Abstract
The objective of this study was to evaluate bone formation after application of different doses of recombinant human bone morphogenetic protein-2 (rhBMP-2) combined with monoolein or poloxamer gels, in critical bone defects of rats. Forty-five Wistar rats were divided into nine treatment groups with five animals each: I: application of 1 µg rhBMP-2 + monoolein; II: 3 µg rhBMP-2 + monoolein; III: 7 µg rhBMP-2 + monoolein; IV: 1 µg rhBMP-2 + poloxamer; V: 3 µg rhBMP-2 + poloxamer; VI: 7 µg rhBMP-2 + poloxamer; VII: monoolein only; VIII: poloxamer only; and IX: critical bone defect only. A critical-sized defect of 6 mm diameter was produced in the left parietal bone and it was filled with gels of the above mentioned treatments. After 2 weeks, the calvarial bones were removed for histological processing. Bone formation in the groups that received poloxamer gel and rhBMP-2 was not significantly different from the control group (IX). Groups receiving monoolein and rhBMP-2 (1 and 3 µg) and those that received only the carriers (VII and VIII) had less bone formation in relation to the control. The association of rhBMP-2 to both poloxamer and monoolein did not exhibit any significant differentiation in bone formation in comparison with the control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 40 years ago, Marshal R. Urist discovered a substance in bone matrix that had inductive properties for the development of bone and cartilage; this substance was named bone morphogenetic protein (BMP; Urist 1965). Since then, over 30 different BMPs have been identified and recombinant human bone morphogenetic proteins (rhBMPs) have been created using molecular biological techniques (Wozney 1989). BMPs belong to the TGFβ superfamily, which comprises some other growth factors such as activins, inhibins or TGFβs. Members of the BMP family can be subdivided into subgroups based on their gene homology and correspondence in protein structure (Kingsley 1994). BMPs act as growth and differentiation factors and as chemotactic agents, stimulating angiogenesis and migration, proliferation, and differentiation of mesenchymal stem cells into cartilage or bone-forming cells in the vicinity of a bone injury (Reddi and Cunningham 1993; Reddi 1997; Issa et al. 2008a). These abilities have attracted the interest of scientists in the orthopaedic and dental fields, anticipating their clinical applications (Johnson et al. 1988; Toriumi et al. 1991).
Among the members of this protein family, recombinant human bone morphogenetic protein-2 (rhBMP-2) has demonstrated its fundamental importance in the process of osteogenesis (Hogan 1996; Issa et al. 2008b). When applied in vivo and in solution form, rhBMP-2 diffuses rapidly from the implantation site, abbreviating its osteoinductive effect (Fujimura et al. 1995). High doses of these proteins applied directly to tissues are not efficient in induction of osteogenesis because they are metabolized in a rapid manner. Thus, the use of smaller doses of rhBMP-2 in a carrier to provide a sustained release would be one way to overcome this problem and achieve satisfactory results (Yasko et al. 1992; Lee et al. 1994).
An ideal BMP carrier should not induce inflammatory or immune reactions, it should be absorbed concomitantly with bone healing, leaving no toxic residues, while also providing a slow delivery of the protein. Carriers should also be easily and cost-effectively manufactured for large-scale production, conveniently sterilized, and should be stable under appropriate storage conditions (Seeherman and Wozney 2005). Various substances have been tested as BMP carriers attempting to fulfill these requirements and many studies have been conducted in this area.
Among potential BMP carrier materials, four main classes of substances have already been evaluated: (1) natural polymers, such as collagen (Inoda et al. 2004; Hsu et al. 2006), chitosan (Issa et al. 2008b) and alginate (Suzuki et al. 2000); (2) inorganic materials, beta-tricalcium phosphate (Maus et al. 2008) and hydroxyapatite (HA; Morisue et al. 2006); (3) synthetic polymers such as polylactic acid (PLA) (Schliephake et al. 2008) and poly(d,l-lactide-co-glycolide)—PLGA (Weber et al. 2002); and (4) allograft and autograft material (Cullinane et al. 2002). In addition, combinations of natural polymers, inorganic salts and synthetic polymers, such as PLGA–HA–fibrin (Kim et al. 2008) and PLGA–chitosan–collagen, have also been evaluated (Shi et al. 2008).
1-Monooleoyl-glycerol, more often referred to as monoolein, is a polar lipid that is commonly used as a food emulsifier. Lipid-based systems can be: (1) eliminated through normal catabolic pathways, thus decreasing the risk of irritation or allergic reactions at the application site—a possibility that may occur when fibers or microparticles are used; (2) prepared from a diversity of lipids; and (3) very versatile concerning the site of administration. Cubic phase gels of monoolein–water are formed at body temperature, are physically stable upon contact with excess of water, and have interesting characteristics for drug delivery (D’Antona et al. 2000).
Poloxamers or Pluronics (BASF, http://www.basf.com) are nonionic, polyoxyethylene–polyoxypropylene–polyoxyethylene (PEO–PPO–PEO) triblock copolymers that have many pharmaceutical applications. This polymer is the base of some systems for the delivery of drugs such as antimicrobials (Sato et al. 2008), local anesthesics (Paavola et al. 2000) and peptides (Barichello et al. 1999; Liu et al. 2007).
Success in the induction of bone formation after rhBMP-2 use may be affected by the carrier, animal model and time until sacrifice of the animal (Zellin and Linde 1997). Dosing of rhBMP-2 is also a significant factor affecting the outcomes obtained. Yasko et al. (1992) observed in rats that a dose of 11 µg rhBMP-2 resulted in significant bone formation, with mechanical, radiographical and histological evidence of the union, while a smaller dose of 1.4 µg produced newly formed bone, but without evidence of union.
The positive characteristics of monoolein and poloxamer gels suggest that they could be employed as good carriers of rhBMP-2 for the induction of bone formation. The aim of this investigation was to assess the quantity and quality of the newly formed bone with different dosages of rhBMP-2 associated with two different carriers—monoolein and poloxamer gels—in critical bone defects created in the calvaria of Wistar rats.
Materials and methods
Substances
The rhBMP-2 used in this study was obtained from the Theodor-Boveri-Institut für Biowissenschaften (Am Hubland, Würzburg, Germany).
The rhBMP-2 was dissolved in phosphate-buffered saline (PBS; pH 7.0) at a 1:1 ratio. Monoolein (Myverol 18–99, 98.1% monoglycerides, Naarden, The Netherlands) gel was prepared in a 7:3 (monoolein:water) ratio as described previously (Lara et al. 2005). Briefly, monoolein was weighed, heated to 45°C and mixed with water at the same temperature. After a resting period, the mixture becomes a transparent viscous mass. Aqueous rhBMP-2 solutions of 1, 3 and 7 µg were prepared, added to the mixture and then applied to the critical bone defects. Poloxamer 407 (Pluronic F-127, BASF, Brazil) gels in a 2:1 (polyoxyethylene:polyoxypropylene) ratio containing rhBMP-2 in 1, 3 and 7 µg per volume were applied to the bone defects.
Animals
All aspects of this work were approved by the local ethical committee, and were performed in accordance with International Law on the use of experimental animals’ use (Process number: 07.1.126.53.3). Forty-five male Wistar rats (n = 45) weighing about 300 g each were selected and allowed to acclimatize for 1 week prior to surgery. A commercial rat chow was used to feed the animals and they were kept on a 12 h light/12 h dark cycle, with lights on at 7:00 a.m., in a temperature controlled room (23 ± 2°C) with water and food ad libitum. The animals were assigned randomly to nine groups with five animals each (n = 5), according to the chosen treatment.
The treatment groups were as follows:
-
Group I: critical bone defect with application of 1 µg rhBMP-2 + monoolein gel;
-
Group II: critical bone defect with application of 3 µg rhBMP-2 + monoolein gel;
-
Group III: critical bone defect with application of 7 µg rhBMP-2 + monoolein gel;
-
Group IV: critical bone defect with application of 1 µg rhBMP-2 + poloxamer gel;
-
Group V: critical bone defect with application of 3 µg rhBMP-2 + poloxamer gel;
-
Group VI: critical bone defect with application of 7 µg rhBMP-2 + poloxamer gel;
-
Group VII: critical bone defect with application of pure monoolein gel;
-
Group VIII: critical bone defect with application of pure poloxamer gel;
-
Group IX: critical bone defect only.
Surgical procedure
The rats were anesthetized with an intraperitoneal injection of a solution of ketamine hydrochloride [75–100 mg/kg body weight (BW)] and xylazine (5–10 mg/kg BW). The scalp hair was shaved, a longitudinal incision was made in the midline of the cranium and the periosteum was elevated to expose the surface of the parietal bones. Under copious irrigation with saline, a critical bone defect of 6 mm diameter was produced in the left parietal bone using a surgical round bur and a high-speed micromotor (Fig. 1a). The bone defects were filled in accordance with the above-mentioned groups. The periosteum and skin were then repositioned and maintained in place with 4-0 silk sutures (Ethicon, Johnson & Johnson, São José dos Campos-SP, Brazil).
Sacrifice and animal perfusion
After 2 weeks, the animals were anaesthetized with urethane 37.5% (1.5 g/kg BW) and submitted to perfusion. The procedure was carried out with a perfusion pump model 550 T2—Samtronic, involving an intracardiac infusion of PBS (0.01 M, pH 7.4; ±200 mL), followed by paraformaldehyde 4% dissolved in PBS solution (0.1 M, pH 7.4). The calvarial bones were then removed for histological processing.
Histological processing and histomorphometric analysis
The calvaria fragments with the critical bone defects enclosed were immersed in 4% paraformaldehyde/0.1 M phosphate-buffer solution for 24 h, decalcified in 5% EDTA-Tris for 30 days and neutralized with a 5% sodium sulfate solution. After embedding in paraffin, the specimens were cut into 6 µm thick sections along the sagittal plane and stained with hematoxylin–eosin. The critical bone defects were observed through a grid containing 90 equidistant points in each analyzed field, adapted to the eyepiece of a light microscope (Leica, Wetzlar, Germany) connected to a digital camera (Olympus DP11, Center Valley, PA), to measure the percentage of newly formed bone by a differential point-counting method (Weibel et al. 1966).
Statistical analysis
Exploratory data analysis was carried out with the results and normality of data was not observed. For this reason, the nonparametric Kruskal–Wallis test was chosen to compare the nine groups. In case of significance in that test, the Dunn’s test was run to compare groups in pairs. The significance level of all tests was established as 5%. All analysis was done in NCSS 2007 (NCSS, Kaysville, UT).
Results
Histological analysis: qualitative
After 2 weeks, the histological analysis showed formation of new bone tissue to different extents, especially at the margin of the lesion, that varied according to the study group.
In group I (1 µg rhBMP-2 + monoolein gel), the periphery of the lesion exhibited newly formed bone in the deep area as an extension of the receptor bone. This tissue presented osteocytes embedded in the bone matrix, with small medullary cavities and some large osteoblasts on the surface of the medullary cavity and on the surface of the osseous trabeculae. In the intermediary portion of this area, a tissue of organized aspect revealed some calibrous vessels, with many capillaries and fibroblasts among organized bundles of collagen fibers. In the core of the lesion, the characteristics of the tissue were similar, except for the absence of newly formed bone tissue in the deep area (Fig. 1b).
a Surgical procedure being performed (critical bone defect). b–d Photomicrographs of bone defects in study groups I–III: b I 1 µg rhBMP-2 combined with monoolein gel [central region, hematoxylin–eosin (H&E) ×250], c II 3 µg rhBMP-2 combined with monoolein gel (central region, H&E ×250), d III (periphery) 7 µg rhBMP-2 combined with monoolein gel (central region, H&E ×250)
In group II (3 µg rhBMP-2 + monoolein gel), the lesion displayed newly formed bone with many osteocytes in wide lacunae embedded in the bone matrix, with many medullary cavities and numerous active osteoblasts on the surface of the osseous trabeculae and on some medullary cavities (Fig. 1c).
In group III (7 µg rhBMP-2-monoolein gel), the lesion revealed islands of newly formed bone as an extension of the marginal bone. The lesion was characterized by many osteocytes embedded in the bone matrix, with many medullary cavities and a large number of active osteoblasts on the surface of the new bone tissue (Fig. 1d).
In group IV (1 µg rhBMP-2 + poloxamer gel), the lesion revealed a thin layer of fibrous connective tissue covering the surface of the residual bone and small osseous trabeculae of newly formed bone. Superficially, this region was covered by a layer of loose connective tissue that was poor in cells and blood vessels. Both fibrous and loose tissues extended to the central region of the lesion. The connective tissue consisted of an amorphous substance surrounded by many macrophages and blood vessels (Fig. 2a).
Photomicrograph of bone defects in study groups: a IV 1 µg rhBMP-2 combined with poloxamer gel (central region, H&E ×250), b V 3 µg rhBMP-2 combined with poloxamer gel (central region, H&E ×250), c VI 7 µg rhBMP-2 combined with poloxamer gel (central region, H&E ×250), d VII monoolein gel (H&E ×250), e VIII poloxamer gel (central region, H&E ×250), f IX critical bone defect only (central region, H&E ×250)
A large amount of new bone was formed in the deep area of the periphery of the lesion in group V (3 µg rhBMP-2 + poloxamer gel). The osseous trabeculae had many osteocytes incorporated in lacunae, with active and inactive osteoblasts covering them. Wide and irregular medullary cavities were surrounded by those trabeculae. A dense connective tissue with many cells was present in the superficial areas of this region, with some islands of amorphous material surrounded by macrophages and multinucleated giant cells. A thick fibrous connective tissue was present in the deepest part of the center of the lesion that was characterized by the presence of blood vessels, many capillaries and cells. In some superficial areas of this region, an amorphous material surrounded by macrophages was observed (Fig. 2b).
In group VI (7 µg rhBMP-2 + poloxamer gel), the deepest area displayed well developed trabeculae of newly formed bone at the periphery of the lesion, with embedded osteocytes, active osteoblasts on their surface and the development of some small ossification centers. On the superficial area of this region, a thick layer of a well organized connective tissue with blood vessels, capillaries and various cells was observed. Newly formed trabecular bone with wide irregular medullary cavities and active osteoblasts on the surface was seen in the deepest part of the central region of the lesion (Fig. 2c).
Group VII (monoolein gel only) revealed a fibrous connective tissue rich in fibroblasts, blood vessels, capillaries and organized collagen fibers, with scarce newly formed bone in the deepest area of the periphery of the lesion. On the surface of the peripheral region, a loose connective tissue was found, with few mononuclear cells, fibroblasts, blood vessels and capillaries. The deepest area of the center of the lesion presented an amorphous material, surrounded by an inflammatory infiltrate rich in cells. On the surface of the central area there were fibroblasts, mononuclear cells, many capillaries and collagen fibers (Fig. 2d).
In group VIII (poloxamer gel only), a thin layer of fibrous connective tissue covered the residual bone in the deepest area of the periphery of the lesion. The superficial area of this region presented loose connective tissue, with just a few cells and blood vessels. There was no evidence of new bone formation. In the center of the lesion, the deep and superficial layers were formed by fibrous connective tissue and loose tissue, respectively. An amorphous material surrounded by various macrophages was observed in the loose connective tissue (Fig. 2e).
In group IX (critical bone defect only without any material insertion), a slender trabecular bone was observed, permeated by osteocytes in wide lacunae. Different cell layers, especially osteoblastic cells, were also seen in the periphery of the defect, close to the periosteum. The connective tissue is rich in blood vessels and fibroblastic cells, representing an extension of the periosteum layer (Fig. 2f).
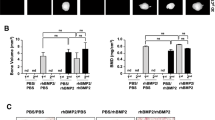
Histological analysis—quantitative
The results are summarized in Fig. 3. A significant difference among the nine groups was observed (Kruskal–Wallis; P < 0.001). The groups were compared in pairs with the Dunn’s test and significance was taken as P < 0.05. The z-values of the Dunn’s test, comparing pairs of treatment groups, are presented in Table 1 and the significantly different pairs are described below:
-
I and IV: both received 1 µg rhBMP-2, but more newly formed bone was found when monoolein was the carrier (group I), in comparison to poloxamer (group IV);
-
I and VII: more bone tissue was formed when 1 µg rhBMP-2 was added to monoolein (I), in comparison to the pure monoolein gel (VII);
-
I and VIII: more bone tissue was formed when 1 µg rhBMP-2 was added to monoolein (I), in comparison to the pure poloxamer gel (VIII);
-
II and VIII: more bone tissue was formed when 3 µg rhBMP-2 was added to monoolein (II), in comparison to the pure poloxamer gel (VIII);
-
III and VII: more bone formation occurred when 7 µg rhBMP-2 + monoolein (III) was compared to monoolein gel only (VII);
-
III and VIII: more bone formation occurred when 7 µg rhBMP-2 + monoolein (III) was compared to poloxamer gel only (VIII);
-
IV and VI: less bone formed with application of 1 µg rhBMP-2 + poloxamer (IV) when compared to 7 µg rhBMP-2 + poloxamer (VI);
-
IV and IX: less bone formation with 1 µg rhBMP-2 + poloxamer (IV) when compared with the control—critical bone defect only (IX);
-
V and VIII: more newly formed bone with 3 µg rhBMP-2 + poloxamer (V) in comparison with poloxamer gel only (VIII);
-
V and IX: less bone was formed with 3 µg rhBMP-2 + poloxamer (V) in comparison with the control—critical bone defect only (IX);
-
VI and VII: more bone tissue formed with 7 µg rhBMP-2 + poloxamer (VI) in comparison to monoolein gel only (VII);
-
VI and VIII: more bone tissue formed with 7 µg rhBMP-2 + poloxamer (VI) in comparison to poloxamer gel only (VIII);
-
VII and IX: less bone was formed in the group of monoolein gel only (VII) compared with the control—critical bone defect only (IX);
-
VIII and IX: less bone was formed in the group of poloxamer gel only (VIII) in comparison with the control—critical bone defect only (IX).
Discussion
The development of suitable carriers for rhBMP-2 will definitely stimulate a revolution in the medical area of bone regeneration, allowing the predominance of regenerative over cicatricial processes (Granjeiro et al. 2005). Semi-solid formulations like the carriers tested here, monoolein and poloxamer gels, have advantages over solid devices: they are prepared more easily, have high biocompatibility and low cost.
Monoolein–water systems are considered a sustained drug delivery system for several drugs. Because of their amphiphilic nature, both hydrophilic and lipophilic drugs can be incorporated. The hydrophilic drugs are solubilized in the water channels, while lipophilic drugs are incorporated in the lipidic layer (Chang and Bodmeier 1997). Issa et al. (2008b) evaluated the quantity and quality of newly formed bone stimulated by rhBMP-2 in combination with monoolein or chitosan as carriers, in critical bone defects created in rat mandibles. The results showed a difference between groups of animals receiving rhBMP-2 or not. New bone was formed in large amounts at the occlusal region, followed by the basal and middle regions, respectively. It was concluded that monoolein was satisfactory for defect filling and controlling of rhBMP-2 release. The present investigation confirmed those previous results.
Poloxamer 407 or Pluronic F127, with a nominal molecular weight of 12,500 and a PEO/PPO ratio of 2:1 by weight, is a copolymer of low toxicity, compatible with other chemicals and it can solubilize drugs with different physicochemical properties. Additionally, aqueous solutions of poloxamer 407, at concentrations of 20% and above, demonstrate thermoreversible gelation behavior, characterized by a critical temperature. Below the critical temperature, the poloxamer solution is in the form of a low-viscosity sol, while above it, when approaching body temperature, a viscous transparent gel is formed. This has made F127 attractive in designing thermoreversible gels for many transdermal, injectable, and controlled delivery drugs. Since F127 forms micelles and can solubilize hydrophobic compounds, these gels are attractive for delivering poorly water-soluble drugs (Schmolka 1972; Pandit and Wang 1998; Bohorquez et al. 1999). Issa et al. (2008a) tested poloxamer 407 as a carrier for rhBMP-2 with a dose of 4 µg of the protein. After periods of 2 and 4 weeks, new bone formation was observed with this carrier. The same findings were observed here, but with different dosages and considering only one period of 2 weeks.
The Wistar rat was selected as the animal model for this study because of its easy handling and care, and because it is not expensive, thus allowing an adequate number of individuals to be studied. The calvarial region was chosen because of its importance to the medical and dental fields, as it is part of the craniofacial complex. Another reason for the choice of this region is that it is an area that is easy to access surgically, and is free of vital structures (nerves, important blood vessels) that might compromise the surgery or the animal’s survival.
In this study, the time interval between the application of rhBMP-2 and animal euthanasia for the assessment of bone formation was established as 2 weeks but, according to the literature (Inoda et al. 2004, 2007; Issa et al. 2008a), longer periods were previously adopted with successful bone tissue formation. The period of 2 weeks adopted in the present study was adequate for the assessment of the initial phase of bone repair process but, according to the quantitative data presented, it was not sufficient to demonstrate the expressive osteoinduction action of the rhBMP-2 in relation to the control groups. This finding is accordance with the cited literature, which showed bone morphogenetic protein action combined with different carriers over longer periods than that used here.
The quantitative analysis in this study revealed a significant augmentation of bone formation when the rhBMP-2 dose was increased from 1 to 7 µg and poloxamer was used as the carrier, while in the groups receiving monoolein no significant differences were observed when a higher dose of rhBMP-2 was administered. When the control was compared to the groups receiving both poloxamer and rhBMP-2, no significant differences in bone formation were found. Comparing the control with the groups receiving monoolein and rhBMP-2, it was observed that significantly less bone was formed in the groups that received 1 and 3 µg, while no differences were found between the control group and the one that received 7 µg rhBMP-2 and monoolein. The control group showed more bone formation than the groups that received only the carriers: monoolein and poloxamer gels. The carriers, in their isolated form, do not have any properties favoring bone repair (Issa et al. 2008a, b).
The qualitative analysis demonstrated that the groups receiving 7 µg rhBMP-2 had larger cells, suggesting a high metabolic activity of osteocytes and osteoblasts. Usually, the connective tissue in these groups was thicker, had more cells and the collagen fibers were present in a more organized fashion. The inductive properties of the rhBMP-2 were also observed in the proliferation of numerous capillaries (Granjeiro et al. 2005; Saito et al. 2005).
The osteoinductive properties of rhBMP-2 are clear, but the optimal dose to be provided for new bone formation is still controversial. In this investigation, lower doses of rhBMP-2 were tested (1, 3 and 7 µg) associated to monoolein and poloxamer gels, and new bone formation was observed with all dosages to different extents. Higher doses (50 µg) have been described in the literature (Inoda et al. 2004), but they are no more effective than lower and sustained doses. In the present study, it was demonstrated that lower doses with a suitable carrier might effect a slow release of the protein, minimizing waste of this expensive material (Saito et al. 2005; Issa et al. 2008a, b).
Conclusion
The association of rhBMP-2 to either poloxamer or monoolein did not reveal any improvements in bone formation in comparison with the control group, in which only a critical sized defect was created in the calvarial bone, perhaps due to the short period of time used in this research precluding observation of expressive rhBMP-2 action.
References
Barichello JM, Morishita M, Takayama K, Nagai T (1999) Absorption of insulin from pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm 184:189–198
Bohorquez M, Koch C, Trygstad T, Pandit N (1999) A study of the temperature-dependent micellization of pluronic F127. J Colloid Interface Sci 216:34–40
Chang C-M, Bodmeier R (1997) Effect of dissolution media and additives on the drug release from cubic phase delivery systems. J Control Release 46:215–222
Cullinane DM, Lietman SA, Inoue N, Deitz LW, Chao EY (2002) The effect of recombinant human osteogenic protein-1 (bone morphogenetic protein-7) impregnation on allografts in a canine intercalary bone defect. J Orthop Res 20:1240–1245
D’Antona P, Parker WO, Zanirato MC, Esposito E, Nastruzzi C (2000) Rheologic and NMR characterization of monoglyceride-based formulations. J Biomed Mater Res 52:40–52
Fujimura K, Bessho K, Kusumoto K, Ogawa Y, Iizuka T (1995) Experimental studies on bone inducing activity of composites of a telopeptide type I collagen as a carrier for ectopic osteoinduction by rhBMP-2. Biochem Biophys Res Commun 208:316–322
Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R (2005) Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res 38:1463–1473
Hogan BL (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594
Hsu HP, Zanella JM, Peckham SM, Spector M (2006) Comparing ectopic bone growth induced by rhBMP-2 on an absorbable collagen sponge in rat and rabbit models. J Orthop Res 24:1660–1669
Inoda H, Yamamoto G, Hattori T (2004) Histological investigation of osteoinductive properties of rh-BMP2 in a rat calvarial bone defect model. J Craniomaxillofac Surg 32:365–369
Inoda H, Yamamoto G, Hattori T (2007) rh-BMP2-induced ectopic bone for grafting critical size defects: a preliminary histological evaluation in rat calvariae. Int J Oral Maxillofac Surg 36:39–44
Issa JP, do Nascimento C, Iyomasa MM et al (2008a) Bone healing process in critical-sized defects by rhBMP-2 using poloxamer gel and collagen sponge as carriers. Micron 39:17–24
Issa JPM, do Nascimento C, Bentley MVLB et al (2008b) Bone repair in rat mandible by rhBMP-2 associated with two carriers. Micron 39:373–379
Johnson EE, Urist MR, Finerman GA (1988) Repair of segmental defects of the tibia with cancellous bone grafts augmented with human bone morphogenetic protein. A preliminary report. Clin Orthop Relat Res 236:249–257
Kim SS, Gwak SJ, Kim BS (2008) Orthotopic bone formation by implantation of apatite-coated poly(lactide-co-glycolide)/hydroxyapatite composite particulates and bone morphogenetic protein-2. J Biomed Mater Res A 87:245–253
Kingsley DM (1994) The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8:133–146
Lara MG, Bentley MVLB, Collett JH (2005) In vitro drug release mechanism and drug loading studies of cubic phase gels. Int J Pharm 293:241–250
Lee SC, Shea M, Battle MA et al (1994) Healing of large segmental defects in rat femurs is aided by RhBMP-2 in PLGA matrix. J Biomed Mater Res 28:1149–1156
Liu Y, Lu WL, Wang JC et al (2007) Controlled delivery of recombinant hirudin based on thermo-sensitive pluronic F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Control Release 117:387–395
Maus U, Andereya S, Gravius S, Ohnsorge JA, Niedhart C, Siebert CH (2008) BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J Biomater Appl 22:559–576
Morisue H, Matsumoto M, Chiba K et al (2006) A novel hydroxyapatite fiber mesh as a carrier for recombinant human bone morphogenetic protein-2 enhances bone union in rat posterolateral fusion model. Spine 31:1194–1200
Paavola A, Kilpelainen I, Yliruusi J, Rosenberg P (2000) Controlled release injectable liposomal gel of ibuprofen for epidural analgesia. Int J Pharm 199:85–93
Pandit NK, Wang D (1998) Salt effects on the diffusion and release rate of propranolol from poloxamer 407 gels. Int J Pharm 167:183–189
Reddi AH (1997) Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev 8:11–20
Reddi AH, Cunningham NS (1993) Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Miner Res 8(Suppl 2):S499–S502
Saito N, Murakami N, Takahashi J et al (2005) Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv Drug Deliv Rev 57:1037–1048
Sato S, Fonseca MJ, Del Ciampo JO, Jabor JR, Pedrazzi V (2008) Metronidazole-containing gel for the treatment of periodontitis: an in vivo evaluation. Braz Oral Res 22:145–150
Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S (2008) Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid—an experimental study in rats. Biomaterials 29:103–110
Schmolka IR (1972) Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res 6:571–582
Seeherman H, Wozney JM (2005) Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev 16:329–345
Shi S, Cheng X, Wang J, Zhang W, Peng L, Zhang Y (2008) RhBMP-2 microspheres-loaded chitosan/collagen scaffold enhanced osseointegration: an experiment in dog. J Biomater Appl 23:331–346
Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y (2000) Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res 50:405–409
Toriumi DM, Kotler HS, Luxenberg DP, Holtrop ME, Wang EA (1991) Mandibular reconstruction with a recombinant bone-inducing factor. Functional, histologic, and biomechanical evaluation. Arch Otolaryngol Head Neck Surg 117:1101–1112
Urist MR (1965) Bone: formation by autoinduction. Science 150:893–899
Weber FE, Eyrich G, Grätz KW, Maly FE, Sailer HF (2002) Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int J Oral Maxillofac Surg 31:60–65
Weibel ER, Kistler GS, Scherle WF (1966) Practical stereological methods for morphometric cytology. J Cell Biol 30:23–38
Wozney JM (1989) Bone morphogenetic proteins. Prog Growth Factor Res 1:267–280
Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA (1992) The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am 74:659–670
Zellin G, Linde A (1997) Importance of delivery systems for growth-stimulatory factors in combination with osteopromotive membranes. An experimental study using rhBMP-2 in rat mandibular defects. J Biomed Mater Res 35:181–190
Acknowledgment
We are grateful to Fapesp for financial support (Process Number: 2007/58338-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdala, P.M.F., Iyomasa, M.M., Sato, S. et al. Osteoinductivity potential of rhBMP-2 associated with two carriers in different dosages. Anat Sci Int 85, 181–188 (2010). https://doi.org/10.1007/s12565-010-0075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-010-0075-5