Abstract
We conducted rearing experiments to examine the cumulative effects of fasting in freshwater and transfer to cold seawater on the growth of juvenile chum salmon. In the first experiment in May 2016, juvenile fish were either fed or fasted in freshwater for 5 days and acclimatized to seawater of either optimal (10 °C) or cold (5 °C) temperature for 10 days with feeding. Despite resumed feeding, fish that were fasted in freshwater and transferred to cold seawater showed the poorest growth. Serum levels of insulin-like growth factor (IGF)-I, a positive indicator of growth, were also the lowest in this group, suggesting a synergistic negative effect of fasting in freshwater and transfer to cold seawater on growth in chum salmon. A similar experiment in May 2017 suggested that the depressed growth and serum IGF-I in cold seawater might be due to the predominant allocation of energy to liver glycogen. On the one hand, serum levels of IGF-binding protein-1b, a negative indicator of growth, were not affected by seawater temperature but by fasting in seawater. The present study shows that relatively short-termed fasting in freshwater critically affects growth of juvenile chum salmon transferred to cold seawater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chum salmon Oncorhynchus keta is an important commercial fish in northern Japan and is sustained largely by intensive releases from hatcheries (Morita et al. 2006; Nagata et al. 2012; Miyakoshi et al. 2013; Kitada 2014). Approximately 1.8 billion juvenile salmon are released from hatcheries in northern Japan each year. Despite significant efforts, there are large regional variations in adult return rates. Moreover, in the past few years, adult return rates on the Pacific side have declined dramatically, with an average return of about 35% (https://salmon.fra.affrc.go.jp/zousyoku/ok_relret.html). Although the reasons for this variation and for the declining pattern in general are unclear, it was suggested that climate change alters the currents and temperatures of coastal waters from spring to summer, affecting the growth of juvenile chum salmon (Morita and Nakashima 2015; Wagawa et al. 2016).
Mark–recapture studies have shown high mortality rates for juvenile chum salmon soon after sea entry (Healey 1982; Bax 1983; Fukuwaka and Suzuki 2002; Wertheimer and Thrower 2007). While the rates of mortality vary depending on the efficiency of the recapture method and on the applied models, it is considerably high, at 2.9–8.1% per day (Fukuwaka and Suzuki 2002; Wertheimer and Thrower 2007). Comparably high mortality rates have also been observed in other salmonids, which is likely a growth-dependent effect (Beamish and Mahnken 2001; Zavolokin and Strezhneva 2013; Tucker et al. 2016). Honda et al. (2017) compared growth histories of juvenile chum salmon of different regional origins caught near the eastern Hokkaido region by analyzing the otolith growth increment. Growth of juveniles from regions closer to eastern Hokkaido (i.e., Kushiro and Tokachi) was poorer than that of individuals from regions far from the sampling site (i.e., Sanriku coast), thus it was hypothesized that individuals from more distant regions may have undergone size-dependent mortality before reaching the sampling site (Honda et al. 2017). Therefore, size at the time of release and subsequent growth are critical factors affecting the survival of juvenile chum salmon. Furthermore, it is important to identify abiotic and biotic factors affecting salmon growth along the coasts.
Sea surface temperature (SST) is a key factor determining the distribution along the coast and the growth of out-migrating juvenile chum salmon (Mayama and Ishida 2003; Nagata et al. 2007, 2016). In early spring when SST is below 8 °C, juveniles are restricted to the littoral zone and may experience considerable competition for food (Nagata et al. 2007). A SST ranging from 7 to 11 °C is optimal for juvenile chum salmon to increase their distribution for active feeding (Nagata et al. 2016). When the SST exceeds 13 °C, juvenile salmon in this area leave the coastal waters and move to the Sea of Okhotsk. Thus, SST can directly affect growth of juvenile chum salmon by altering metabolic rates (Gabillard et al. 2005; Kaeriyama et al. 2007) and indirectly through affecting the degree of food competition.
So far, size at release, SST, and abundance of zooplankton were the three major parameters explaining regional adult return rates (Nagata et al. 2007, 2016; Saito and Nagasawa 2009; Saito et al. 2009, 2010, 2011). A mismatch of SST and the timing of release can cause substantial mortality. Thus, hatchery managers attempt to release juveniles weighing more than 1 g when the coastal seawater temperature can be expected to maximize the probability of survival (i.e., 7–11 °C). However, Saito and Nagasawa (2009) and Saito et al. (2010) predicted that also other factors would affect the survival rates of juvenile and young chum salmon, which were not included in their prediction models for chum salmon stocks. In order to establish the optimal management scheme for hatchery releases, it would be necessary to identify other factors and their interactions with size at release, SST, and abundance of zooplankton.
The effect of the freshwater environment on growth of juvenile chum salmon released from hatcheries has received little attention. Released juveniles spend days to weeks in the rivers, depending on the watershed (Kasugai et al. 2014), and a substantial proportion of juveniles may die before sea entry (Morita et al. 2015). Nutritional and physiological conditions can be expected to affect survival and growth of remaining individuals. However, it remains unknown how the cumulative effects of the freshwater and seawater environments and their complex interactions affect growth of juvenile salmon. In order to disentangle and assess the combined effects of freshwater and seawater environments on growth of juvenile salmon, rearing experiments under controlled conditions are needed and tools to monitor short-term changes in growth would be required.
Fish growth is predominantly regulated by the growth hormone (GH)-insulin-like growth factor (IGF)-I system (Reinecke 2010). GH from the pituitary gland promotes growth directly by affecting the target tissues and indirectly through inducing hepatic production of IGF-I. Circulating IGF-I was proposed to be a useful index of growth in several fish taxa, including the salmonids (Picha et al. 2008; Beckman 2011). Typically, individual growth rate is positively correlated with serum/plasma IGF-I. In a previous study, we showed that serum IGF-I was a robust marker for the assessment of the growth status of juvenile chum salmon and reflects the growth rate of the past 5–10 days (Kaneko et al. 2015; Taniyama et al. 2016). IGF-I measurements should be analyzed with care as the circulating concentrations may be affected by a rapid change in water temperature, independently of growth (Beckman et al. 2004b); however, despite this methodological limitation, IGF-I measurements can provide useful information on the recent and current growth status in changing environments.
IGF-binding proteins (IGFBPs) are also useful indices of growth in salmonids (Beckman et al. 2004a; b; Shimizu et al. 2006; Kawaguchi et al. 2013). IGFBPs modulate the actions of circulating IGF-I by inhibiting its binding to the receptor and/or prolonging the half-life of IGF-I and delivering it to the receptor (Rajaram et al. 1997; Shimizu and Dickhoff 2017; Allard and Duan 2018). Three major circulating IGFBPs have been identified in salmon (Shimizu et al. 2005, 2011a, b), and one of them, IGFBP-1b, has been used as a negative index of growth in juvenile chum salmon (Taniyama et al. 2016; Kaneko et al. 2019). The concentrations of this protein are increased under catabolic conditions such as fasting and stress, and are negatively correlated with growth rate. IGFBP-1b and IGF-I may differ regarding sensitivity to changes in the metabolic state or in the environment. Our experiments suggest that combining IGFBP-1b and IGF-I measurements improves methodological sensitivity and accuracy in order to assess salmon growth in the field (Kaneko et al. 2019).
Using endocrine growth indices, the present study aimed to unravel the combined effects of feeding status during the freshwater phase and SST on the growth of juvenile chum salmon under laboratory conditions.
Materials and methods
Fish
Chum salmon fry were transferred from a local hatchery in northeastern Hokkaido (Kamisato Hatchery; Tsubetu, Abashiri-gun, Japan) to an indoor rearing facility at the Faculty of Fisheries Sciences, Hokkaido University, in May 2016 and 2017. Fish were reared in glass tanks (60 × 29.5 × 36 cm) filled with 60 L freshwater in a temperature-controlled room (10 °C), and each tank had a closed circulation system with a filtration unit in the upper half (GEX, Osaka, Japan). Until the start of the experiment, the fish were fed a commercial diet ad libitum once per day (Marubeni Nisshin Feed Co. Ltd., Tokyo, Japan). All experiments were carried out in accordance with the guidelines of the Hokkaido University Animal Care and Use Committee.
Rearing experiment 1: effects of fasting in freshwater and feeding in cold seawater
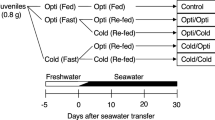
The experimental design is shown in Fig. 1. In May 2016, a total of 102 fish were placed in two 60 L tanks and were acclimated for 1 week and fed as described above. The fish in one tank were fed the commercial diet once per day, provided at an amount of 3.0% of the body weight per day for 5 days; the fish in the other tank were fasted for 5 days. The fish were acclimated to artificial seawater ([SW]; Tetra Marine Salt Pro; Spectrum Brands Inc., Madison, WI, USA) by gradually increasing the salinity to 31–34 g kg−1 (100% SW), as follows. On day 6, the first half of each group was transferred to two 40 L tanks (45 × 30 × 30 cm) filled with 50% SW at 10 °C. The second half of each group was transferred to a different temperature-controlled room (5 °C) and was placed in two 40 L tanks filled with 50% SW that was kept at 9 °C using a portable heater. On day 7, a quarter of the 50% SW was removed from the tanks and the same amount of 145% SW was added to produce 75% SW. The water temperature of two 75% SW tanks was reduced from 9 to 7 °C. On day 8, the fish were transferred to another set of tanks filled with 100% SW at 10 °C or 5 °C, and ten to twelve fish from each group were placed in two 40 L tanks to produce replicates. Fish were fed for 4 days during the acclimatization period and for an additional 6 days after that. The combinations of feeding/fasting in freshwater and optimal/cold seawater thus produced four treatment groups: fed at optimal temperature (Opti), fasted (Fast)/Opti, fed at cold temperature (Cold), and Fast/Cold. The experiment was repeated in June 2016. Mortality during the two experiments was within two fish/treatment.
Designs of rearing experiments. Experiment 1 (Exp 1) were conducted in May and June 2016. Juvenile chum salmon were either fed or fasted (Fast) for 5 days, transferred to either optimal (10 °C; Opti) or cold (5 °C) seawater, and maintained at the same temperature with feeding. Experiment 2 (Exp 2) was conducted in late May 2017
Eight fish from each treatment were sampled at 0, 5, 10, and 15 days after the start of the experiment. Fish were anesthetized using 3.3% 2-phenoxyethanol (Kanto Chemical, Tokyo, Japan). Fork length (FL) and body weight (BW) were measured, after which the tail was cut, and blood was drawn using plain 10 or 20 μl glass tubes (Microcap; Drummond Scientific Company, Broomall, PA, USA). Blood was left to clot at 4 °C overnight and was then centrifuged at 10,000 rpm for 15 min. Serum was collected and stored at −80 °C until use. The gill filaments were collected, frozen immediately on dry ice, and stored at −80 °C. Condition factor (K) was calculated as: (BW (g)) × 100/(FL (cm))3.
Rearing experiment 2: effects of fasting in freshwater and fasting in cold seawater
The experimental design is shown in Fig. 1. In late May 2017, a total of 200 fish were placed in two 60 L tanks and were allowed to acclimatize for 1 week and fed as described above. Fish in one tank were fed once per day using the commercial diet, provided at 3.0% of the body weight per day for 5 days; fish in the other tank were fasted for 5 days. The fish were acclimated to 100% SW as described above. Fish were either fed at 2.0% of the body weight per day or fasted during the acclimatization period for 4 days and for an additional 6 days after the transfer. The combinations of feeding or fasting in seawater and optimal or cold seawater produced four treatment groups: Opti/Fed, Opti/Fast, Cold/Fed, and Cold/Fast. An additional group which was fed throughout the experiment and transferred to optimal seawater was used as a control. Mortality during the experiment was within two fish/treatment. Sixteen fish from each treatment were sampled 0, 5, 10, and 15 days after the beginning of the experiment as described above. Additionally, livers were collected and weighed. Samples of the livers were then excised, weighed, frozen on dry ice, and stored at −80 °C until use.
Na+, K+-ATPase (NKA) activity assay
Gill NKA activity was measured according to Quabius et al. (1997) with a minor modification (i.e., correction of a wrong concentration of sulfuric acid from 0.66 mM to 0.66 M). The protein concentration was measured using a BCA (bicinchoninic acid) Protein Assay Kit (Thermo Scientific, IL, USA). Enzymatic activity was expressed as Pi (µmol) per mg protein per h.
Time-resolved fluoroimmunoassay (TR-FIA)
For measuring IGF-I, serum was first extracted using acid–ethanol, as described by Shimizu et al. (2000). IGF-I was quantified using a TR-FIA, based on the method described by Small and Peterson (2005) and using recombinant salmon/trout IGF-I (GroPep Bioreagents Pty Ltd, Adelaide, Australia) as a standard. Time-resolved fluorescence was measured using a Wallac ARVO SX or Wallac ARVO X4 multilabel counter (PerkinElmer, Waltham, MA, USA).
Serum IGFBP-1b levels were quantified by TR-FIA as described by Fukuda et al. (2015). Briefly, a competitive method was employed following a procedure for DELFIA immunoassays (PerkinElmer). First, serum samples were incubated at 4 °C overnight with antiserum against purified salmon IGFBP-1b in a 96-well microtiter plate coated with goat anti-rabbit IgG (PerkinElmer). Biotinylated salmon IGFBP-1b was added to each well and incubated at 4 °C overnight. After washing with DELFIA wash buffer (PerkinElmer), each well received europium-labeled streptavidin (PerkinElmer) and DELFIA Enhancement Solution (PerkinElmer). Time-resolved fluorescence was measured at 615 nm using a Wallac ARVO X4 multilabel counter (PerkinElmer).
Glycogen content
Liver glycogen content was measured according to Dreiling et al. (1987). Briefly, liver pieces (20–30 mg) were homogenized in cold 10% perchloric acid (Sigma-Aldrich) and centrifuged at 10,000 rpm and 4 °C for 5 min. The supernatants were mixed with an I2-KI solution containing saturated CaCl2. The absorbance was measured at 460 nm using an ARVO X4 multilabel counter. Values are expressed as a percentage of the liver weight (g).
Statistical analyses
The effect of fasting in freshwater was assessed using a t test. Results of experiment 1 on days 10 and 15 were analyzed using a three-way ANOVA (fasting × temperature × time) with JMP software (SAS Institute Inc., Cary, NC, USA). When significant effects were found, differences were tested using a one-way ANOVA followed by a Fisher’s protected least significant difference (PLSD) test. Differences among groups were considered significant at P < 0.05. Results of experiment 2 were analyzed using a three-way ANOVA and excluding the control group. Differences among groups including the control group were then tested using a one-way ANOVA followed by a Fisher’s PLSD test, as described above.
Results
Rearing experiment 1: effects of fasting in freshwater and feeding in cold seawater
Fasting for 5 days in freshwater did not affect FL but affected BW and K in May 2016 (Online Resource, Table S1). Fasting in freshwater consistently affected FL and BW in seawater but not on K. Seawater temperature also had overall effects on BW and K. On day 10 after the transfer to seawater, FL and BW were lowest in the Fast/Cold group whereas K was low in both, the Fed/Cold and Fast/Cold groups (Fig. 2a, c, e). In June, fasting for 5 days in freshwater did not affect FL and BW but affected K (Online Resource, Table S1). There were consistent effects of fasting in freshwater, seawater temperature, and time on FL and BW in seawater, whereas no respective effects on K were observed. On day 10 after transfer to seawater, FL and BW were lowest in the Fast/Cold group, whereas K did not differ between groups (Fig. 2b, d, f).
Effects of fasting in freshwater and transfer to cold seawater on fork length (FL; a, b), body weight (BW; c, d) and condition factor (K; e, f) in May (a, c, e) and June (b, d, f) 2016. Juvenile chum salmon were either fed or fasted (Fast) for 5 days, transferred to either optimal (10 °C; Opti) or cold (5 °C) seawater, and maintained at the same temperature with feeding. Value are expressed as means ± SE (n = 8). Asterisks (*) and daggers (†) indicate parameters and their combinations having overall effect and interaction, respectively. Groups sharing the same letter are not significantly different
There was no effect of fasting for 5 days in freshwater on gill NKA activity (fed: 8.9 ± 0.6 µmol Pi/mg/h; fasted: 9.1 ± 0.7 µmol Pi/mg/h; n = 8). However, fasting in freshwater had a positive effect on gill NKA activity 10 days after the transfer to optimal or cold seawater (Table 1). In June, a positive effect of fasting for 5 days in freshwater on gill NKA activity was observed (fed: 8.9 ± 0.5 µmol Pi/mg/h; fasted: 11.0 ± 0.7 µmol Pi/mg/h; n = 8). Gill NKA activity was highest in the Cold/Fed group 5 days after the transfer to seawater, however, this difference was not observed 10 days after the transfer (Table 1).
In May, fasting in freshwater significantly affected serum IGF-I levels (fed: 10.6 ± 1.0 ng/ml; fasted: 4.9 ± 2.5 ng/ml; n = 8). Serum IGF-I levels were lowest in the Fast/Cold group 5 days after the transfer to seawater, and they were not restored 10 days after the transfer (Fig. 3a). No significant effect of fasting in freshwater on serum IGF-I was observed in June (fed: 16.3 ± 5.7 ng/ml; fasted: 5.2 ± 2.0 ng/ml; n = 8). The Fast/Cold group showed a trend of low IGF-I levels 5 days after the transfer, which was, however, not significant (Fig. 3b). No significant difference in IGF-I levels was observed between groups 10 days after the transfer.
Effects of fasting in freshwater and transfer to cold seawater on serum insulin-like growth factor (IGF)-I in May (a) and June (b) 2016. Juvenile chum salmon were either fed or fasted (Fast) for 5 days, transferred to either optimal (10 °C; Opti) or cold (5 °C) seawater, and maintained at the same temperature with feeding. Value are expressed as means ± SE (n = 8). Asterisks (*) indicate parameters having overall effect. Groups sharing the same letter are not significantly different
Rearing experiment 2: effects of fasting in freshwater and in cold seawater
Fasting for 5 days in freshwater did not affect FL but affected BW and K in the end of May 2017 (Online Resource, Table S2). There were overall effects of fasting in seawater on FL, BW, and K. On day 10 after the transfer to seawater, FL and BW values of the Cold/Fed group were significantly lower than those of the Opti/Fed group (Fig. 4a, b). In contrast, K was affected by feeding conditions, but no significant effect of seawater temperature was observed (Fig. 4c). No significant differences in feeding rates were observed between the fed groups (Online Resource, Fig. S1).
Effects of fasting in freshwater and transfer to cold seawater with or without feeding on fork length (FL; a), body weight (BW; b) and condition factor (K; c) in late May 2017. Juvenile chum salmon were either fasted (Fast) for 5 days, transferred to either optimal (10 °C; Opti) or cold (5 °C) seawater, and maintained at the same temperature with or without feeding. Value are expressed as means ± SE (n = 8). Asterisks (*) and daggers (†) indicate parameters and their combinations having overall effect and interaction, respectively. Groups sharing the same letter are not significantly different
No significant effect of fasting for 5 days in freshwater on gill NKA activity was observed (fed: 6.3 ± 1.1 µmol Pi/mg/h; fasted: 6.5 ± 0.3 µmol Pi/mg/h; n = 8). Seawater temperature and feeding condition in seawater produced no significant effect on gill NKA (Table 2). However, fasting in freshwater had a significant positive effect on gill NKA activity (Table 2).
Fasting in freshwater had a negative effect on serum IGF-I levels (fed: 11.7 ± 1.1 ng/ml; fasted: 4.8 ± 1.2 ng/ml; n = 8). Serum IGF-I levels of the Cold/Fed group were lower than those of the Opti/Fed group 10 days after the transfer to seawater and were similar to those of fish fasted in seawater (i.e., Cold/Fast and Opti/Fast) (Fig. 5a). Serum IGFBP-1b levels were higher in fish fasted in freshwater (fed: 22.4 ± 3.5 ng/ml; fasted: 188.1 ± 23.9 ng/ml; n = 8). After transfer to seawater, serum IGFBP-1b levels were higher in fasted fish regardless of the seawater temperature (Fig. 5b). Fasting in freshwater for 5 days significantly reduced liver glycogen content (fed: 17.1 ± 2.6 mg/g liver weight; fasted: 6.5 ± 0.7 mg/g liver weight; n = 8). Liver glycogen contents were restored by resumption of feeding in seawater at optimal temperature, and fed fish in cold seawater had the highest liver glycogen content, which was sevenfold higher than that of fed fish (Fig. 5c).
Effects of fasting in freshwater and transfer to cold seawater with or without feeding on serum insulin-like growth factor (IGF)-I (a), serum IGF-binding protein (IGFBP)-1b (b) and liver glycogen content (c) in late May 2017. Juvenile chum salmon were either fasted (Fast) for 5 days, transferred to either optimal (10 °C; Opti) or cold (5 °C) seawater, and maintained at the same temperature with or without feeding. Value are expressed as means ± SE (n = 8). Asterisks (*) and daggers (†) indicate parameters and their combinations having overall effect and interaction, respectively. Groups sharing the same letter are not significantly different
Discussion
The release of juvenile salmon during times of unfavorable environmental conditions is a major issue for hatchery programs of chum salmon, as it can substantially affect the degree of growth-dependent mortality in juveniles during the early marine life stage (Nagata et al. 2007, 2016; Saito et al. 2010, 2011). However, the respective mechanisms leading to growth retardation of juveniles are not well understood. Juvenile chum salmon are released from hatcheries to rivers from where they migrate to the ocean; therefore, the timing of juvenile release and their physiological and nutritional condition before entering seawater are key for a successful transition to marine life. However, it is challenging to disentangle the effects of freshwater and seawater environments in the field. The present study examined their combined effects under controlled laboratory conditions.
Effects of fasting in freshwater and feeding in cold seawater
Five days of fasting in freshwater is below the starvation tolerance limit in juvenile chum salmon weighing 1–1.5 g, which is approximately 30 days (Kaeriyama and Kumagai 1984; Ban et al. 1996); however, this time span is sufficient to reduce BW and K in most cases. Such effects on BW lasted for at least 10 days after resumption of feeding in both seawater temperatures. Low seawater temperature inhibited the restoration of K. The prolonged effects of fasting in freshwater on BW and K may be one way by which low seawater temperature exerts its negative effect on juvenile chum salmon in coastal waters.
Growth retardation in cold seawater in fish fasted in freshwater was unlikely a direct effect of failed acclimatization to seawater. Plasma ion levels and osmolality were not measured in the current study; however, equal or higher gill NKA activity in fish fasted in freshwater suggests that fish acclimatized well to seawater, irrespective of the temperature. In agreement with this, Ban et al. (1996) reported that juvenile chum salmon fasted in freshwater for 20 days retained the ability to maintain plasma sodium ion concentrations 24 h after seawater transfer. In their study, however, fasting in freshwater for 20 days reduced the 24 h survival rate in seawater, suggesting high energetic costs of maintaining ion levels. Although the fasting period (5 days) in the present study was shorter than those used by Ban et al. (1996), the energetic costs for hypo-osmoregulation must be considered in this comparison. A notable result of the present study was that fasting in freshwater had a positive effect on gill NKA activity. This positive effect of fasting in freshwater was also observed after the transfer to both cold and optimal seawater; however, the mechanism behind this increase in gill NKA activity in fasted fish remains to be unraveled.
One of the major findings of the present study was that growth status, as determined based on serum IGF-I levels, was lowest in fish fasted in freshwater and transferred to cold seawater in May. In coho salmon O. kisutch, cold freshwater had a negative effect on circulating IGF-I for at least 2 weeks, irrespective of the feeding status (Larsen et al. 2001; Beckman et al. 2004b). In the present study, cold seawater showed no negative effect on serum IGF-I as long as fish were fed in both freshwater and seawater. Although fish were fed after the transfer to cold seawater, the preceding fasting period in freshwater apparently hampered subsequent growth. Our results are partially in line with the findings of Picha et al. (2014) who examined the effects of water temperature and fasting on circulating IGF-I and growth of a hybrid striped bass Morone chrysops × M. saxatilis. Fasting at 24 °C decreased plasma IGF-I levels in the hybrid striped bass, and resumption of feeding at 14 °C did not restore IGF-I levels for at least 1 month (Picha et al. 2014), which suggests slow restoration of growth functions in cold water. In the present study, low temperature likely reduced the metabolic rate of juvenile salmon which slowed the recovery of serum IGF-I levels and body size; however, a possible interaction effect of salinity and water temperature should be considered in future studies.
The cumulative effect of fasting in freshwater and cold seawater on serum IGF-I was somewhat different in June. In this experiment, a tendency of reduced serum IGF-I levels was observed in fish fasted in freshwater and transferred to cold seawater, which was, however, not statistically significant. One reason for this was the large variation in serum IGF-I levels within a treatment. Fish used in the experiment in June were larger than those used in May, thus large fish may be more tolerant to fasting in freshwater. A different possible explanation may be a seasonal increase in the baseline levels of serum IGF-I, as IGF-I levels were generally higher in June than in May. Seasonal changes in serum IGF-I levels were also observed in out-migrating juvenile chum salmon caught in the coastal waters of the Abashiri region, northeast Hokkaido (Kaneko et al. 2015, 2019). The prolonged effect of fasting in freshwater may thus be size/stage- and/or season-dependent.
Effects of fasting in freshwater and in cold seawater
A similar experiment was conducted in late May 2017 and confirmed a synergistic negative effect of fasting in freshwater and cold seawater on BW of juvenile chum salmon. However, BW of fish fasted in freshwater and fed in cold water recovered to some extent, as it was heavier than in fish fasted in cold seawater. In addition, K did not differ between seawater temperatures when fish were fed, suggesting they reduced growth but not condition. These results support the assumption that the cumulative effect of fasting in freshwater and cold seawater synergistically slows the growth of juvenile salmon.
The response of serum IGF-I in fish of about 1 g was similar to that observed in May 2016: IGF-I levels were low when fish were fasted in freshwater and transferred to cold seawater. Moreover, the reduced IGF-I levels 10 days after resumption of feeding were still as low as those in fish fasted in seawater, indicating slow recovery of growth functions. These results suggest that juvenile chum salmon of about 1 g are more susceptible to fasting in freshwater and unfavorable seawater temperatures have a strong adverse effect during the early down-migration season.
The present study used serum IGFBP-1b as a negative index of growth, and this marker produced a different picture of endocrine responses to the treatments. In general, circulating IGFBP-1b levels in fish are high under catabolic conditions such as fasting and stress (Kelley et al. 2001; Shimizu and Dickhoff 2017). A change in water temperature can also affect IGFBP-1b levels in coho salmon (Shimizu et al. 2006). In the present study, serum IGFBP-1b was not affected by seawater temperature, whereas feeding status had an effect: when fish were fasted, IGFBP-1b levels increased. Growth is generally correlated positively with serum IGF-I and negatively with IGFBP-1b; thus these markers respond differently to fasting and environmental changes (Shimizu et al. 2006; Kawaguchi et al. 2013; Kaneko et al. 2019). In the present study, IGF-I was a stronger indicator of growth, whereas IGFBP-1b was a better indicator of the feeding status. Therefore, measuring both IGFBP-1b and IGF-I is a useful approach to assess short- and medium-term growth and feeding status in the field.
The results of liver glycogen suggest an adaptive response to varying food availability and water temperature in juvenile chum salmon. Liver glycogen serves as a short-term energy reservoir for fish to cope with acute stress (Vijayan and Moon 1992). Liver glycogen content is generally decreased in salmonids by short-term fasting (Sheridan and Mommsen 1991; Vijayan and Moon 1992; Misaka et al. 2004). In the present study, the liver glycogen content in juvenile chum salmon fasted for 5 days in freshwater was decreased, as observed in other salmonids. However, these fish showed substantially increased levels of liver glycogen after the transfer to cold seawater and resumption of feeding. This over-compensation regarding liver glycogen content at a time of reduced body weight suggests that available energy was mobilized to produce and/or accumulate liver glycogen. Based on these results, we hypothesize that when fish in poor nutritional condition in freshwater enter cold seawater, growth is sacrificed for the benefit of accumulating glycogen in the liver to anticipate potential stress. However, it is not known how cold seawater affects energy allocation to either metabolism or growth, which is a subject of future studies.
Perspective
The freshwater stage may exert a stronger effect on the survival of juvenile chum salmon than previously assumed. Morita et al. (2015) found a positive correlation between river temperature during the fry stage and fry-to-adult survival rate of hatchery-reared chum salmon in the Chitose River in Hokkaido, emphasizing the importance of temperature-related mortality of juveniles in freshwater. Juvenile chum salmon released from hatcheries in eastern Hokkaido spend 5–26 days in the river (Kasugai et al. 2014), indicating that juveniles need to eat during the downstream migration. One of the possible causes of temperature-related mortality in freshwater may be low availability and digestibility of prey items such as aquatic insects (Morita et al. 2015). In addition, Takahashi et al. (2016) suggested that river water temperature affects foraging efficiency of juvenile chum salmon released from hatcheries. Our finding that poor feeding status in freshwater and subsequent low seawater temperature severely affected growth of juvenile chum salmon in seawater may be a possible consequence of low river temperature reducing food intake and digestibility and leading to high mortality in cold seawater through reducing serum IGF-I levels. To test this, studies are needed to experimentally examine the cumulative effect of freshwater temperature, feeding status in freshwater, and seawater temperature on growth of juvenile chum salmon. If this hypothesis is confirmed, low river water temperature would affect feeding/nutritional status of down-migrating juveniles, making them more susceptible to the adverse effects of low seawater temperature.
In conclusion, the present study indicates that fasting in freshwater severely affects the growth of juvenile salmon when they enter cold seawater, even when food is available. This finding emphasizes the importance of monitoring the feeding status of down-migrating juvenile chum salmon to assess the potential effect of unfavorable coastal water conditions.
References
Allard JB, Duan CM (2018) IGF-binding proteins: why do they exist and why are there so many? Front Endocrinol 9:117
Ban M, Hasegawa H, Ezure M (1996) Effects of starvation and refeeding on physiological condition of juvenile chum salmon, Oncorhynchus keta. Sci Rep Hokkaido Salmon Hatch 50:117–123 (in Japanese with English abstract)
Bax NJ (1983) Early marine mortality of marked juvenile chum salmon (Oncorhynchus keta) released into Hood Canal, Puget Sound, Washington, in 1980. Can J Fish Aquat Sci 40:426–435
Beamish RJ, Mahnken C (2001) A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog Oceanogr 49:423–437
Beckman BR, Shimizu M, Gadberry BA, Cooper KA (2004a) Response of the somatotropic axis of juvenile coho salmon to alterations in plane of nutrition with an analysis of the relationships among growth rate and circulating IGF-I and 41 kDa IGFBP. Gen Comp Endocrinol 135:334–344
Beckman BR, Shimizu M, Gadberry BA, Parkins PJ, Cooper KA (2004b) The effect of temperature change on the relations among plasma IGF-I, 41-kDa IGFBP, and growth rate in postsmolt coho salmon. Aquaculture 241:601–619
Beckman BR (2011) Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol 170:233–252
Dreiling CE, Brown DE, Casale L, Kelly L (1987) Muscle glycogen: comparison of iodine binding and enzyme digestion assays and application to meat samples. Meat Sci 20:167–177
Fukuda M, Kaneko N, Kawaguchi K, Hevroy EM, Hara A, Shimizu M (2015) Development of a time-resolved fluoroimmunoassay for salmon insulin-like growth factor binding protein-1b. Comp Biochem Physiol A 187:66–73
Fukuwaka M, Suzuki T (2002) Early sea mortality of mark-recaptured juvenile chum salmon in open coastal waters. J Fish Biol 60:3–12
Gabillard J-C, Weil C, Rescan P-Y, Navarro I, Gutierrez J, Le Bail P-Y (2005) Does the GH/IGF system mediate the effect of water temperature on fish growth? A review. Cybium 29:107–117
Healey MC (1982) Timing and relative intensity of size-selective mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can J Fish Aquat Sci 39:952–957
Honda K, Kawakami T, Suzuki K, Watanabe K, Saito T (2017) Growth rate characteristics of juvenile chum salmon Oncorhynchus keta originating from the Pacific coast of Japan and reaching Konbumori, eastern Hokkaido. Fish Sci 83:987–996
Kaeriyama M, Yatsu A, Noto M, Saitoh S (2007) Spatial and temporal changes in the growth patterns and survival of Hokkaido chum salmon populations in 1970–2001. N Pac Anadr Fish Comm Bull 4:251–256
Kaeriyama M, Kumagai I (1984) Tolerance of starvation in chum salmon fry. Bull Kesennuma Miyagi Pref Fish Exp Stn 7:11–14 (in Japanese with English abstract)
Kaneko N, Taniyama N, Inatani Y, Nagano Y, Fujiwara M, Torao M, Miyakoshi Y, Shimizu M (2015) Circulating insulin-like growth factor I in juvenile chum salmon: relationship with growth rate and changes during downstream and coastal migration in northeastern Hokkaido, Japan. Fish Physiol Biochem 41:991–1003
Kaneko N, Torao M, Koshino Y, Fujiwara M, Miyakoshi Y, Shimizu M (2019) Evaluation of growth status using endocrine growth indices, insulin-like growth factor (IGF)-I and IGF-binding protein-1b, in out-migrating juvenile chum salmon. Gen Comp Endocrinol 274:50–59
Kasugai K, Takeuchi K, Miyakoshi Y, Nagata M (2014) Estimation of number of downstream migrating chum salmon fry in the Nishibetsu River in 2006. Sci Rep Hokkaido Fish Res Inst 85:37–40 (in Japanese with English abstract)
Kawaguchi K, Kaneko N, Fukuda M, Nakano Y, Kimura S, Hara A, Shimizu M (2013) Responses of insulin-like growth factor (IGF)-I and two IGF-binding protein-1 subtypes to fasting and re-feeding, and their relationships with individual growth rates in yearling masu salmon (Oncorhynchus masou). Comp Biochem Physiol A 165:191–198
Kelley KM, Haigwood JT, Perez M, Galima MM (2001) Serum insulin-like growth factor binding proteins (IGFBPs) as markers for anabolic/catabolic condition in fishes. Comp Biochem Physiol B 129:229–236
Kitada S (2014) Japanese chum salmon stock enhancement: current perspective and future challenges. Fish Sci 80:237–249
Larsen DA, Beckman BR, Dickhoff WW (2001) The effect of low temperature and fasting during the winter on metabolic stores and endocrine physiology (insulin, insulin-like growth factor-I, and thyroxine) of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol 123:308–323
Mayama H, Ishida Y (2003) Japanese studies on the early ocean life of juvenile salmon. N Pac Anadr Fish Comm Bull 3:41–67
Misaka N, Mizuno S, Miyakoshi Y, Takeuchi K, Takami T, Kasahara N (2004) Changes of tryglyceride and glycogen levels in the liver of underyearling masu salmon Oncorhynchus masou during starvation. Nippon Suisan Gakkaishi 70:168–174 (in Japanese with English abstract)
Miyakoshi Y, Nagata M, Kitada S, Kaeriyama M (2013) Historical and current hatchery programs and management of chum salmon in Hokkaido, northern Japan. Rev Fish Sci 21:469–479
Morita K, Saito T, Miyakoshi Y, Fukuwaka MA, Nagasawa T, Kaeriyama M (2006) A review of Pacific salmon hatchery programmes on Hokkaido Island, Japan. ICES J Mar Sci 63:1353–1363
Morita K, Ayumi N, Kikuchi M (2015) River temperature drives salmon survivorship: is it determined prior to ocean entry? R Soc Open Sci 2:140312
Morita K, Nakashima A (2015) Temperature seasonality during fry out-migration influences the survival of hatchery-reared chum salmon Oncorhynchus keta. J Fish Biol 87:1111–1117
Nagata M, Miyakoshi Y, Ando D, Fujiwara M, Sawada M, Shimada H, Asami H (2007) Influence of coastal seawater temperature on the distribution and growth of juvenile chum salmon, with recommendations for altered release strategies. N Pac Anadr Fish Comm Bull 4:223–235
Nagata M, Miyakoshi Y, Urabe H, Fujiwara M, Sasaki Y, Kasugai K, Torao M, Ando D, Kaeriyama M (2012) An overview of salmon enhancement and the need to manage and monitor natural spawning in Hokkaido, Japan. Environ Biol Fish 94:311–323
Nagata M, Miyakoshi Y, Fujiwara M, Kasugai K, Ando D, Torao M, Saneyoshi H, Irvine JR (2016) Adapting Hokkaido hatchery strategies to regional ocean conditions can improve chum salmon survival and reduce variability. N Pac Anadr Fish Comm Bull 6:73–85
Picha ME, Turano MJ, Beckman BR, Borski RJ (2008) Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-Binding proteins as potential growth indicators in fish. N Am J Aquacult 70:196–211
Picha ME, Biga PR, Galt N, McGinty AS, Gross K, Hedgpeth VS, Siopes TD, Borski RJ (2014) Overcompensation of circulating and local insulin-like growth factor-1 during catch-up growth in hybrid striped bass (Morone chrysops × Morone saxatilis) following temperature and feeding manipulations. Aquaculture 428:174–183
Quabius ES, Balm PHM, Bonga SEW (1997) Interrenal stress responsiveness of tilapia (Oreochromis mossambicus) is impaired by dietary exposure to PCB 126. Gen Comp Endocrinol 108:472–482
Rajaram S, Baylink DJ, Mohan S (1997) Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18:801–831
Reinecke M (2010) Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J Fish Biol 76:1223–1254
Saito T, Nagasawa K (2009) Regional synchrony in return rates of chum salmon (Oncorhynchus keta) in Japan in relation to coastal temperature and size at release. Fish Res 95:14–27
Saito T, Shimizu I, Seki J, Nagasawa K (2009) Relationship between zooplankton abundance and the early marine life history of juvenile chum salmon Oncorhynchus keta in eastern Hokkaido, Japan. Fish Sci 75:303–316
Saito T, Shimizu I, Seki J, Kaga T, Hasegawa E, Saito H, Nagasawa K (2010) Can research on the early marine life stage of juvenile chum salmon Oncorhynchus keta forecast returns of adult salmon? A case study from eastern Hokkaido, Japan. Fish Sci 76:909–920
Saito T, Kaga T, Hasegawa E, Nagasawa K (2011) Effects of juvenile size at release and early marine growth on adult return rates for Hokkaido chum salmon (Oncorhynchus keta) in relation to sea surface temperature. Fish Oceanogr 20:278–293
Sheridan MA, Mommsen TP (1991) Effects of nutritional state on in vivo lipid and carbohydrate metabolism of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol 81:473–483
Shimizu M, Swanson P, Fukada H, Hara A, Dickhoff WW (2000) Comparison of extraction methods and assay validation for salmon insulin-like growth factor-I using commercially available components. Gen Comp Endocrinol 119:26–36
Shimizu M, Dickey JT, Fukada H, Dickhoff WW (2005) Salmon serum 22 kDa insulin-like growth factor-binding protein (IGFBP) is IGFBP-1. J Endocrinol 184:267–276
Shimizu M, Beckman BR, Hara A, Dickhoff WW (2006) Measurement of circulating salmon IGF binding protein-1: assay development, response to feeding ration and temperature, and relation to growth parameters. J Endocrinol 188:101–110
Shimizu M, Kishimoto K, Yamaguchi T, Nakano Y, Hara A, Dickhoff WW (2011a) Circulating salmon 28- and 22-kDa insulin-like growth factor binding proteins (IGFBPs) are co-orthologs of IGFBP-1. Gen Comp Endocrinol 174:97–106
Shimizu M, Suzuki S, Horikoshi M, Hara A, Dickhoff WW (2011b) Circulating salmon 41-kDa insulin-like growth factor binding protein (IGFBP) is not IGFBP-3 but an IGFBP-2 subtype. Gen Comp Endocrinol 171:326–331
Shimizu M, Dickhoff WW (2017) Circulating insulin-like growth factor binding proteins in fish: Their identities and physiological regulation. Gen Comp Endocrinol 252:150–161
Small BC, Peterson BC (2005) Establishment of a time-resolved fluoroimmunoassay for measuring plasma insulin-like growth factor I (IGF-I) in fish: effect of fasting on plasma concentrations and tissue mRNA expression of IGF-I and growth hormone (GH) in channel catfish (Ictalurus punctatus). Domest Anim Endocrinol 28:202–215
Takahashi S, Hasegawa K, Ito H, Ban M, Miyauchi Y (2016) Comparisons of growth of chum salmon fry released into rivers of which temperature and prey abundance conditions were different. Nippon Suisan Gakkaishi 82:559–568 (in Japanese with English abstract)
Taniyama N, Kaneko N, Inatani Y, Miyakoshi Y, Shimizu M (2016) Effects of seawater transfer and fasting on the endocrine and biochemical growth indices in juvenile chum salmon (Oncorhynchus keta). Gen Comp Endocrinol 236:146–156
Tucker S, Hipfner JM, Trudel M (2016) Size- and condition-dependent predation: a seabird disproportionately targets substandard individual juvenile salmon. Ecology 97:461–471
Vijayan MM, Moon TW (1992) Acute handling stress alters hepatic glycogen metabolism in food-deprived rainbow trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 49:2260–2266
Wagawa T, Tamate T, Kuroda H, Ito SI, Kakehi S, Yamanome T, Kodama T (2016) Relationship between coastal water properties and adult return of chum salmon (Oncorhynchus keta) along the Sanriku coast, Japan. Fish Oceanogr 25:598–609
Wertheimer AC, Thrower FP (2007) Mortality rates of chum salmon during their early marine residency. In: Grimes CB, Brodeur RD, Haldorson LJ, McKinnell SM (eds) The ecology of juvenile salmon in the northeast Pacific Ocean: regional comparisons. American Fisheries Society, Bethesda, pp 233–247
Zavolokin AV, Strezhneva EV (2013) Size-selective mortality of Sea of Okhotsk pink salmon in the ocean in the winter and spring. Russ J Mar Biol 39:501–508
Acknowledgements
We thank the staff of the Kitami Salmon Enhancement Programs Association for providing juvenile chum salmon and Hayato Saneyoshi for transferring experimental fish. This work was supported by Hokusui Foundation and JSPS KAKENHI Grant number JP18K05801.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakamura, S., Kaneko, N., Nonaka, T. et al. Fasting in freshwater severely affects growth of juvenile chum salmon when entering cold seawater. Fish Sci 85, 655–665 (2019). https://doi.org/10.1007/s12562-019-01313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01313-3