Abstract
We examined the combined effects of fasting in freshwater and water temperature during the transition from freshwater to seawater on growth/metabolic parameters in juvenile chum salmon Oncorhynchus keta. Juveniles of 1 g in freshwater were first subjected to a 5-day fasting period at 10 or 5 °C, acclimated to either 10 or 5 °C seawater and fed ad libitum for 30 days. Control fish were transferred from 10 °C freshwater to 10 °C seawater and fed ad libitum throughout the experimental period. Serum insulin-like growth factor (IGF)-I was measured to evaluate growth status/potential and liver glycogen as an index of energy storage. Fasting in freshwater for 5 days negatively affected body size. Growth of juveniles kept at colder temperatures was retarded in seawater for at least 20 days, which may partly be explained by a lower feeding rate in cold seawater. Serum IGF-I levels were lower in fasted fish in freshwater at both temperatures and colder seawater had a negative effect on restoring serum IGF-I levels after refeeding for 20 days. Liver glycogen content was low in fish fasted in freshwater for 5 days. After refeeding in seawater for 10 days, liver glycogen content increased significantly in juveniles kept at colder temperatures. Colder water temperatures in both salinities positively affected glycogen content for 30 days after transfer to seawater, suggesting that juveniles allocated energy stores to the liver rather than growth under suboptimal feeding and temperature conditions. The findings of the present study suggested that relatively cold freshwater could negatively affect juvenile chum salmon growth soon after sea entry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chum salmon Oncorhynchus keta is an important commercial fish along the coasts of Hokkaido and northern Japan. They are obligatory anadromous fish: hatching in freshwater, remaining in rivers for a few to several months depending on the region and river system, and migrating to the ocean in the spring of their first year (Salo 1991; Urawa et al. 2018). Chum salmon resources in Japan are mainly sustained by intensive hatchery releases (Morita et al. 2006; Nagata et al. 2012; Miyakoshi et al. 2013; Kitada 2014). In Hokkaido, chum salmon fry/juveniles are reared in hatcheries until they reach about 1 g and are then released into the rivers (Seki 2005, 2013). Released chum salmon juveniles can adapt to seawater through hypo-osmoregulatory changes (Kashiwagi and Sato 1969; Wong et al. 2019). Despite their ability to hypo-osmoregulate, juvenile chum salmon are vulnerable to environmental fluctuations, such as food abundance, water temperature, and predation pressure owing to their small size. Recent declines in adult return rates along Hokkaido coasts have been mainly attributed to climate change (Urawa et al. 2018). Therefore, hatchery managers need to adjust the timing of releases to match favorable river and coastal conditions and/or make hatchery fish more robust against environmental fluctuations (Mizuno 2012; Nagata et al. 2016).
High mortalities of juvenile chum salmon occur during the early phase of their marine life (Healey 1982; Bax 1983; Saito et al. 2011). Estimations by mark-recapture of juvenile chum salmon suggested that the daily mortality soon after sea entry ranged from 2.9% to 8.1% (Fukuwaka and Suzuki 2002; Wertheimer and Thrower 2007). Such high mortality may be size/growth dependent; smaller sized juveniles or those with poor growth are more susceptible to predation and energy deficit under changing environments (Honda et al. 2017, 2020; Duncan and Beaudreau 2019). A critical size and critical period hypothesis has been proposed to explain the high mortality of juvenile salmon, which states that juveniles must attain a certain size by a certain period to survive during early marine life (Beamish and Mahnken 2001; Farley et al. 2007). Although the validity of the hypothesis has been recently challenged (Beacham et al. 2017, 2018), there are several studies suggesting the high mortality of juvenile salmon is growth dependent along coastal waters (Moss et al. 2005; Duffy and Beauchamp 2011; Zavolokin and Strezhneva 2013; Tucker et al. 2016; Duncan and Beaudreau 2019).
The growth of fish is influenced by environmental factors. Water temperature is a primary abiotic factor affecting fish growth by influencing the distribution and abundance of prey items and fish metabolism. Water temperature affects metabolic rates, and therefore fish growth. The standard metabolic rate increases exponentially as the water temperature increases, whereas the maximal metabolic rate reaches a peak at a certain temperature and declines thereafter. The difference between the maximal and standard metabolic rate is the aerobic scope, which is a reflection of the capacity of organisms to work and/or grow (Claireaux and Chabot 2016). Correlation analyses revealed that a prolonged period of optimal coastal water temperature increased the fry-to-adult survival rates of hatchery-reared chum salmon (Morita and Nakashima 2015). This implies that cold spring or/and hot summer would reduce juvenile chum salmon growth and, in turn, expose them to a risk of growth-dependent mortality. Nagata et al. (2007) reported coastal seawater surface temperatures ranging from 8 to 13 °C are optimal for growth and off-shore movement in juvenile chum salmon.
Water temperature also influences fish growth by altering endocrine system activity, such as the growth hormone (GH)-insulin-like growth factor (IGF)-I system (Gabillard et al. 2005; Reinecke 2010). IGF-I is mainly produced by the liver in response to GH stimulation from the pituitary gland and mediates GH actions (Daughaday and Rotwein 1989). IGF-I is also produced by peripheral tissues, such as muscle and exerts its mitogenic action in autocrine and paracrine systems (Le Roith et al. 2001; Ohlsson et al. 2009). In several fishes, including salmonids, endocrine IGF-I is valuable as a growth index because its circulating level generally correlates with individual growth rate (Beckman et al. 2004a, b; Picha et al. 2008; Beckman 2011). The validity of serum IGF-I as a growth index has been confirmed for juvenile chum salmon, and the juvenile growth status in the coastal area of the Abashiri region, eastern Hokkaido, has been evaluated (Kaneko et al. 2015, 2019; Taniyama et al. 2016).
Using rearing experiments, we previously assessed the effects of feeding status and seawater temperature on juvenile chum salmon growth (Nakamura et al. 2019). In that study, transfer to cold seawater (5 °C) had moderate negative effects on body size and serum IGF-I. However, when juveniles were fasted in freshwater for 5 days and then transferred to cold seawater, their growth was severely affected for 10 days even after refeeding. In addition, serum IGF-I levels were not recovered in these fish, and liver glycogen content was very high (Nakamura et al. 2019). These results suggested that when juvenile chum salmon experienced fasting during downstream migration and entered cold seawater, they sacrificed growth and stored energy in the liver to prepare for future nutritional deficit. However, because the previous rearing experiments were relatively short (10 days), their longer-term effects on growth retardation need to be addressed.
In addition to the importance of coastal water temperature for the growth of juvenile chum salmon, river water temperature may also affect growth performance (Morita et al. 2015; Takahashi et al. 2016). Morita et al. (2015) found a positive correlation between river water temperature, the number of downriver migrating fry, and the number of adult returns in the Chitose River, central Hokkaido, Japan. Their study highlights the importance of river water temperature on the survival of juvenile chum salmon in the river and/or coast. However, the mechanism by which river water temperature influences the survival of juvenile chum salmon before or/and after sea entry is not clear. The present study examined the combined effects of freshwater and seawater temperatures on the growth of juvenile chum salmon fasted in freshwater by a rearing experiment utilizing growth and metabolic indices.
Materials and methods
Fish
Juvenile chum salmon (fork length 5 cm and body weight 0.8 g) were transferred in May 2019 from a local hatchery in northeastern Hokkaido (Kamisato Hatchery; Tsubetu, Abashiri-gun, Hokkaido, Japan) to an indoor rearing facility at the Faculty of Fisheries Sciences, Hokkaido University (Hakodate, Hokkaido, Japan). Fish were reared in 60-L freshwater (FW) glass aquariums (size 60 × 29.5 × 36 cm) in a temperature-controlled room (10 °C), and each tank had a closed circulation system with filtration in the upper half (Jex, Osaka, Japan). Until the beginning of the experiment, fish were fed to satiety once daily on a commercial diet (Marubeni Nisshin Feed Co. Ltd., Tokyo, Japan). The following experiments were carried out in accordance with the guidelines of the Hokkaido University Animal Care and Use Committee.
Rearing experiment
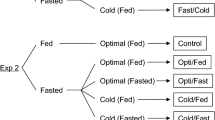
In late May 2019, a total of 240 fish (approximately 1 g) were equally distributed into eight 40-L tanks (30 fish/tank; size 45 × 30 × 30 cm) filled with freshwater and acclimated for 1 week with feeding as described above. Fish in four tanks were kept at 10 °C and fasted for 5 days. Fish in the other four tanks were also fasted while being acclimated from 10 to 5 °C over 3 days, followed by two additional days at 5 °C. After the 5-day fasting period in freshwater, fish were acclimated to artificial seawater (SW; TetraMarin Salt Pro; Spectrum Brands Inc., Tokyo, Japan) by gradually increasing the salinity to 31–34 g kg−1 (100% SW) as follows. On day 6, the first two tanks of each group were transferred to four 40-L tanks filled with 50% SW at 10 °C. The second half of each group was brought to another temperature-controlled room (5 °C) and transferred to four 40-L tanks filled with 50% SW that was maintained at 9 °C using a portable heater. On day 7, a quarter of 50% SW was removed from the tanks and the same amount of 145% SW was added to make 75% SW. The water temperature of the two 75% SW tanks was reduced from 9 to 7 °C. On day 8, fish were transferred to another set of tanks filled with 100% SW at 10 or 5 °C, and 10–12 fish from each group were further placed into two 40-L tanks to have replicates. Fish were fed during the acclimatization period for 4 days and for an additional 26 days. These treatments created four groups based on rearing water temperatures in FW and SW: optimal FW temperature and optimal SW (Opti/Opti), Opti/Cold, Cold/Opti, and Cold/Cold (Fig. 1). Additional fish placed in two 40-L tanks filled with 10 °C FW were acclimated to 10 °C SW as described above and fed once daily at libitum throughout the experimental period for 35 days, which served as a control group. Feeding rate (%) per tank was calculated as follows:
The design of the rearing experiment conducted in late May 2018. Juvenile chum salmon were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater, and maintained at the same temperature with feeding. Control fish were fed and kept at the optimal temperature throughout the experiment
Eight or 13 (Cold/Cold) fish from each treatment were sampled before and 10, 20 and 30 days after transfer to seawater (Fig. 1). Fish were anesthetized using 3.3% 2-phenoxyethanol (Kanto Chemical, Tokyo, Japan). After FL and BW were measured, the tail was cut and blood was withdrawn using 10- or 20-μL plain glass tubes (Microcap; Drummond Scientific Company, Broomall, PA, USA). Blood was allowed to clot overnight at 4 °C and centrifuged at 10,000 rpm for 15 min. Serum was collected and stored at −80 °C until use. For the Cold/Cold group, blood was collected from 13 fish because some individuals in this group were too small to collect enough blood for analysis. When necessary, sera from two individuals with similar body size were pooled for hormone measurement. Gills and livers were collected, immediately frozen on dry ice, and stored at −80 °C.
Na+,K+-ATPase activity assay
Gill NKA activity was measured according to Quabius et al. (1997) with minor modifications (i.e., correction of an incorrect concentration of sulfuric acid). Protein concentration was measured using a BCA (bicinchoninic acid) Protein Assay Kit (Thermo Scientific, IL, U.S.A.). The activity was expressed as Pi (µmol) per protein (mg) per time (h).
Time-resolved fluoroimmunoassay (TR-FIA) for IGF-I
To measure IGF-I, serum was first extracted with an acid–ethanol, as described by Shimizu et al. (2000). IGF-I was quantified by TR-FIA, based on the method described by Small and Peterson (2005), using recombinant salmon/trout IGF-I (GroPep Bioreagents Pty Ltd, Adelaide, Australia) as a standard. Time-resolved fluorescence was measured using a Wallac ARVO SX or Wallac ARVO X4 multilabel counter (PerkinElmer, Waltham, MA, USA). When serum volume was too small, sera from two individuals were pooled.
Glycogen content
Liver glycogen content was measured according to Dreiling et al. (1987). Briefly, liver pieces (20–30 mg) were homogenized in cold 10% perchloric acid (Sigma-Aldrich) and centrifuged at 10,000 rpm for 5 min at 4 °C. The supernatants were reacted with I2-KI solution containing saturated CaC2. The absorbance at 460 nm was measured using an ARVO X4 multilabel counter. Values are expressed as mg per liver weight (g).
Statistical analysis
The effects of fasting in freshwater on body size and physiological parameters were analyzed using a one-way analysis of variance (ANOVA) using the JMP program (SAS Institute Inc., Cary, NC, USA). When significant effects were found, treated groups were compared with the control group by Tukey's honestly significant difference (HSD) test. The effects of freshwater and seawater temperatures within each sampling point on body size and physiological parameters were analyzed using a two-way ANOVA. Differences among groups, including the control group, were identified using a one-way ANOVA followed by Tukey's HSD test. Differences among groups were considered significant at P < 0.05.
Results
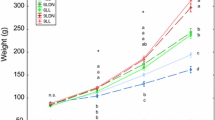
Fasting for 5 days in freshwater had negative effects on FL (F2,40 = 8.67, Residual = 0.058, P = 0.0008), BW (F2,40 = 26.13, Residual = 0.013, P < 0.0001), and K (F2,40 = 16.05, Residual = 0.002, P < 0.001) regardless of water temperature (Fig. 2). There were negative main effects of low freshwater temperature on FL (P = 0.0468), BW (P = 0.0051), and K (P = 0.0233) 10 days after seawater transfer, whereas no interactions between freshwater and seawater temperatures were detected (Fig. 3). Thereafter, low seawater temperature mostly had negative main effects on FL (P = 0.0004 on day 20; P < 0.0001 on day 30) and BW (P = 0.004 on day 20; P = 0.0008) without temperature interaction, but not on K (Fig. 3). Control fish were larger and heavier than the other fish throughout the experimental period for 30 days (Fig. 3, Online Resource, Table S1). However, K became similar in all groups from 20 days after seawater transfer (Fig. 3, Online Resource, Table S1).
Effects of a 5-day fasting period in freshwater at optimal (Opti; 10 °C) and cold (5 °C) temperature on fork length (FL; a), body weight (BW; b) and condition factor (K; c) in juvenile chum salmon. Control fish were fed ad libitum at the optimal temperature. Values are expressed as means ± SE (n = 8). Asterisks indicate a significant difference from control group (Tukey’s HSD, P < 0.05)
Combined effects of freshwater and seawater temperatures on fork length (FL; a), body weight (BW; b) and condition factor (K; c) in juvenile chum salmon. Treated groups were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater and fed ad libitum for 30 days. Control fish were fed and kept at the optimal temperature throughout the experiment. Values are expressed as means ± SE (n = 8). Asterisks indicate freshwater (FW) or seawater (SW) temperature had a main effect in two-way ANOVA at a given time point. Groups sharing the same letter are statistically not different each other (Tukey’s HSD, P < 0.05)
Feeding rates after transfer to seawater appeared to vary among groups and over time; however, having only one duplicated tank (i.e., n = 2) did not allow statistical comparisons between groups (Fig. 4). When the data on feeding rate were grouped by freshwater temperature experienced (n = 4) for statistical analysis, the feeding rate during the first 10 days was lower in fish held in cold freshwater (P = 0.0295). Feeding rates during 11–20 days and 21–30 days after seawater transfer were lower in fish reared in cold seawater (11–20 days: P < 0.0001; 21–30 days: P = 0.0033).
Combined effects of freshwater and seawater temperatures on feed intake by juvenile chum salmon. Treated groups were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater and fed ad libitum for 30 days. Control fish were fed and kept at the optimal temperature throughout the experiment. Values are expressed as means ± SE (n = 2)
There was no effect of fasting for 5 days in freshwater on gill NKA activity (Fig. 5). At 10 and 20 days after seawater transfer, there was no interaction but a negative main effect of low freshwater temperature on gill NKA activity (P = 0.0078 on day 10; P < 0.0001 on day 20), although its effect was positive on day 30 (P = 0.050; Fig. 6).
Effects of 5-day fasting in freshwater at optimal (Opti; 10 °C) and cold (5 °C) temperature on gill Na+,K+-ATPase (NKA) activity in juvenile chum salmon. Control fish were fed ad libitum at the optimal temperature. Values are expressed as means ± SE (n = 8). Asterisks indicate significant difference from control group (Tukey’s HSD, P < 0.05)
Combined effects of freshwater and seawater temperatures on gill Na+,K+-ATPase (NKA) activity in juvenile chum salmon. Treated groups were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater and fed ad libitum for 30 days. Control fish were fed and kept at the optimal temperature throughout the experiment. Values are expressed as means ± SE (n = 8). Asterisks indicate freshwater (FW) temperature had a main effect in two-way ANOVA at a given time point. Groups sharing the same letter are statistically not different each other (Tukey’s HSD, P < 0.05)
Serum IGF-I levels were lower in fish fasted for 5 days in freshwater regardless of water temperature (F2,24 = 42.53, Residual = 21.30, P < 0.0001; Fig. 7). There was no interaction but a negative main effect of low seawater temperature on serum IGF-I on days 10 (P < 0.0001) and 20 (P = 0.0003), whereas its effect was not seen on day 30 (Fig. 8, Online Resource, Table S1).
Effects of5-day fasting in freshwater at optimal (Opti; 10 °C) and cold (5 °C) temperature on serum insulin-like growth factor (IGF)-I levels in juvenile chum salmon. Control fish were fed ad libitum at the optimal temperature. Values are expressed as means ± SE (n = 8). Asterisks indicate significant difference from control group (Tukey’s HSD, P < 0.05)
Combined effects of freshwater and seawater temperatures on serum insulin-like growth factor (IGF)-I levels in juvenile chum salmon. Treated groups were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater and fed ad libitum for 30 days. Control fish were fed and kept at the optimal temperature throughout the experiment. Values are expressed as means ± SE (n = 5–8). Due to the small serum volume, some samples were pooled. Numbers of the samples analyzed for each group are shown in parentheses. Asterisks indicate seawater (SW) temperature had a main effect in two-way ANOVA at a given time point. Groups sharing the same letter are statistically not different each other (Tukey’s HSD, P < 0.05)
Liver glycogen content was low in fish fasted for 5 days in freshwater at both water temperatures when compared to that in fed fish (F2,24 = 23.30, Residual = 19.04, P < 0.0001; Fig. 9). There were positive main effects of low freshwater (P = 0.0149) and low seawater (P = 0.0013) on liver glycogen 10 days after seawater transfer (Fig. 10). In addition, an interactive effect of freshwater and seawater was seen on live glycogen content (P = 0.0031), being three times higher in fish kept at low freshwater and seawater temperatures than in other groups (Fig. 10). There was no interaction but a positive main effect of low seawater temperature 20 days (P < 0.0001) and 30 days (P = 0.0128) after transfer. A positive effect of low freshwater temperature was seen on 30 days after transfer (P = 0.0079) and the fish kept at low temperatures maintained higher levels of liver glycogen for 30 days after transfer (P = 0.0023; Fig. 10, Online Resource, Table S1).
Effects of 5-day fasting in freshwater at optimal (Opti; 10 °C) and cold (5 °C) temperature on liver glycogen content in juvenile chum salmon. Control fish were fed ad libitum at the optimal temperature. Values are expressed as means ± SE (n = 8). Asterisks indicate significant difference from control group (Tukey’s HSD, P < 0.05)
Combined effects of freshwater and seawater temperatures on liver glycogen content in juvenile chum salmon. Treated groups were fasted for 5 days in freshwater at optimal (Opti; 10 °C) or cold (5 °C) temperature, transferred to optimal or cold seawater and fed ad libitum for 30 days. Control fish were fed and kept at the optimal temperature throughout the experiment. Values are expressed as means ± SE (n = 8). Asterisks indicate freshwater (FW) and/or seawater (SW) temperatures had main effects or interaction in two-way ANOVA at a given time point. Groups sharing the same letter are statistically not different each other (Tukey’s HSD, P < 0.05)
Discussion
The present study is an expansion of Nakamura et al. (2019), who reported the synergetic negative effect of fasting in freshwater and transfer to cold seawater on the growth of juvenile chum salmon in a short-term. In the present study, we further examined the effect of freshwater temperature in addition to that of seawater temperature for a longer period compared to the previous study (1 month compared to 10 days). The results of the present study highlight the importance of both freshwater and seawater temperature for growth of juveniles during the first month of their ocean life.
Fasting 1 g juvenile chum salmon in freshwater for 5 days was long enough to suppress FL, BW, and K, regardless of water temperature, when compared to the fed control at optimal temperature. Refeeding during and after transfer to seawater at optimal temperature allowed the juveniles to catch up with the control fish in terms of body size 20 days after transfer, although the control fish showed better growth thereafter. Our results support the suggestion of Nakamura et al. (2019) that the feeding status of juvenile chum salmon in the river is a factor affecting growth after sea entry.
Cold freshwater inhibited the growth of fasted fish for at least 10 days after transfer to seawater. This finding implies that river water temperature and food availability influence the growth of juvenile chum salmon during residency in the estuary and shoreline. Morita et al. (2015) found positive correlations between water temperature in the Chitose River during the downstream migration period, the number of down-migrating chum salmon fry, and the fry-to-adult survival rate. Their results suggested that, despite chum salmon spending most of their life in the ocean, river temperature determines juvenile survival. However, it is unknown if cold river water temperature directly increases juvenile chum salmon mortality during their freshwater residency or indirectly by reducing their growth capacity after entry into the ocean. Moreover, both scenarios are possible.
Cold seawater also affected the overall growth of juvenile chum salmon that experienced fasting in freshwater. We previously reported the combined effect of fasting in freshwater and transfer to cold seawater in a short-term experiment (10 days; Nakamura et al. 2019), and the results of the present study indicated that it lasts for a longer period (more than 30 days) in cold seawater. Cold seawater appeared to suppress the appetite of fish fasted in freshwater, which was possibly caused retarded growth, although its mechanism is unknown. These findings again emphasize the importance of nutritional conditions of juvenile chum salmon during their river residency.
Gill NKA activity in fish in seawater was influenced by the freshwater temperature experienced. Enhanced branchial NKA activity is essential for juvenile chum salmon to adapt to seawater. Juvenile anadromous salmon undergo smoltification when the river-type parr transfers to the ocean-type smolts by increasing gill NKA activity while in freshwater (Hoar 1988; McCormick et al. 2013). High gill NKA activity was maintained or further increased upon sea entry (Hoar 1988; McCormick et al. 2013). However, in the present study, fish that were fasted in cold freshwater did not show increased gill NKA activity, whereas that in the other groups was increased. Such a negative effect lasted for at least 20 days. It is not known if reduced gill NKA activity affects juvenile chum salmon growth during that period. The reason for the positive effect on gill NKA of cold freshwater 30 days after transfer to seawater is unknown. In addition, it is worth noting that in situ branchial NKA activity might differ if assayed at the respective acclimated temperatures.
Serum IGF-I responded well to fasting in freshwater and cold seawater temperatures, and may provide a mechanistic basis for growth retardation under these conditions. As expected, serum IGF-I levels were low in fish fasted for 5 days in freshwater. Its levels were restored within 20 days with refeeding in optimal seawater, whereas it took more than 20 days in cold seawater. Given that the feeding rate in fish held in cold freshwater was low, the decrease in serum IGF-I levels is possibly owing to the reduced food intake rather than a direct action of cold water temperature on IGF-I. Although a similar finding was reported by Nakamura et al. (2019), it was a short-term assessment for 10 days and the present study shows that its effect lasted more than 20 days. These findings suggested that when coastal seawater temperature is low, juveniles have reduced growth capacity, as reflected in low circulating IGF-I.
Liver glycogen content in refed fish was considerably increased by the combination of cold freshwater and cold seawater, indicating that excess energy was stored in the liver. Liver glycogen is an important energy reserve for fish to cope with acute stress (Vijayan and Moon 1992; Vijayan et al. 1993). Thus, the fish that experienced fasting in cold freshwater and entered cold seawater might prepare for future stressful conditions by accumulating energy. However, partitioning more energy to glycogen would sacrifice growth. In addition, these fish did not eat well after transfer to cold seawater so their growth was further suppressed. Such compensatory mechanisms may protect fish from acute stress, but expose them to a higher risk for growth-dependent mortality.
The mechanism by which liver glycogen dramatically increased in fish subjected to fasting and cold temperatures is not known at present, but the balance between synthesis, breakdown, and glycogen storage is important. In temperate euryhaline milkfish Chanos chanos, hypothermal stress (18 °C compared to 28 °C) in freshwater accelerated the breakdown of liver glycogen to maintain the energy balance of the body, whereas no changes were seen in fish in seawater (Chang et al. 2018), indicating an interactive effect of water temperature and salinity on metabolism. In the present study, the situation is more complicated because of interactions among feeding status, water temperature, and salinity. However, analyzing enzymes, such as glycogen synthase and phosphorylase, involved in the synthesis and breakdown of glycogen in the liver should be useful to understand glycogen metabolism, which is a subject of future study.
Morita et al. (2015) proposed multiple possible pathways to explain the impact of cold river temperature on juvenile chum salmon survival. Cold river temperature limits the availability of prey items in the river, reduces digestion efficiency of those prey items in the river, directly causes thermal damage, and/or increases predation risk by reducing metabolism and swimming speed. The present study revealed that cold freshwater prevented juvenile chum salmon from restoring growth and serum IGF-I after the 5-day fasting period by reducing appetite and partitioning more energy to the liver, which provides a mechanistic basis for the impact of cold river temperature on juvenile chum salmon growth, although the other possibilities are not mutually exclusive.
The findings of the present study may provide additional considerations on hatchery rearing strategies and parameters to be monitored in the spring. Size at release (i.e., > 1 g) has been one of the most important traits of hatchery-reared chum salmon because of the size-dependent survival during off-shore migration (Saito et al. 2010; Honda et al. 2017, 2020). Releasing fish > 1 g has been recommended in Hokkaido (Seki 2005, 2013). Kasugai et al. (2014) estimated the period taken for downriver migration by chum salmon fry released into the Nishibetsu River, eastern Hokkaido, to be 5–26 days and suggested that nutritional status during that period affected their survival before entry into the sea. The present study further suggests that even for fish around 1 g, poor feeding status in freshwater in addition to low river water temperature would suppress growth in the estuaries and coasts, even when seawater temperatures are optimal and food is available. Therefore, rearing chum salmon juveniles with nutritional supplementation and forage training would make them more robust against low prey abundance in the river, and monitoring river water temperature in addition to coastal water temperature would help determine the optimum release time.
In conclusion, the present study indicated that the negative effects of fasting in freshwater and transfer to cold seawater on the growth of juvenile chum salmon lasts more than 20 days, and cold freshwater also affects growth soon after sea entry. Thus, in addition to coastal seawater temperature at the time of release, the water temperature and food availability in the rivers is important for hatchery release.
References
Bax NJ (1983) Early marine mortality of marked juvenile chum salmon (Oncorhynchus keta) released into Hood Canal, Puget Sound, Washington, in 1980. Can J Fish Aquat Sci 40:426–435
Beacham TD, Neville CM, Tucker S, Trudel M (2017) Is there evidence for biologically significant size-selective mortality of coho salmon during the first winter of marine residence? Trans Am Fish Soc 146:395–407
Beacham TD, Araujo HA, Tucker S, Trudel M (2018) Validity of inferring size-selective mortality and a critical size limit in Pacific salmon from scale circulus spacing. PLoS ONE 13:e0199418
Beamish RJ, Mahnken C (2001) A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog Oceanogr 49:423–437
Beckman BR (2011) Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol 170:233–252
Beckman BR, Shimizu M, Gadberry BA, Cooper KA (2004a) Response of the somatotropic axis of juvenile coho salmon to alterations in plane of nutrition with an analysis of the relationships among growth rate and circulating IGF-I and 41 kDa IGFBP. Gen Comp Endocrinol 135:334–344
Beckman BR, Shimizu M, Gadberry BA, Parkins PJ, Cooper KA (2004b) The effect of temperature change on the relations among plasma IGF-I, 41-kDa IGFBP, and growth rate in postsmolt coho salmon. Aquaculture 241:601–619
Chang C-H, Huang J-J, Yeh C-Y, Tang C-H, Hwang L-Y, Lee T-H (2018) Salinity effects on strategies of glycogen utilization in livers of euryhaline milkfish (Chanos chanos) under hypothermal stress. Font Physiol 9:81
Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through fry’s concept of aerobic metabolic scope. J Fish Biol 88:232–251
Daughaday WH, Rotwein P (1989) Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10:68–91
Dreiling CE, Brown DE, Casale L, Kelly L (1987) Muscle glycogen: comparison of iodine binding and enzyme digestion assays and application to meat samples. Meat Sci 20:167–177
Duffy EJ, Beauchamp DA (2011) Rapid growth in the early marine period improves the marine survival of Chinook salmon (Oncorhynchus tshawytscha) in Puget Sound, Washington. Can J Fish Aquat Sci 68:232–240
Duncan DH, Beaudreau AH (2019) Spatiotemporal variation and size-selective predation on hatchery- and wild-born juvenile chum salmon at marine entry by nearshore fishes in Southeast Alaska. Mar Coast Fish 11:372–390
Farley EV, Moss JH, Beamish RJ (2007) A review of the critical size, critical period hypothesis for juvenile Pacific salmon. N Pac Anadr Fish Comm Bull 4:311–317
Fukuwaka M, Suzuki T (2002) Early sea mortality of mark-recaptured juvenile chum salmon in open coastal waters. J Fish Biol 60:3–12
Gabillard JC, Weil C, Rescan PY, Navarro I, Gutierrez J, Le Bail PY (2005) Does the GH/IGF system mediate the effect of water temperature on fish growth? A review. Cybium 29:107–117
Healey MC (1982) Timing and relative intensity of size-selective mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can J Fish Aquat Sci 39:952–957
Hoar WS (1988) The physiology of smolting salmonids. In: Hoar WS, Randall D (eds) Fish physiology 11B. Academic Press, Orland, pp 274–343
Honda K, Kawakami T, Suzuki K, Watanabe K, Saito T (2017) Growth rate characteristics of juvenile chum salmon Oncorhynchus keta originating from the Pacific coast of Japan and reaching Konbumori, eastern Hokkaido. Fish Sci 83:987–996
Honda K, Shirai K, Komatsu S, Saito T (2020) Sea-entry conditions of juvenile chum salmon Oncorhynchus keta that improve post-sea-entry survival: a case study of the 2012 brood-year stock released from the Kushiro River, eastern Hokkaido, Japan. Fish Sci 86:783–792
Kaneko N, Taniyama N, Inatani Y, Nagano Y, Fujiwara M, Torao M, Miyakoshi Y, Shimizu M (2015) Circulating insulin-like growth factor I in juvenile chum salmon: relationship with growth rate and changes during downstream and coastal migration in northeastern Hokkaido, Japan. Fish Physiol Biochem 41:991–1003
Kaneko N, Torao M, Koshino Y, Fujiwara M, Miyakoshi Y, Shimizu M (2019) Evaluation of growth status using endocrine growth indices, insulin-like growth factor (IGF)-I and IGF-binding protein-1b, in out-migrating juvenile chum salmon. Gen Comp Endocrinol 274:50–59
Kashiwagi M, Sato R (1969) Studies on the osmoregulation of the chum salmon, Oncorhynchus keta (Walbaum). I. The tolerance of eyed period eggs, alevins and fry of the chum salmon to sea water. Tohoku J Agric Res 20:41–47
Kasugai K, Takeuchi K, Miyakoshi Y, Nagata M (2014) Estimation of number of downstream migrating chum salmon fry in the Nishibetsu River in 2006. Sci Rep Hokkaido Fish Res Inst 85:37–40 (In Japanese with English abstract)
Kitada S (2014) Japanese chum salmon stock enhancement: current perspective and future challenges. Fish Sci 80:237–249
Le Roith D, Bondy C, Yakar S, Liu JL, Butler A (2001) The somatomedin hypothesis: 2001. Endocr Rev 22:53–74
McCormick SD (2013) Smolt physiology and endocrinology. In: McCormick SD et al (eds) Euryhaline fishes. Academic Press, Oxford, UK, pp 199–251
Miyakoshi Y, Nagata M, Kitada S, Kaeriyama M (2013) Historical and current hatchery programs and management of chum salmon in Hokkaido, northern Japan. Rev Fish Sci 21:469–479
Mizuno S (2012) Studies on improvement of seed production techniques in Salmonids and Osmerids. Aqua-Biosci 5:103–143
Morita K, Nakashima A (2015) Temperature seasonality during fry out-migration influences the survival of hatchery-reared chum salmon Oncorhynchus keta. J Fish Biol 87:1111–1117
Morita K, Saito T, Miyakoshi Y, Fukuwaka MA, Nagasawa T, Kaeriyama M (2006) A review of Pacific salmon hatchery programmes on Hokkaido Island, Japan. ICES J Mar Sci 63:1353–1363
Morita K, Nakashima A, Kikuchi M (2015) River temperature drives salmon survivorship: is it determined prior to ocean entry? Roy Soc Open Sci 2:140312
Moss JH, Beauchamp DA, Cross AD, Myers KW, Farley EV, Murphy JM, Helle JH (2005) Evidence for size-selective mortality after the first summer of ocean growth by pink salmon. Trans Am Fish Soc 134:1313–1322
Nagata M, Miyakoshi Y, Ando D, Fujiwara M, Sawada M, Shimada H, Asami H (2007) Influence of coastal seawater temperature on the distribution and growth of juvenile chum salmon, with recommendations for altered release strategies. N Pac Anadr Fish Comm Bull 4:223–235
Nagata M, Miyakoshi Y, Urabe H, Fujiwara M, Sasaki Y, Kasugai K, Torao M, Ando D, Kaeriyama M (2012) An overview of salmon enhancement and the need to manage and monitor natural spawning in Hokkaido, Japan. Environ Biol Fish 94:311–323
Nagata M, Miyakoshi Y, Fujiwara M, Kasugai K, Ando D, Torao M, Saneyoshi H, Irvine JR (2016) Adapting Hokkaido hatchery strategies to regional ocean conditions can improve chum salmon survival and reduce variability. N Pac Anadr Fish Comm Bull 6:73–85
Nakamura S, Kaneko N, Nonaka T, Kurita D, Miyakoshi Y, Shimizu M (2019) Fasting in freshwater severely affects growth of juvenile chum salmon when entering cold seawater. Fish Sci 85:655–665
Ohlsson C, Mohan S, Sjögren K, Tivesten Å, Isgaard J, Isaksson O, Jansson JO, Svensson J (2009) The role of liver-derived insulin-like growth factor-I. Endocr Rev 30:494–535
Picha ME, Turano MJ, Beckman BR, Borski RJ (2008) Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. N Am J Aquacult 70:196–211
Quabius ES, Balm PHM, Bonga SEW (1997) Interrenal stress responsiveness of tilapia (Oreochromis mossambicus) is impaired by dietary exposure to PCB 126. Gen Comp Endocrinol 108:472–482
Reinecke M (2010) Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J Fish Biol 76:1233–1254
Saito T, Shimizu I, Seki J, Kaga T, Hasegawa E, Saito H, Nagasawa K (2010) Can research on the early marine life stage of juvenile chum salmon Oncorhynchus keta forecast returns of adult salmon? A case study from eastern Hokkaido, Japan. Fish Sci 76:909–920
Saito T, Kaga T, Hasegawa E, Nagasawa K (2011) Effects of juvenile size at release and early marine growth on adult return rates for Hokkaido chum salmon (Oncorhynchus keta) in relation to sea surface temperature. Fish Oceanogr 20:278–293
Salo EO (1991) Life history of chum salmon (Onocorhynchus keta). In: Groot C, Margolis L (eds) Pacific salmon life histories. UBC Press, Vancouver, BC, Canada, pp 231–310
Seki J (2005) Study of characteristics of feeding habitat of juvenile chum salmon and their food environment in the Pacific coastal waters, central part of Hokkaido. Bull Nat Salmon Resour Ctr 7:1–104 (In Japanese with English abstract)
Seki J (2013) Development of hatchery techniques for releasing juvenile chum salmon in Japan. J Fish Technol 6:69–82 ((In Japanese with English abstract))
Shimizu M, Swanson P, Fukada H, Hara A, Dickhoff WW (2000) Comparison of extraction methods and assay validation for salmon insulin-like growth factor-I using commercially available components. Gen Comp Endocrinol 119:26–36
Small BC, Peterson BC (2005) Establishment of a time-resolved fluoroimmunoassay for measuring plasma insulin-like growth factor I (IGF-I) in fish: effect of fasting on plasma concentrations and tissue mRNA expression of IGF-I and growth hormone (GH) in channel catfish (Ictalurus punctatus). Domest Anim Endocrinol 28:202–215
Takahashi S, Hasegawa K, Ito H, Ban M, Miyauchi Y (2016) Comparisons of growth of chum salmon fry released into rivers of which temperature and prey abundance conditions were different. Nippon Suisan Gakkaishi 82:559–568 (In Japanese with English abstract)
Taniyama N, Kaneko N, Inatani Y, Miyakoshi Y, Shimizu M (2016) Effects of seawater transfer and fasting on the endocrine and biochemical growth indices in juvenile chum salmon (Oncorhynchus keta). Gen Comp Endocrinol 236:146–156
Tucker S, Hipfner JM, Trudel M (2016) Size- and condition-dependent predation: a seabird disproportionately targets substandard individual juvenile salmon. Ecology 97:461–471
Urawa S, Beacham TD, Fukuwaka M, Kaeriyama M (2018) Ocean ecology of chum salmon. In: Beamish RJ (ed) The ocean ecology of Pacific salmon and Trout. American Fisheries Society, Bethesda, MD, pp 161–317
Vijayan MM, Moon TW (1992) Acute handling stress alters hepatic glycogen metabolism in food-deprived rainbow trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 49:2260–2266
Vijayan MM, Maule AG, Schreck CB, Moon TW (1993) Hormonal control of hepatic glycogen metabolism in food-deprived, continuously swimming coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 50:1676–1682
Wertheimer AC, Thrower FP (2007) Mortality rates of chum salmon during their early marine residency. In: Grimes CB, Brodeur RD, Haldorson LJ, McKinnell SM (eds) The ecology of juvenile salmon in the northeast Pacific Ocean: regional comparisons. American Fisheries Society, Bethesda, MD, pp 233–247
Wong MKS, Nobata S, Hyodo S (2019) Enhanced osmoregulatory ability marks the smoltification period in developing chum salmon (Oncorhynchus keta). Comp Biochem Physiol A 238:110565
Zavolokin AV, Strezhneva EV (2013) Size-selective mortality of Sea of Okhotsk pink salmon in the ocean in the winter and spring. Russ J Mar Biol 39:501–508
Acknowledgements
We thank the staff of the Kitami Salmon Enhancement Programs Association for providing juvenile chum salmon. This work was supported by grants from the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number 18K05801, and the JSPS Bilateral Joint Research Project (Open Partnership with Norway) Grant Number JPJSBP120209901. We acknowledge Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oikawa, J., Nakamura, S., Kaneko, N. et al. Effects of fasting and water temperatures during transition from freshwater to seawater on juvenile chum salmon growth and metabolism. Fish Sci 87, 579–588 (2021). https://doi.org/10.1007/s12562-021-01526-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-021-01526-5