coals towards oxidizing gases is an important property in connection with their uses such as combustion, gasification and carbonization processes. There are many properties that govern the reactivities of coals, and which also contribute to the heterogeneity of coals, for example, carbon content, mineral matter (both quantity and composition), particle size and porosity. The gasification process is exceedingly complex and many competing processes contribute to the thermal analysis and gas evolution curves. In this study, the coal sample is subjected to dynamic heating rate until 950 °C in water vapour, blended mixture of water vapour, air and oxygen ambience, and water vapour and air ambience, which is illustrated in Figs. 4, 5 and 6. The isothermal conditions are maintained for about 15 min until the mass loss rate reaches to constant value. The residual mass of the burn out samples at water vapour ambience infers that the complete burn out of the coal and char samples occurred at 950 °C when compare the Figs. 1 and 4. Figure 4 shows the mass loss and its derivative of the coal and char at water vapour ambience. The first zone of weight loss, temperatures below 700 °C and conversion upto 20 %, is the pyrolysis (decomposition) stage; these characteristics are not affected by the presence of water vapour environment. The initial stages of coal devolatilisation is occurred in the temperature ranges from 350 to 550 °C, maximum at around 450 °C as shown in Fig. 4. The total mass losses are not affected by the coal particle sizes but the reaction starts on later stage for larger sized particles. The maximum mass loss rate |(dm/dt)2max for the coal volatilization is coincided for 98 and 925 µm particles. During this stage, the evolution of H2 occurred at 350 °C for 98 µm coal, whereas for 925 µm coal it is shifted to 450 °C due to the thermal and size effects. The evolution of CO and CO2 is maximum at 450 °C. The release of CO and H2 at higher temperature is probably due to the cracking of heavy hydrocarbons (secondary pyrolysis), whereas its release at the lower temperature is due to primary pyrolysis [23]. The hydrocarbons are evolved in the primary pyrolysis (500–600 °C), and the secondary pyrolysis phase (∼640 °C) resulting mainly in CO and H2 generation [35], the volume content increased as the temperature increased, as shown in Figs. 7, 8a and 9a. In this stage, above 600 °C, abundant H2 comes from condensation of aromatic and hydroaromatic structures or the decomposition of heterocyclic compound [26, 36]. However, the release of CO2 is decreased as the temperature increased.
Figure 6 shows the temporal weight losses of 98 µm coal and char particles at gasification related conditions. The gasification process of the coal started above 800 °C at water vapour environment which is demonstrated by Wilson [37], ascertained in Fig. 6. The gasification process of the coal starts around 700 °C at blended mixtures of water vapour, air and oxygen ambience. The evolution of different volatile products could provide information on the pyrolysis and gasification reactions occurring during thermal decomposition and gasification of coal, which is shown in Figs. 7, 8a and 9a. Figures 7, and 8a show the average concentration of evolved gases (H2, CO, and CO2) from coal devolatilization and gasification at water vapour ambience. The gasification reaction of the char particles commenced around 850 °C for both water vapour and blended mixtures of water vapour and air/oxygen ambiences. This ignition delay may be caused by differences in heat transfer and kinetic rates of char decomposition [34]. The rate of weight loss (DTG) of the coal and char sample is almost same at water vapour ambience (Fig. 4). However in the water vapour, oxygen and air blended ambiences, significant differences in coal and char mass loss rates are observed, as shown in Fig. 5. As a result, the oxygen content influence in the blended ambiences which leads to higher reactivity of the smaller sized samples. However, comparing the effects of coal size on the similar ambient, the Tig occurs at lower temperature for smaller sized particles which is followed in char gasification also. The evolution of CO commences at 750 °C, reaches its peak at 850 °C, and then decreases rapidly, shown in Figs. 7 and 8a. The evolution of H2 commences at 600 °C with the continuation of the pyrolysis process, reaches its peak at 900 °C during gasification process, and then decreases rapidly. The release of the CO2 is reached the peak at 880 °C in the gasification process. The size effect of the coal is not significant in the gas evolution profile at the water vapour ambience when compare the Figs. 7 and 8a. The major gaseous species evolution of the char sample is also shown similar trends with coal, but at higher temperatures. In the blended ambiences of water vapour, oxygen and air, the CO content is substantially reduced, and minor reduction in the hydrogen evolution during the char gasification process as illustrated in Figs. 8b and 9b. Similar tendencies occurred for CO2 evolution also. The major peaks of the gas evolution occurred at 825 °C, the point at which the water vapour decomposition rate increasing. However, there is slight shift in gasification process towards combustion process due to the presence of oxygen in the blended ambience and its reactivity potential. These trends are occurred in char gasification also, as shown in Figs. 8b and 9b. The complete burn out of both the coal and char sample occurs at 950 °C to keep the isothermal conditions for longer time, as shown in the Fig. 6. The mass loss is preserved even if the temperature is increased to 1,050 °C. This clearly demonstrates that the high ash coal gasification can be carried out at around 950 °C using the blended gaseous water vapour, oxygen and air with efficient carbon conversion. These results will be helpful for researchers to understand the differences of coal and char particles gasification, gases evolution process, appropriate gasification temperature, and to develop new coal utilization technologies by the use of high ash coals.
3.3 Kinetic analysis
The non-isothermal kinetic study of mass loss during the gasification and combustion process is extremely complex, because of the presence of numerous components and their parallel and consecutive reactions. The activation energy (E) of the high ash coal is determined for low temperature thermal decomposition and high temperature combustion and gasification processes using the Arrhenius method. The curves of mass conversion against time are plotted for all experimental conditions until the curves display straight line. A regression analysis with the least square method is used to determine the best straight line. There is no agreement regarding the variation of the gasification rate with conversion. Typically, these curves are plotted between the maximum rate between 20 and 60 %. But, in the present case only report the gasification rate at a specific value of conversion. Particularly for coal and char gasification in water vapour ambience, the conversion level is accounted between 20 and 40 %, because the remaining conversion is followed in isothermal regime as shown in Fig. 6. The Arrhenius method calculation is shown in Fig. 10, and the results are displayed in Table 2. The activation energy of the char particles is higher than the coal particles under oxygen ambience. Activation energy calculated during the combustion of the Indian char is in the range of 395–405 kJ/mol, which is on the higher side. This exhibits the lower reactivity of char during combustion at lower temperatures which is explained in detail at combustion tests. The activation of the coal particles are varied from 192 to 261 kJ/mol for the different size coals which is in consistency with the reported range of values by Wang et al. [31] and Ming-gao et al. [38] for coal combustion under various oxygen concentrations and temperatures respectively. The activation energy of the char is less when compared with similar size of coals under water vapour ambience, since the reaction started at higher temperatures. But, this trend is occurred in other way at blended mixture of water vapour, air and oxygen ambience to similar size coal and char particles, which is due to the ease of gas penetration through the particles and difference in reaction mechanisms. The gasification of coal started early in these ambience. The size effects in the activation energy are consistent for char particles, i.e., smaller sized particles exhibits lower activation energy, but not for coal particles at water vapour and blended mixture of water vapour ambiences. These differences may be appeared due to the variations in conversion level and reaction mechanisms over the selected temperature ranges.
4 Conclusion
The pyrolysis, combustion, and gasification behaviors of high ash Indian coal and char with different sizes are investigated using TG–MS method. These results indicated that the ignition temperature, temperature of maximum mass loss rate and burn out temperatures of chars are occurred at higher temperature range when compare with similar sizes of coal. The smaller sized coal particles clearly exhibits devolatilisation and combustion stages in subsequent temperature ranges under oxygen ambiences which is identified through mass spectrometer results of the evolved gaseous species. With the higher sized coal particles, these processes are continuously occurred. As the size of the particle is increased, burn out temperature is also increased which is expected due to the lower reactivity of bigger sized particles. The size effects of the coal are not significant during the pyrolysis stage of the coal. The gasification process of the coal started above 800 °C at water vapour ambience. The gasification process of the coal started around 700 °C at blended mixtures of water vapour, air and oxygen ambience. The gas evolution profile of the coal implies that the size effect is not significant in the water vapour ambience. The gasification reaction of the char particles commenced around 850 °C for both water vapour and blended mixtures of water vapour and air/oxygen ambiences. The complete burn out of both the coal and char sample occured at 950 °C which demonstrated the suitable gasification temperature with higher carbon conversion rate and gasification efficiencies for high ash coal. The activation energies of the char particles are higher than the coal particles under oxygen ambience. The activation energy in gasification process are not consistent for different sized coal and char particles which is due to the variation in reaction temperature, gas penetration through the particles and reaction mechanisms. These results will be helpful for researchers to understand the high ash coal gasification process and its kinetic parameters.
References
International Energy Agency, Key World Energy Statistics, IEA, 2010. www.iea.org/ stats/index.asp
Couch, G.: Coal to liquids, IEA Clean Coal Centre (2008)
BP Statistical review of world energy.: http://www.bp.com. September 2010
World Coal Institute.: Coal: liquid fuels, http://www.worldcoal.org/resources/wca-publications/ 2006
Deutch, J., Moniz, J.: The future of coal: options for a carbon-constrained world. Massachusetts Institute of Technology Interdisciplinary Study (2007)
Wall, T., Liu, Y., Spero, C., Elliott, L., Khare, S., Rathnam, R.: An overview on oxyfuel coal combustion state of the art research and technology development. Chem. Eng. Res. Des. 87, 1016–1103 (2009)
Nali, M., Corana, F., Montanari, L., Pellegrini, L.: A pyrolysis-gas chromatography/mass spectrometry study on coals. J. Anal. Appl. Pyrolysis 29, 15–23 (1994)
Groenewoud, W.M., de Jong, W.: The thermogravimetric analyser—coupled—Fourier transform infrared/mass spectrometry technique. Thermochim. Acta 286, 341 (1996)
Sonobe, T., Worasuwannarak, N., Pipatmanomai, S.: Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process. Technol. 89, 1371–1378 (2008)
Worasuwannarak, N., Sonobe, T., Tanthapanichakoon, W.: Pyrolysis behaviors of rich straw, rice husk and corncob by TG–MS technique. J. Anal. Appl. Pyrolysis 78, 265–271 (2007)
Miura, K., Mae, K., Shimada, M., Minami, H.: Analysis of formation rates of sulfur containing gases during the pyrolysis of various coals. Energy Fuels 15, 629–636 (2001)
Kaisersberger, E., Post, E.: Practical aspects for the coupling of gas analytical methods with thermal-analysis instruments. Thermochim. Acta 295, 73–93 (1997)
Van Heek, K.H., Hodek, W.: Structure and pyrolysis behaviour of different coals and relevant model substances. Fuel 73, 886 (1994)
Hu, H., Zhou, Q., Zhu, S., Meyer, B., Krzack, S., Chen, G.: Product distribution and sulfur behavior in coal pyrolysis. Fuel Process. Technol. 85, 849–861 (2004)
Gryglewicz, G.: Effectiveness of high temperature pyrolysis in sulfur removal from coal. Fuel Process. Technol. 46, 217–226 (1996)
Zhou, Q., Hu, H., Liu, Q., Zhu, S., Zhao, R.: Effect of atmosphere on evolution of sulfur containing gases during coal pyrolysis. Energy Fuels 19, 892–897 (2005)
Karaca, S.: Desulfurization of a Turkish lignite at various gas atmospheres by pyrolysis: effect of mineral matter. Fuel 82, 1509–1516 (2003)
Czaplicki, A., Smolka, W.: Sulfur distribution within coal pyrolysis products. Fuel Process. Technol. 55, 1–11 (1998)
Campoy, M., Gomez-Barea, A., Vidali, F.B., Ollero, P.: Air-steam gasification of biomass in a fluidised bed: process optimisation by enriched air. Fuel Process. Technol. 90, 677–685 (2009)
Mahajan, O.P., Akira, T., Nelson, J.R., Walker Jr, P.L.: Differential scanning calorimetry studies on coal and hydrogenation of coals. Fuel. 56, 33–39 (1977)
Morris, R.M.: Effect of particle size and temperature on volatiles produced from coal by slow pyrolysis. Fuel 69, 776–779 (1990)
Jayaweera, S.A.A., Moss, J.H., Thwaites, M.W.: The effect of particle size on the combustion of weardale coal. Thermochim. Acta 152, 215–225 (1989)
Ciuryla, V.T., Weimer, R.F., Bivans, D.A., Motika, S.A.: Ambient-pressure thermogravimetric characterization of four different coals and their chars. Fuel 58, 748–754 (1979)
Kök, M.V., Esber, E., Ozbas, O., Hicyilmaz, K.C.: Effect of particle size on coal pyrolysis. J. Anal. Appl. Pyrolysis 45, 103–110 (1998)
Hanson, S., Patrick, J.W., Walker, A.: The effect of coal particle size on pyrolysis and steam gasification. Fuel 81, 531–537 (2002)
Arenillas, A., Rubiera, F., Pis, J.J.: Simultaneous thermogravimetric–mass spectrometric study on the pyrolysis behaviour of different rank coals. J. Anal. Appl. Pyrolysis. 50, 31–46 (1999)
Arenillas, A., Rubiera, F., Pevida, C., Pis, J.J.: Thermogravimetric—mass spectrometric study on the evolution of nitrogen compounds during coal devolatilisation. J. Anal. Appl. Pyrolysis 65, 57–70 (2002)
Seo, D.K., Parka, S.S., Kim, Y.T., Hwang, J., Tae-U, Yu.: Study of coal pyrolysis by thermo-gravimetric analysis (TGA) and concentration measurements of the evolved species. J. Anal. Appl. Pyrolysis 92, 209–216 (2011)
Solomon, P.R., Serio, M.A., Suuberg, E.M.: Coal pyrolysis: experiments, kinetic rates and mechanisms. Prog. Energy Combust. Sci. 18, 133–220 (1992)
Kök, M.V.: Temperature-controlled combustion and kinetics of different rank coal samples. J. Therm. Anal. Calorim. 79, 175–180 (2005)
Wang, C., Yongbo, Du, Che, D.: Reactivities of coals and synthetic model coal under oxy-fuel conditions. Thermochim. Acta 553, 8–15 (2013)
Comming, J.W.: Reactivity assessment of coals via a weighted mean activation energy. Fuel 631, 1436 (1984)
Jiménez, F., Mondragón, F., López, D.: Structural changes in coal chars after pressurized pyrolysis. J. Anal. Appl Pyrolysis 95, 164–170 (2012)
Qian, W., Xie, Q., Huang, Y., Dang, J., Sun, K., Yang, Q., Wang, J.: Combustion characteristics of semicokes derived from pyrolysis of low rank bituminous coal. Int. J. Min. Sci. Technol. 22, 645–650 (2012)
Serio, M.A., Hamblen, D.G., Markham, J.R., Solomon, P.R.: Kinetics of volatile product evolution in coal. pyrolysis: experiment and theory. Energy Fuels 1, 138–152 (1987)
Arenillas, A., Rubiera, F., Pis, J.J., Cuesta, M.J., Iglesias, M.J., Jiménez, A., Suárez-Ruiz, I.: Thermal behavior during the pyrolysis of low rank perhydrous coals. J. Anal. Appl. Pyrolysis 68–69, 371–385 (2003)
Wilson.: Method for increasing steam decomposition in a coal gasification process US patent, No. 4,786,291 (1998)
Ming-gao, Y., Yan-min, Z., Chang, L.: Thermal analysis of kinetics of coal oxidation. Procedia Earth Planet. Sci. 1, 341–346 (2009)
Acknowledgments
This research work is a part of OPTIMASH project which is financially supported by EU-FP7, European union under the seventh frame work programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayaraman, K., Gökalp, I. Thermal characterization, gasification and kinetic studies of different sized Indian coal and char particles. Int J Adv Eng Sci Appl Math 6, 31–40 (2014). https://doi.org/10.1007/s12572-014-0104-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12572-014-0104-z