Abstract
The greasyback shrimp Metapenaeus ensis is widely distributed along the coast of India and the West Pacific where it is an important fisheries species. We have examined seasonal changes in ovarian development, spermatogenesis, and mating of Me. ensis in histological studies and by external observations on specimens collected in Ise Bay, its northernmost habitat. Ovaries were found to be previtellogenic from February to May, with the first signs of development being the accumulation of yolk in oocytes in late May. Ovarian shadow ratios were high during the period late July to mid-September. The formation of cortical rods in the peripheries of oocytes and germinal vesicle breakdown were observed in ovaries from late June to September. Male shrimps had sperm in the testes during the period early June to early October, and female shrimps had spermatophores in spermatheca after early July. In late July, some post-spawn female shrimps had exogenous vitellogenic oocytes in their ovaries, indicating that ovarian development had been repeated in preparation for the next spawning. Ovarian shadow ratios, which were positively correlated with gonadosomatic indices and ovarian development, seem to be a useful marker to determine ovarian development. Our results indicate that mating in Me. ensis started in early July and that the spawning season ranged from July to September with more than two cycles of spawning in Ise Bay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
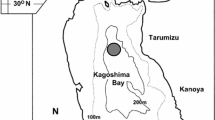
The greasyback shrimp Metapenaeus ensis belongs to the Penaeidae family and is widely distributed along the coast of India and the West Pacific (India, Sri Lanka, Australia, Southeast Asian countries, China, Taiwan, and Japan). In Japan, they inhabit the coastal waters of central Honshu and southwards (Hayashi 1992). Ise Bay, a semi-closed sea area located in the central part of Honshu (Fig. 1), is the northernmost fishing ground of Me. ensis on the Pacific side, along with Mikawa Bay. The annual catch of Me. ensis in Ise Bay landed in Mie Prefecture ranged from 0.9 to 30 tons between 1992 and 2012 (Mizuno 2014). Reared juveniles are released every year for stock enhancement (Yamane 2014a). Metapenaeus ensis has become an important commercial species for bottom trawl fisheries not only in Japan but also in many other countries, and many studies on its life history, in particular its spawning season, have been conducted in various regions around the world, including the waters off the coast of Japan. It is generally considered that penaeid shrimps in tropical waters have two major spawning periods in a single year: spring and autumn (Garcia 1988). However, the annual spawning frequency of penaeid shrimps remains controversial. Various studies have reported that Me. ensis in tropical and subtropical regions has two spawning seasons per year or, alternatively, that it spawns throughout the whole year (Crocos et al. 2001; Samphan et al. 2016). In contrast, its spawning season along the coast of Japan has been reported to be about 3 months long, extending from June to September, although there are some differences among regions (Tokuda et al. 1997). As in Japan, in the coastal area of central Queensland, Australia, Me. ensis has been reported to spawn mainly in summer (Courtney et al. 1989). Since the spawning season of Me. ensis in Japan is actually shorter than that in tropical and subtropical regions, it is widely accepted that this shrimp may have a life cycle with a single spawning season rather than multiple ones, although no substantial evidence has been reported so far.
Females of closed-thelycum penaeid shrimps mate immediately after molting and receive the spermatophore containing sperm from males (Hudinaga 1942; Dall et al. 1990). The stopper/plug that forms on the spermatophore can be observed on the outside of the female copulatory organ (thelycum), which is located at the base of the fifth pair of legs (Primavera 1985). Females of the kuruma prawn Marsupenaeus japonicus are observed with a spermatophore throughout the year (Hudinaga and Kurata 1971). Shrimp hatcheries in Japan that raise juveniles for release exclusively use mature females with a spermatophore that were caught in the wild to obtain fertilized eggs, while the mating process with males does not necessarily occur in hatcheries. Likewise, in Me. ensis, only females with a spermatophore are used to produce juveniles (Yamane 2014a), indicating that males do not need to be present at the time of spawning and fertilization. This has led to research on the reproductive ecology of Me. ensis being biased toward females; for example, histological analysis of ovaries (Courtney et al. 1989; Crocos et al. 2001), external estimation of ovarian developmental stages using the ovarian shadow (Chu et al. 1993), and investigation of gonadosomatic index (GSI) (Abe et al. 1995; Tokuda et al. 1997), whereas information pertaining to males is limited. In Ise Bay and Osaka Bay, female Me. ensis do not have a spermatophore except during the spawning season (Abe et al. 1995; Yamane 2014b; Ariyama and Sano 2015), suggesting that Me. ensis in Ise Bay will mate mainly during the spawning season. Although it is speculated that males have a seasonal reproductive cycle, similar to that of females, actual annual changes in spermatogenesis remain largely unknown.
The purpose of this study was to clarify the reproductive cycle of both Me. ensis males and females in Ise Bay by externally observing the ovarian shadow and by conducting histological analyses of the ovary and testis. In addition, to identify the period of mating in Ise Bay, we also investigated the relationship between the presence or absence of the spermatophore in females, as well as the ovarian maturation stage.
Materials and methods
Animals and sampling
Shrimp (Me. ensis) caught by a small-type trawl net in Ise Bay were obtained or observed at local fish markets (Fig. 1) around Ise Bay from 2007 to 2014 (Table 1). These shrimp were immediately transported to the Mie Prefectural Fish Farming Center and used for analyses. Since the smallest individual with ovaries in development throughout the study period weighed 8.0 g (body length [BL]: 84.0 mm, carapace length [CL]: 22.6 mm), only females weighing > 8.0 g were considered for this study.
Ovarian shadow ratio and ovarian developmental stage
The shadow of the ovary, which was photographed from the back, was observed by shining a light on the ventral surface of the shrimp. The ovarian shadow ratio (OSR) was calculated according to Sakiyama et al. (2013) as: OSR (%) = width of the ovary (part of the first abdominal segment)/width of the first abdominal segment × 100. The ovarian developmental stages were determined based on the OSR: stage A, invisible or unclear; stage B, 30–40; stage C, 40–55; stage D, > 55 (Fig. 2 upper part).
Histology
For the histological analyses, pieces of the midportion of the ovaries (first and second abdominal segments) or whole testes were fixed in Davidson's fixative solution, embedded in paraffin after dehydration through a series of ethanol and xylene, and sectioned at a thickness of 5 μm, following which the sections were stained with hematoxylin and eosin. Oocyte development was classified into six stages according to previously reported criteria (Kawazoe et al. 2000; Okumura et al. 2007): oogonium, previtellogenic oocyte (oocyte with homogeneously hematoxylin-stained cytoplasm), endogenous vitellogenic oocyte (oocyte with eosin-negative vesicles in the cytoplasm and enveloped by follicle cells), exogenous vitellogenic oocyte (oocyte with eosin-positive yolk globules in the cytoplasm), early-maturing oocyte (oocyte with cortical rods around the periphery of the oocyte plasma membrane), and late-maturing oocyte (oocyte after germinal vesicle breakdown [GVBD]). The criterion on which histological determination of the ovarian developmental stages was based was the relative abundance of the most advanced type of oocyte in the ovary: previtellogenic stage, endogenous vitellogenic stage, exogenous vitellogenic stage, maturation stage, or GVBD. In this study, the exogenous vitellogenic stage was further divided into before and after the clear formation of oocyte developmental groups (clusters). The developmental stages of spermatogenesis were determined according to King (1944): spermatogonia, primary spermatocyte, secondary spermatocyte, spermatid, and spermatozoa. The GSI was calculated using the following formula: GSI = (gonad weight/body weight) × 100. The relationship between ovarian tissue, GSI, and OSR was examined.
The incidence of spermatophores in females
The presence of a spermatophore implanted on the female thelycum (Fig. 3a, b) was confirmed by external observation, and when a spermatophore was not observed by eye, the spermatheca was examined further for the presence of a spermatophore (Fig. 3c) under a stereomicroscope after dissecting the female genital organs. To examine the relationship between the existence of a spermatophore and ovarian development, the ovarian developmental stage was also determined by the ovarian shadow, and in the case of stage A, histological observation was used to distinguish whether it was post-spawn or not.
Statistics
The GSI of each developmental stage is shown as mean ± standard deviation (SD), and multiple comparisons were made using the Steel–Dwass’s multiple comparison test (P < 0.05) by Excel-Toukei 2012 (Social Survey Research Information Co., Ltd., Japan).
Results
Relationship between ovarian tissue, GSI and OSR of female Me. ensis
The results of the study using samples collected from 2007 to 2009 show that there was a significant difference in the mean GSI values among developmental stages, from the previtellogenic to the maturation stages, but that there was no significant difference between the maturation and GVBD stages (Table 2). The GSI was highly correlated with the OSR (GSI = 0.3233 × OSR − 6.6326; r = 0.903; Fig. 2, lower part). Ovarian shadows were not visible in stage A during the previtellogenic and endogenous vitellogenic stages but were visible after the beginning of exogenous vitellogenesis (stages B–D in the exogenous vitellogenic stage, stages C, D in the maturation and GVBD stages (Table 2).
Seasonal changes in ovarian shadow of female Me. ensis
Seasonal changes in the ovarian shadow were investigated using samples collected from 2011 to 2012 (Table 1); the results are shown in Fig. 4. All females were in stage A from January to May. In mid-June, individuals at stage B first appeared, followed by the emergence in early July of stage C individuals and in late July of stage D individuals. The proportion of stage D individuals then increased rapidly, reaching 48% in early August and remaining at > 30% until early September, following which time the number of individuals in stage D decreased and were not observed in October. Individuals in stages B and C appeared until middle October and late October, respectively, but did not appear in November.
Seasonal changes in ovarian histology
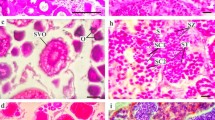
Seasonal changes in the histology of ovaries were observed in a few to ten females at each sampling time, using a 2012 sample (Table 1). In February, the ovary was filled with oogonia and previtellogenic oocytes, and cells that are believed to be the precursor of follicular cells elocalized along the connective tissue that separates the ovarian lobules (Fig. 5a). There were no obvious histological changes in the ovaries between individuals obtained in February and those obtained in early May. In contrast, in late May, large oocytes appeared along the connective tissue, indicating the beginning of endogenous vitellogenesis (Fig. 5b). In June, individuals that had reached the exogenous vitellogenic stage began to appear (Fig. 5c). In the middle of June, individuals with an advanced exogenous vitellogenic stage ovary appeared (Fig. 5d), and most of the exogenous vitellogenic oocytes were similar in size. In late June, individuals with a mature ovary with cortical rods in the peripheries of oocyte cytoplasm were observed (Fig. 5e, f). In late July, individuals having traces of mature eggs remaining in the ovarian cavity were identified, indicating that they were at a post-spawn stage; these individuals also had endogenous and eosin-stained exogenous vitellogenic oocytes (Fig. 5g, h). The histology of ovaries from individuals obtained from August to September was similar to that of those obtained in July. In early October, most individuals that carried exogenous vitellogenic stage oocytes had oocytes with disrupted nuclei and cytoplasm (Fig. 5i). On the other hand, some individuals had oocytes in the GVBD stage and in the exogenous vitellogenic stage with no cortical rods (Fig. 5j), showing asynchronous oocyte development that was not observed in individuals obtained during late June. In addition, there were a few oocytes with collapsed nuclei and cytoplasm. In November, the ovary was filled with oogonia and previtellogenic oocytes, similar to the ovary of individuals observed in February (Fig. 5a).
Seasonal changes in ovarian histology in shrimp collected in 2012: February (a), late May (b), early June (c), middle of June (d), late June (e, f), late July (g, h), and early October (i, j). Magnified area in a, b and f indicate the oogonium, follicle cells, and cortical rod, respectively. ao Atretic oocyte, GVBD oocytes at germinal vesicle breakdown stage, cr cortical rod, end endogenous vitellogenic oocyte, exo exogenous vitellogenic oocyte, fc follicle cell, n nuclear, oc ovarian cavity, og oogonium, pre previtellogenic oocyte, usm unspawned mature oocyte. Scale bars: 100 μm
Seasonal changes in testis histology
Seasonal changes in testis histology were observed in a few to ten males at each sampling time, using a 2012 sample (Table 1). In March, the testis consisted of two kinds of seminal lobules: one was surrounded by the basal lamina, and the other contained spermatogonia and nurse cells (Fig. 6a). In late April, individuals whose seminal lobule was filled with spermatogonia and primary spermatocytes appeared (Fig. 6b), and in early June, some individuals appeared with mature sperm in the seminal lobule (Fig. 6c). The process of spermatogenesis was asynchronous among seminal lobules in the same individual (Fig. 6d). The status of testis development was observed from June to September. In early October, the spot area where sperm had been released into the seminiferous tubules became hollow, and an individual with a seminal lobule containing only spermatogonia appeared. In the hollowed-out seminal lobule, nurse cells gathered and surrounded the cavity (Fig. 6e). In November, seminal lobules were filled with nurse cells, but they were not in the process of spermatogenesis (Fig. 6f).
Relationship between ovarian maturation stages and mating ratio
The existence of spermatophores on each sampling day was examined using a 2014 sample, as shown in Fig. 7. From 29 May to 25 June, no individuals in this sample possessed a spermatophore; on 2 July, 40% of individuals possessed a spermatophore, and the ratio increased rapidly, reaching 92% on 31 July. The ratio of individuals possessing a spermatophore for each ovarian maturation stage is shown in Table 3. The ratio for stage A individuals was low (15.8%), but that of stage B through stage D individuals, especially stages C and D individuals, was high (88.3% and 97.7%, respectively). Histological analysis revealed that the ovarian tissues in individuals with a soft carapace (1 individual was fixed at 14:00 on 31 July and 2 individuals were fixed at 4:30 on 28 August; with all 3 individuals considered to be immediately post molt) were in the endogenous vitellogenic stage or in the onset stage of exogenous vitellogenesis.
Discussion
Annual reproductive cycle of female and male Me. ensis in Ise Bay
In this study, based on histological and external observation of ovaries, we determined that Me. ensis in Ise Bay spawns from July to September, with peak spawning occurring in early August to early September. This result is consistent with previous reports showing that the spawning season of Me. ensis is during the summer in Japan (Ikematsu 1959; Abe et al. 1995; Tokuda et al. 1997). In the 2012 sample, females with mature oocytes with cortical rods appeared in late June. As GVBD and ovulation are known to begin a short time after the formation of cortical rods in oocytes (Anderson et al. 1984; Suitoh et al. 1996), this observation indicated that spawning would likely occur in July. On the other hand, in the 2011 sample, > 80% of females had an invisible ovarian shadow (stage A) in early July, and females with a large ovarian shadow (stage D) appeared in late July. These results indicate that spawning appeared to have occurred later in 2011. Differences between years might be due to environmental factors, such as water temperature of the habitat. Changes in the temperature of the bottom water (depth: 14.88 m) in Nakayam Channel (indicated by the star in Fig. 1) from April to September in 2011 and 2012 are shown in Electronic Supplementary Material (ESM) Fig. 1 (Ise Environmental Database Web: http://www.isewan-db.go.jp/; accessed 22 Aug 2021). The daily water temperature from April to June 2011 was 0.1–4.2 ℃ lower than that for the same period in 2012, and this lower water temperature was presumably responsible for the delay in the start of spawning in 2011. Likewise for Me. ensis in Osaka Bay, the spawning season in 1993 was later than that in 1991 and 1992, and it has been speculated that the delay was caused by the lower water temperature due to the cold summer in 1993 (Abe et al. 1995). In the present study, there were a few females with mature oocytes with cortical rods in early October, but only some of these oocytes with an advanced stage of yolk accumulation showed cortical rod formation, while the remainder of the ovary remained in an exogenous vitellogenic stage (Fig. 5j), indicating the loss of synchrony in oocyte development. Such asynchrony was not observed in females during July and August. In addition, there were females with regressed oocytes in early October (Fig. 5i). From these results, we concluded that spawning of Me. ensis in Ise Bay in 2011 had ended by October of that year.
In general, environmental factors, such as water temperature and day length, are thought to affect the beginning and end of the spawning season in penaeid shrimps (Kanazawa 1982). July, when the spawning season of Me. ensis in Ise Bay begins, is the time when the water temperature rises and the day length begins to shorten. Based on the difference in the timing of spawning initiation between 2011 and 2012 in Ise Bay, water temperature may have a greater influence on the ovarian development and prespawning molt than day length. September, which is the end of the spawning season, is the time when water temperatures begin to fall and day length becomes shorter. Further study is needed to determine whether water temperature or day length is the more important factor influencing spawning.
The spawning season of Me. ensis in Ise Bay (July–September) is later than that in other areas of Japan; for example, the spawning season is from late June to mid-August in the Ariake Sea (Ikematsu 1959), from late June to late August in the Buzen Sea (Tokuda et al. 1997), and from late June to early September in Osaka Bay (Abe et al. 1995). The main environmental factor influencing the start of the spawning season is considered to be an increase in water temperature, suggesting that the water temperature in Ise Bay may be lower than that in these other areas. Because Me. ensis spends the winter in deep water in bays and moves to shallow coastal areas around June for spawning in Osaka Bay (Abe et al. 1995), the bottom water temperatures of the deep water areas considered to be wintering areas and the shallow water areas considered to be spawning areas were compared between Osaka Bay (Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture 2013, 2014, 2015, 2016) and Ise Bay (Mie Prefecture Fisheries Research Institute 2011, 2012, 2014, 2015, 2016) for the months of March to October from 2011 to 2014 (ESM Fig. 2). From June to October, the bottom water temperatures in the deep waters tended to be higher in Osaka Bay than in Ise Bay, while from March to June, the bottom water temperatures in the shallow waters tended to be lower in Osaka Bay than in Ise Bay and, conversely, higher from July to October. Shrimps are considered to migrate from deep waters to shallow waters for spawning around June (Abe et al. 1995), so the higher water temperature in Osaka Bay between the deep waters in June and shallow waters after July may have affected the earlier start of the spawning season. In addition, most of Ise Bay has a depth of < 35 m, while Osaka Bay has areas that are > 50 m in depth. This difference in the depth of water of the overwintering areas may also have contributed to the difference in the start of the spawning season. Since the main environmental factor for the end of the spawning season is unknown, the reason for the late end of the spawning season in Ise Bay is unclear. Abe et al. (1995) reported that a delay in the start of the spawning season results in a delay in the end of the spawning season. The end of the spawning season in waters other than those of Ise Bay is from August to early September, when water temperatures are at their highest, suggesting that high water temperatures may have an effect on the end of the spawning season.
The variation in the start of the spawning season between years observed in this study may have had an impact on shrimp stock abundance. In years with low water temperature from early spring to summer, such as 2011, the spawning season shortened, which may have reduced the total abundance of egg-laying. In terms of water temperature, the relationship between water temperature (both surface and bottom waters) and the following year’s catch was investigated in Osaka Bay by Abe et al. (1995) who pointed out that when low water temperatures are observed in the previous year, the catch in the following year tends to decrease, and vice versa. Similarly, in Ise Bay, it is expected that the water temperature will affect the stock fluctuation in the following year—that is, the catch of the next year when the spawning season is shortened as in 2011 will decrease, but to prove this, further research is required.
The OSR showed a strong positive correlation with the GSI (r = 0.903), similar to the result of a previous study on Ma. japonicus (r2 = 0.803; Sakiyama et al. 2013). The OSR is easy to observe and is a useful indicator to estimate ovarian development in Me. ensis. It can be used as a market survey of Me. ensis catches to determine the state of ovarian maturity as an alternative to the GSI and histological analyses.
Our histological observations of ovaries appear to indicate that female Me. ensis of Ise Bay spawn more than twice within one spawning season. Oocytes in the endogenous and exogenous vitellogenic stages were found in the ovaries of females that had regressing mature eggs and that appeared to be in the post-spawn stage. Oocytes in the endogenous and exogenous vitellogenic stages start to accumulate yolk in preparation for the next spawning. This condition of the ovaries is the same as that reported for Ma. japonicus, which spawns multiple times in one spawning season (Suitoh et al. 2014). The ovarian developmental processes of penaeids have been well described for Ma. japonicus (Yano 1988), Penaeus monodon (Tan-Fermin and Pudadera 1989), and the fleshy prawn Fenneropenaeus chinensis (Oka and Shirahata 1965). Based on the criteria of ovarian developmental mode in teleost fish (Kurita 2010), some penaeids, such as Ma. japonicus and P. monodon, are categorized into “group-synchronous oocyte development”, in which a part of the oocytes starts vitellogenesis as a cluster group and eggs are laid once or more during the spawning season (Makinouchi et al. 1994; Suitoh et al. 2014). In Me. ensis, the diameter of oocytes accumulating yolk was almost uniform, and the oocytes were divided into two major groups: previtellogenic and exogenous vitellogenic stages (Fig. 5d). Based on these observations, ovarian maturation of Me. ensis was categorized into “group-synchronous oocyte development”, as for Ma. japonicus and P. monodon.
Ovary maturation in Me. ensis is very consistent to that in Ma. japonicus but has the different morphological characteristics of cortical rods after GVBD. In Ma. japonicus, the cortical rods grow in size during oogenesis, changing in shape from spherical to elliptical (Hudinaga 1942; Yano 1988). In contrast, cortical rods in the ovary of Me. ensis do not change morphologically, even after GVBD. The contents of cortical rods are released into seawater during spawning and form a layer of jelly that surrounds the eggs. The roles of the jelly layer are widely accepted as attracting sperm, preventing polyspermy, and protecting eggs from environmental stimuli that are unsuitable for fertilization and embryonic development (Hudinaga 1942; Clark et al. 1980; Pongtippatee-Taweepreda et al. 2004). In Me. ensis, smaller cortical rods could result in a smaller jelly layer, but the differences in physiological roles are unknown.
In our study, the smallest female Me. ensis with developing ovaries observed during this study weighed 8.0 g (CL: 22.6 mm, BL: 84.0 mm). The smallest size of a female Me. ensis with a stopper/plug was reported to be 25.9 mm in CL in a female Me. ensis collected in the Ariake Sea (Ikematsu 1959), 76 mm in BL in one collected in Osaka Bay (Ariyama and Sano 2015), and 20.5 mm in CL in one collected in the waters off Australia (Courtney et al. 1989). The smallest size of female Me. ensis in the process of yolk accumulation was also reported to be 24.2 mm in CL (Australia; Courtney et al. 1989). Although the results obtained in this study differed slightly from those reported in these studies, they can be considered as the biological minimum size of female Me. ensis. In Ise Bay, female Me. ensis are thought to start reproduction at around a weight of 8 g and BL of 80 mm.
Males having a seminal lobule filled with spermatids were observed from early June to September. During this period, males are ready for mating in Ise Bay. At the end of the spawning season (in early October), a hollow part where sperm had been released was observed in the testis, indicating that there was no supply of new mature sperm; in other words, spermatogenesis had terminated. Taken together, these results indicate that spermatogenesis of Me. ensis in the Ise Bay population occurred only during the spawning season. Since the testis of male Me. ensis is small and did not show a distinct change in size during testis development, gonadal shadow by lighting from the ventral surface was not used for males.
Relationship of timing between mating and ovarian maturation stage
In this study, the ratio of individuals with a spermatophore increased rapidly in July, indicating that mating occurred in July. A similar trend has been reported in Osaka Bay, where the ratio of female Me. ensis with stopper/plug increased rapidly in July, reaching 80–100% within 1 month (Abe et al. 1995, Ariyama and Sano 2015). Females of closed-thelycum penaeid shrimp mate and receive a spermatophore containing sperm from males immediately after molting (prespawning molt) when their shells are soft (Hudinaga 1942; Dall et al. 1990). Female Me. ensis will mate for the first time that year following the first molt in July. Females may have molted before July, but they did not mate at that time. In general, females need to attract males with pheromones for mating. When females mate, their ovaries are in the endogenous vitellogenic stage or at the onset stage of exogenous vitellogenesis; this status of ovarian development might be associated with attracting males. Unlike Me. ensis, Ma. japonicus females have a pair of spermatophores in the spermatheca throughout the year (Hudinaga and Kurata 1971). The spawning period of Ma. japonicus is long, from March to November (Suitoh et al. 2014), and in order to use spermatophores for spawning in March, female Ma. japonicus likely need to mate and receive spermatophores before the winter when they do not molt due to low water temperature. The difference in the length of the spawning period between Me. ensis and Ma. japonicus could lead to the difference in the period during which females have spermatophores in spermatheca.
We found only one virgin Me. ensis female that had reached sexual maturity (1 of 44 individuals in stage D; Table 3). Even in Ma. japonicus, it has been reported that some females with soft shells (thought to be immediately after molting) have not mated (7 of 1424 individuals; Hudinaga 1942). These findings indicate that there may be a certain percentage of females in closed-thelycum penaeid which were not able to mate after the pre-spawning molt. The proportion of such female shrimps may be lower in years with a large population (male and female encounter rate is high) and higher in years with a smaller population (male and female encounter rate is low). Alternatively, they may be permanently present during the spawning season regardless of population size. In any case, it is very interesting to note that many males and females encounter and mate during a very limited time period, just after molting, in the vast natural ocean.
In general, reproduction in decapod crustaceans, including prawns, is primarily regulated by endocrine factors secreted from the X organ-sinus gland complex in the eyestalks (Katayama et al. 2013). Although several reproduction-related peptide hormones, such as vitellogenesis-inhibiting hormone and molt-inhibiting hormone, have been identified in Ma. japonicus (Katayama et al. 2013), there are also areas in which research is lacking: for example, the molecules/genes responsible for the prespawning molt in females and those responsible for the promotion and termination of gonad maturation in both females and males. As mentioned previously, the Me. ensis population in Ise Bay has a short period for spawning (about 3 months), and the emergence rate of mature females reaches about 50% in the peak spawning season. On the other hand, the spawning season of Ma. japonicus population in the same area is longer than that of Me. ensis, extending from March to November, and the emergence rate of mature females is about 20%, even in the peak spawning season (Suitoh et al. 2014). In addition, female Me. ensis have a distinct prespawning molt period compared to Ma. japonicus which are found to have stopper/plug (spermatophores) throughout the year. These results suggest that Me. ensis has the advantage of individuals at specific physiological states, such as pre- and postspawning molt and GVBD that are synchronized well, being easy to obtain. The approaches used in this study and the findings of the study will be valuable in subsequent research aimed at shedding light on the endocrine and molecular mechanisms of reproduction in penaeid species and may enable proper stock management by adjusting the number of Me. ensis juveniles released according to the water temperature from early spring to the spawning season.
References
Abe T, Kusakabe T, Nabeshima Y, Tsujino K (1995) Fisheries biology of the greasyback shrimp Metapenaeus ensis in Osaka Bay. Bull Osaka Pref Fish Exp Stat 9:57–76 (in Japanese)
Anderson SL, Chang ES, Clark WH Jr (1984) Timing of postvitellogenic ovarian changes in the ridgeback prawn Sicyonia ingentis (Penaeidae) determined by ovarian biopsy. Aquaculture 42:257–271
Ariyama H, Sano M (2015) Growth, reproduction and ontogenetic migration of the greasyback shrimp Metapenaeus ensis in Osaka Bay Japan. Plankton Benthos Res 10(1):55–66
Chu KH, Tam YK, Chung CK, Ng WL (1993) Morphometric relationships and reproductive maturation of the shrimp, Metapenaeus ensis, from commercial catches in Hong Kong. Fish Res 18:187–197
Clark WH Jr, Lynn JW, Yudian AI, Persyn HO (1980) Morphology of the cortical reaction in the eggs of Penaeus aztecus. Biol Bull 158:175–186
Courtney AJ, Dredge MCL, Masel JM (1989) Reproductive biology and spawning periodicity of endeavour shrimps Metapenaeus endeavouri (Schmitt 1929) and Metapenaeus ensis (de Haan, 1850) from a central Queensland (Australia) fishery. Asian Fish Sci 3:133–147
Crocos PJ, Park YC, Die DJ, Warburton K, Manson F (2001) Reproductive dynamics of endevour prawns, Metapenaeus endevouri and M. ensis, in Albatross Bay, Gulf of Carpentaria, Australia. Mar Biol 138:63–75
Dall W, Hill BJ, Rothlisberg PC, Sharples DJ (eds) (1990) The biology of the Penaeidae. Advances in marine biology, vol 27. London, Academic Press
Garcia S (1988) Tropical penaeid prawns. In: Culland JA (ed) Fish population dynamics. Hoboken, Wiley
Hayashi K (1992) Nihonsanebirui no Bunrui to Seitai, I. Konsaiamoku (Taxonomy and ecology of shrimps in Japan, I. Dendrobranchiata). Seibutsu Kenkyusha Co Ltd, Tokyo (in Japanese)
Hudinaga M (1942) Reproduction, development and rearing of Penaeus japonicus Bate. Jpn J Zool 10:305–393
Hudinaga M, Kurata H (1971) The biology of Peaneus japonicus. In: Imai T, Ino T, Kuroki M, Hudinaga M, Yamamoto G (eds) Shallow sea complete aquaculture. Kouseisha Kouseikaku, Tokyo, pp 297–343 (in Japanese)
Ikematsu W (1959) On the life-history of Metapenaeus monoceros (FABRICIUS) in Ariake Sea. Rep Invest Ariake Sea 5:19–29 (in Japanese)
Kanazawa A (1982) Induction of maturation and spawning by external environmental factors 6 Crustaceans. In: The Japanese Society of Fisheries Science (ed) Regulation of maturation and spawning in aquatic animals. Fisheries Science series 41. Kouseisha Kouseikaku, Tokyo, pp 80–89 (in Japanese)
Katayama H, Ohira T, Nagasawa H (2013) Crustacean peptide hormones: structure, gene expression and function. Aqua-BioSci Monogr 6(2):49–90
Kawazoe I, Jasmani S, Shih TW, Suzuki Y, Aida K (2000) Purification and characterization of vitellin from the ovary of kuruma prawn, Penaeus japonicus. Fish Sci 66:390–396
King JE (1944) A study of the reproductive organs of the common marine shrimp, Penaeus setiferus (Linnaeus). Biol Bull 94:244–262
Kurita Y (2010) Influence of spatio-temporal changes in stock reproductive potential on the recruitment levels of fish. Bull Jpn Soc Fish Oceanogr 74:4–18 (in Japanese)
Makinouchi S, Samuel L, John H, Tatam S (1994) Interrelationship between maturation/spawning cycle and molting cycle of the pond-reared Penaeus monodon. Aquaculture 42(1):33–40
Mie Prefecture Fisheries Research Institute (2011) Senkai Teisen Chousa. H22 Data of Fishing and Oceanographic Conditions (data for 2011). Mie Prefecture Fisheries Research Institute, Mie (in Japanese)
Mie Prefecture Fisheries Research Institute (2012) Senkai Teisen Chousa H23. Data of fishing and oceanographic conditions (data for 2011). Mie Prefecture Fisheries Research Institute, Mie (in Japanese)
Mie Prefecture Fisheries Research Institute (2014) Senkai Teisen Chousa. H24 Data of fishing and oceanographic conditions (data for 2012). Mie Prefecture Fisheries Research Institute, Mie (in Japanese)
Mie Prefecture Fisheries Research Institute (2015) Senkai Teisen Chousa H25. Data of fishing and oceanographic conditions (data for 2013). Mie Prefecture Fisheries Research Institute, Mie (in Japanese)
Mie Prefecture Fisheries Research Institute (2016) Senkai Teisen Chousa H26. Data of fishing and oceanographic conditions (data for 2014). Mie Prefecture Fisheries Research Institute, Mie (in Japanese)
Mizuno K (2014) Life history of the greasyback shrimp Metapeaneus ensis at the end of Ise Bay (at the mouths of the Kiso Three River). In: Okumura T, Suitoh K (eds) Maturation and hatching of kuruma prawn Marsupenaeus japonicus. Aichi Sea-Farming Institute, Tahara, pp 68–70 (in Japanese)
Oka M, Shirahata S (1965) Studies on Penaeus orientalis KISHINOUYE-II: morphological classification of the ovarian eggs and the maturity of the ovary. Bull Faculty Fish Nagasaki Univ 18:30–40 in Japanese)
Okumura T, Yamano K, Sakiyama K (2007) Vitellogenin gene expression and hemolymph vitellogenin during vitellogenesis, final maturation, and oviposition in female kuruma prawn, Marsupenaeus japonicus. Comp Biochem Physiol A 147:1028–1037
Pongtippatee-Taweepreda P, Chavadej J, Plodpai P, Pratoomchart B, Sobhon P, Weerachatyanukul W, Withyachumnarnkul B (2004) Egg activation in the black tiger shrimp Penaeus monodon. Aquaculture 234:183–198
Primavera JH (1985) A review of maturation and reproduction in closed thelycum penaeids. In: Taki Y, Primavera JH, Llobrera JA (eds) Proceedings of the first international conference on the culture of penaeid prawns/shrimps, Iloilo City, 4–7 December 1984, pp 47–64
Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture (2013) Senkai Teisen Chousa H23 Zigyou Shiryousyuu (data for 2011). Research Institute of Environment Agriculture and Fisheries, Osaka Prefecture (in Japanese)
Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture (2014) Senkai Teisen Chousa. H23 Zigyou Shiryousyuu (data for 2012). Research Institute of Environment Agriculture and Fisheries, Osaka Prefecture (in Japanese)
Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture (2015) Senkai Teisen Chousa. H23 Zigyou Shiryousyuu (data for 2013). Research Institute of Environment Agriculture and Fisheries, Osaka Prefecture (in Japanese)
Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture (2016) Senkai Teisen Chousa H23 Zigyou Shiryousyuu (data for 2014). Research Institute of Environment Agriculture and Fisheries, Osaka Prefecture (in Japanese)
Sakiyama K, Shimizu D, Tahara D (2013) Evaluation of maturity of the kuruma prawn Marsupenaeus japonicus by ovarian shadow ratio. Aquaculture Sci 61(1):119–120 (in Japanese)
Samphan P, Sukree H, Reunchai T (2016) Population dynamics of the greasyback shrimp (Metapenaeus ensis, De Haan, 1844) in the Songkhla Lake, Songkhla Province Thailand. J Agric Tech 12(1):75–89
Suitoh K, Arakawa T, Ito H (1996) Maturity observation using biopsy for broodstock of kuruma prawn Penaeus japonicus. Saibai Giken 25(1):27–35 (in Japanese)
Suitoh K, Okumura T, Yamane F, Tsuge A, Ogura Y, Yamano K (2014) Initiation and termination of spawning season of female kuruma prawn Marsupenaeus japonicus in Western Enshu-nada Japan. Aquaculture Sci 62(3):295–305 (in Japanese)
Tan-Fermin JD, Pudadera RA (1989) Ovarian maturation stages of the wild giant tiger prawn, Penaeus monodon Fabricius. Aquaculture 77:229–242
Tokuda M, Hamada T, Satou H (1997) The maturity of offshore greasyback prawn (Metapenaeus ensis) in Buzen Sea. Bull Fukuoka Fish Mar Tech Res Cen 7:9–14 (in Japanese)
Yamane F (2014a) History of juvenile production and release of the greasyback shrimp Metapeaneus ensis in Japan. In: Okumura T, Suitoh K (eds) Maturation and hatching of kuruma prawn Marsupenaeus japonicas. Aichi Sea-Farming Institute, Tahara, pp 15–19 (in Japanese)
Yamane F (2014b) Ovarian development in the greasyback shrimp Metapeaneus ensis in the wild. In: Okumura T, Suitoh K (eds) Maturation and hatching of kuruma prawn Marsupenaeus japonicas. Aichi Sea-Farming Institute, Tahara, pp 82–84 (in Japanese)
Yano I (1988) Oocyte development in the kuruma prawn Penaeus japonicus. Mar Biol 99:547–553
Acknowledgements
We thank all the staff members of the Mie Prefectural Fish Farming Center who assisted with this survey over the years. We also thank the staff of the Fisheries Technology Institute, Japan Fisheries Research and Education Agency, for their help in preparing tissue samples of the gonads.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamane, F., Suitoh, K., Okumura, T. et al. Annual reproductive cycle of the greasyback shrimp Metapenaeus ensis in Ise Bay, Japan. Fish Sci 88, 63–73 (2022). https://doi.org/10.1007/s12562-021-01569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01569-8