Abstract
Classically, a single renal artery supplies, and a single renal vein drains each kidney. The morphology and variations in the renal vascular structures are of great importance when performing any type of renal surgery. The present case describes a rare combination of renal vasculature variation in a formalin-fixed, Chinese male cadaver. In this case, the left kidney is drained by a main renal vein (MRV) and an inferior renal vein (IRV), the latter might be the remnant of the left dorsal renal vein during the embryonic period. Two sets of renal arteries are present in this case, possibly due to persistent mesonephric arteries during embryonic development. Describing such anatomical variations is not only of academic interest but also important to help radiologists with the correct interpretation of image examinations and for surgeons to be prepared in advance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The morphology and variations in the renal vascular structures are of great importance when performing any type of renal surgery (e.g., nephrectomy, in kidney transplantation) (Hekimoglu and Ergun 2022). In this regard, the role of the primary tributaries of the renal vein and their relationship to the branches of the renal artery is important. Knowledge of such vascular abnormalities is also of uttermost importance in laparoscopic surgery when entering the paraaortic region, as the repair of renal vessels is much more difficult compared to open surgery, often causing hemorrhage, a need for transfusion, or conversion to laparotomy (Hostiuc et al. 2019).
There are three main types of anatomical variants of renal veins: multiple renal veins, in which are identifiable two or more renal veins, either uni- or bilaterally; retroaortic left renal vein (RLRV), in which the renal vein has a retroaortic course before entering the inferior vena cava; and circumaortic left renal vein (CLRV), in which two or more renal veins are forming a ring around the aorta (Hostiuc et al. 2019; Bouali et al. 2014; Hazırolan et al. 2011). According to multiple classic literature, the overall prevalence for retroaortic renal vein is 3% (CI 2.4–3.6%) (Damen et al. 2024), for circumaortic renal vein—3.5% (CI 2.8–4.4%) (Tanka et al. 2018), and for multiple renal veins − 16.7% (14.3–19.2%), much higher on the right 16.6 (14.2–19.1%) than on the left side 2.1 (1.3–3.2%) (Hoff, et al. 2020). As for the arterial supply of kidneys, previous studies revealed that 73.79% of kidneys were supplied by a single renal artery, 25.72% by double renal arteries, and 0.49% by triple renal arteries. In the present case, we present a rare combination of both multiple renal veins and multiple renal arteries on the left side and their complex anatomical relationships.
Case report
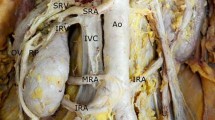
During a routine educational dissection of a formalin-fixed 74-year-old male Chinese cadaver, we found unusual arteriovenous structures at the left renal hilum. After opening the posterior parietal peritoneum, we found 5 separate vessels passing through the renal hilum.
We observed two veins draining the left kidney instead of one. The main renal vein (MRV) is formed immediately medial to the renal hilum by the union of three of its tributaries. Besides collecting the testicular vein (TV) inferiorly and inferior suprarenal vein superiorly, MRV also collects an inferior renal vein (IRV) originating from the inferior part of the left kidney, which travels posterior to both the testicular artery (TA) and TV. Near to its junction with the MRV, IRV crosses the TA anteriorly lateral to the abdominal aorta (AA) and courses upwards until it merges with MRV.
As for arterial supply, the renal artery originates from AA posteroinferior to the MRV and gives off two equal-sized branches. The superior branch of the renal artery, superior renal artery (SRA) detours anterior-laterally and crosses the anterior surface of the MRV and subsequently enters the renal hilum anterior-inferiorly to both MRV and the inferior branch of the renal artery, inferior renal artery (IRA). IRA extends laterally posterior to both MRV and the SRA and subsequently enters the renal hilum.
Another point worth noticing, in this case, is that MRV is jammed between SRA and IRA, theocratically making it difficult for venous blood to drain out of the left kidney. However, we did not find any distinct size differences between the left and right kidneys.
Discussion
Classically, a single renal artery supplies, and a single renal vein drains each kidney. However, this study reports a unique variation where the left kidney is supplied by double renal arteries and drained by two sets of renal veins. This study belongs to the case of multiple renal veins (16.7%), but with several differences. In our case, besides double renal veins, the left kidney is supplied by double renal arteries.
Accessory renal arteries are clinically the most important variations in renal vascular anatomy, occurring in one-third of patients. Multiple renal arteries are either unilateral or bilateral, accounting for approximately 30% and 10% of the patient population, respectively (Abdessater et al. 2022; Magaribuchi et al. 2020; Mao and Li 2015).
Variations in renal vessels may occur due to the atypical position of the kidneys during cephalic migration of the kidneys in embryogenesis. In addition, the number of mesonephric arteries feeding the kidney decreases to one during embryonic development, and abnormalities in this process may cause an increase in the number of ectopic arteries (Ozkan et al. 2006). As for the renal veins, on the 72nd day of a fetus, the renal veins are formed by the anastomosis of the supracardinal and subcardinal veins (two renal veins are formed, ventral and dorsal). Subsequently, the dorsal vein usually degenerates, and the ventral vein remains and becomes the renal vein. The caudal extent of the subcardinal veins forms the gonadal vessels (Mathews et al. 1999). In this case, according to the anatomical position, the left inferior renal vein (Fig. 1) might be the remnant of the left dorsal renal vein during the embryonic period. Various studies showed the right renal vein to be more often multiple, compared to the left renal vein and the main reason postulated for the increased prevalence of double right renal vein compared to the left renal vein is the complex embryogenesis on the left side, discouraging the retention of additional left-sided renal veins (Mathews et al. 1999; Anson and Daseler 1961; Ballesteros et al. 2014; Mankhause and Khalique 1986). However, in this case, the double renal vein is on the left side. In this regard, during the kidney donation process, the left kidney is a more favorable option due to its longer vascular pedicle. And if the left kidney of the donor or the donee has a varied vasculature, then the right kidney should be considered for harvesting. The anatomy and surgery manuals often overlook these anatomical variants, increasing the risk for less experienced surgeons to cause damage during surgeries or kidney transplants. Hence, the awareness of the possible variations of the renal vasculature is necessary for adequate surgical management.
Anterior view of the abdominal cavity. Notes: Anterior view of the posterior abdominal wall. For the names of the described structures, refer to Table 1
Another point worth noticing, in this case, is that MRV is jammed between SRA and IRA, theocratically making it difficult for venous blood to drain out of the left kidney. However, we did not find any distinct size differences between the left and right kidneys and this is probably due to the existence of the IRV as an extra vein helps drain the kidney.
Our report focuses on a cadaver preserved in formaldehyde, allowing for a clear and visual examination of the entire variance site during the dissection process and exploration of the course of blood vessels. However, due to limited resources, we only have one case available, limiting us to conducting a case report and preventing us from conducting a systematic comparative study with more general discoveries.
Describing such anatomical variations is not only of academic interest but also important to help radiologists with the correct interpretation of image examinations and for surgeons to be prepared in advance. We believe that complex anatomical variations with direct clinical implications such as those described in the present study are worth publication.
Data availability
Not applicable.
References
Abdessater M et al (2022) Anatomical variations of the renal artery based on the surgeon’s direct observation: a French perspective. Morphologie 106(352):15–22
Anson BJ, Daseler EH (1961) Common variations in renal anatomy, affecting blood supply, form, and topography. Surg Gynecol Obstet 112:439–449
Ballesteros LE, Saldarriaga V, Ramirez LM (2014) Morphologic evaluation of the renal veins: a study with autopsy material from Colombian subjects. Rom J Morphol Embryol 55(1):77–81
Bouali O et al (2014) Study of renal veins by multidetector-row computed tomography scans. Morphologie 98(323):161–165
Damen NS et al (2024) Anatomical variants of the retroaortic left renal vein. Ann Anat 251:152170
Hazırolan T et al (2011) CT angiography of the renal arteries and veins: normal anatomy and variants. Diagn Interv Radiol 17(1):67–73
Hekimoglu A, Ergun O (2022) Evaluation of renal vascular variations with computed tomography. African J Urol 28(1):21
Hostiuc S et al (2019) Anatomical variants of renal veins: a meta-analysis of prevalence. Sci Rep 9(1):10802
Magaribuchi T, Kobayashi T, Terai A (2020) Anatomical variation of the origin of the right renal artery : assessment with an angle of the origin and ventral protrusion. Hinyokika Kiyo 66(2):37–40
Mankhause W, Khalique A (1986) The adrenal and renal mass and their connection with Azygos and lumber vein. J Anat 146:105–115
Mao QH, Li J (2015) An accessory renal artery originating from the testicular artery, a rare variant. Indian J Surg 77(6):549–550
Mathews R et al (1999) Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology 53(5):873–880
Ozkan U et al (2006) Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol 12(4):183–186
Tanka M, Tuka F, Abazaj E (2018) Circumaortic right renal vein with multiple vascular anomalies. Radiol Case Rep 13(4):778–781
Hoff, M., et al., Anastomosis of dual renal transplant veins. J Surg Case Rep, 2020. 2020(9): p. rjaa310.
Author information
Authors and Affiliations
Contributions
Conceptualization, AR; imaging, BL, AR; methodology, ZJ, YC, YS, BL, AR; visualization, AR and SA; writing—original draft, AR, ZJ; writing—review & editing, AR; resources, BL; project administration, AR supervision, BL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Z., Chen, Y., Shi, Y. et al. A rare combined variation of left renal vasculature in a human cadaver: embryological basis and clinical significance. Anat Sci Int (2024). https://doi.org/10.1007/s12565-024-00798-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12565-024-00798-y