Abstract
The energy partitioning in juvenile chub mackerel was assayed using the apparent digestibility, growth performance, oxygen consumption rate (ḾO2), and swimming speed with feeding on an artificial diet. Fifty-four juveniles (16.6 g) were divided into two 2500 L tanks and reared for 2 weeks. The ḾO2 after the first, second, and the third feeding elevated 1.4, 1.9 and 1.9 fold higher than that of under fasting, and returned to the pre-feeding level within 4, 4, and 9 h, respectively. The post-feeding swimming speed fluctuation of juveniles was significantly correlated with their ḾO2 fluctuation. In contrast, although there was no remarkable change in ḾO2 under fasting for all day, their swimming velocity continued to decline slowly from sunset to sunrise. Energy partitioning rates for fecal, urinary and branchial, heat increment and voluntary activity, standard metabolism, and retained energy were calculated as 7.4, 8.7, 35.9, 23.5, and 24.5 % of total ingested energy, respectively. The results revealed that juvenile chub mackerel shifted respiratory strategy from ram-ventilation to branchial ventilation from sunset to sunrise. Moreover, chub mackerel juveniles, one of the typical carnivorous fish, distribute more energy for maintenance resulting in lower energy allocation for growth as compared with other aquaculture fishes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chub mackerel (Scomber japonicas) is one of the commercial target species, widely distributed in the Atlantic, Indian, and North Pacific Ocean [1, 2]. In Japanese coastal waters, chub mackerel stock has been decreasing from 1980 and still remains at the low level. Therefore, restriction has been imposed on commercial catch by the total allowable catch system (TAC) since 1997 to prevent both overfishing and stock decrement. Recently, several researches to establish the artificial reproduction of chub mackerel have been conducted, and already succeeded in full-cycle culture in land-based tanks without hormone injection [3, 4]. Further studies related to egg collection method and mass seeding production have also been continued [4–6].
Although much effort has been undertaken to establish sustainable aquaculture for chub mackerel in recent years in thinking about its great consumer demands and future perspectives, the unavailability of a suitable formulated feed is a serious bottleneck. For chub mackerel husbandry, the formulated feed for other fish has been used. Since there is species-specific requirement of nutrients and energy, advance knowledge of the characteristics of energy utilization by chub mackerel would help to establish a suitable formulated feed to facilitate the sustainable aquaculture.
Bioenergetics approaches such as energy partitioning is helpful to establish a cost-effective artificial formulated feed based on the energy or nutrients utilization of fish species. By this approach, it has been revealed that red sea bream (Pagrus major) and tiger puffer (Takifugu rubripes) consume more protein for their maintenance than lipids [7]. Conversely, Pacific bluefin tuna (PBT) (Thunnus orientalis) and yellowtail (Seriola quinqueradiata) mainly consume lipid for maintenance to retain protein for their growth [8, 9]. Moreover, Ohnishi et al. [9] showed that juveniles PBT adjust their swimming speed and maintenance energy with feeding due to a ram-ventilator. In the case of chub mackerel, since there have been only a few studies on the partial assessment of energy partitioning [10], little is known about the whole image of energy partitioning, detail change of daily energy expenditure, and utilization of each nutrient. Chub mackerel belongs to the same scombridae family as PBT. The diet for PBT juveniles has been composed of enzyme treated fish meal (EFM) as a protein source. To provide an opportunity to compare the energy partitioning with PBT, the diet in this study was also composed of EFM as a protein source.
In this study, we revealed the time series variation of metabolic rate and energy partitioning of juvenile chub mackerel through monitoring the changes in oxygen consumption rate (ḾO2) and swimming speed during a 2-week feeding trial. This study aimed to determine the characteristic of ingested energy allocation for different purposes in chub mackerel to contribute to the further development of sustainable aquaculture.
Materials and methods
Experimental animals, feed and husbandry
Fifty-four artificially produced chub mackerel juveniles (12.1 ± 1.1 cm TL, 16.6 ± 5.0 g BW) were obtained from the Fisheries Laboratories, Kinki University, Shirahama, Wakayama, Japan. Fifty-four juveniles were divided into two groups [tank 1 (24 fish) and 2 (30 fish)], and were stocked into each of two 2500 L circular experimental tanks (2.0 m diameter × 0.8 m depth; E-25B, Earth Business Corporation, Tokyo, Japan). The juveniles were reared for 2 weeks. Tank 1 was used to measure both daily metabolic rate and swimming speed fluctuation. Tank 2 was used to assay the apparent digestibility coefficient (ADC) and growth performance. The remaining six fish in tank 2 were sampled at the start of the feeding trial to measure both mean initial body weight and carcass proximate compositions in the feeding trial. The fish were fed an experimental diet (Table 1) with pellets 1.5 mm diameter and 3.0 mm length containing 0.5 % chromium oxide (Cr2O3) as an inert marker [9, 11]. The diet was produced by a laboratory pellet machine (Meat-chopper, Iizuka Industrial Co. Ltd., Tokyo, Japan), freeze-dried (Freezone 2.5 plus, Labconco Corporation, Missouri, USA) and stored at −40 °C until use. During the feeding trial, the diet was given 3 times a day at 08:00, 13:00, and 18:00 until apparent satiation. Fluorescent lights (60 W × 2) were situated at 30 cm above the water surface at the center of tank to provide 24 h lighting in order to measure swimming speed. During the experiments, oxygenated seawater was supplied to each tank at 1 L min−1, and dissolved oxygen (DO) and water temperature were 7.6 ± 0.6 mg L−1 and 27.1 ± 1.0 °C, respectively.
Measurement of metabolic rate and swimming speed
Metabolic rate, expressed as energy expenditure during an hour, was measured from ḾO2. Before measurement of ḾO2, fish were starved for 24 h. The experimental tank 1 was filled with filtered seawater and sealed with a transparent sheet (2 m diameter, 3 mm thickness). The DO was measured every 10 min for a day using an optical DO meter (Pro-ODO, YSI Nanotech, Japan), and ḾO2 (mgO2 kg−0.80 h−1) was hourly calculated from the decrement in DO in the experimental tank. The sensor of the DO meter was set at 30 cm below the water surface and 50 cm from edge of the tank. Seawater in the experimental tank was partially replaced every 3 h to maintain levels of DO above 90 % saturation. However, the water flow was stopped during the remaining period. Our observation after water exchange confirmed that the DO was almost similar throughout the tank, suggesting uniform dispersion of newly introduced seawater. The measurement was repeated 5 times under both feeding and fasting conditions.
The swimming behavior of juvenile chub mackerel was recorded for 5 min at 25 min intervals using a video camera (HDR-XR350 V, SONY, Tokyo, Japan) set at 3 m above the experimental tank. For calculation of swimming speed, 5 sections of each video clip, in which fish showed swimming behavior without gliding or burst activity, were selected randomly. Selected sections were then converted into a series of still images (2304 × 1296 pixel) in which folk lengths (FL) and distances moved (D) within 2 s were measured using Image-J image analysis software (NIH, Maryland, USA). Swimming speed (FL s−1) was calculated using the following formula:
Measurement of growth performance, ADC and energy partitioning
The growth performance was determined by measuring six fish at the start and all surviving fish at the end of the rearing trial. Feces were collected every day from the bottom of experimental tank 2 by siphoning at 3–4 h after each feeding. At 30 min after each feeding, the tank bottom was cleaned to remove uneaten feeds immediately after feeding. Collected feces were stored in a freezer at −40 °C, and used for calculation of ADC. The energy budget for different purposes was calculated by using all data of ḾO2, ADCs, growth performance, ingested energy, and nutrients.
Calculation of growth parameters and energy budget
Proximate compositions of diet, carcass, and feces were analyzed by the method of the AOAC (1984) [12], and the gross energy was assayed by an automated oxygen bomb calorimeter (IKA-Werke, Staufen, Germany). The sugar content of the experimental diet was assayed by the phenol–sulfuric acid method [13]. ADCs were assayed by the method of Fukuhara and Tsukahara [14].
The growth parameters, retention efficiency of protein (PRE), lipid (LRE) and energy (ERE), and ADCs were calculated by following formulas:
From the reviews of NRC (15), energy budget for gross energy in feed (GE), fecal energy (FE), digestible energy (DE), urinary and branchial energy (UE + ZE), metabolizable energy (ME), energy of heat increment. and voluntary activity (H i E + H j E), net energy (NE), standard metabolism (SME), and retained energy (RE) were calculated by following formulas [14]:
where ∆ḾO2 fast indicates the daily ḾO2 (mgO2 kg−1 24 h−1) under fasting conditions. Utilized energy was estimated from ḾO2 with the conversion rate of 1 mg of consumed oxygen equal to 14.32 J of energy [16]. UE + ZE as nitrogen compounds excreted from gill and urine were tentatively considered as ammonia, multiplied by 24.9 kJ g−1 NH3 [17].
Statistical analysis
Correlation regression analysis was used to determine the relationship between post-feeding swimming speed and ḾO2. The ḾO2 and swimming speed of each time were compared between feeding and fasting conditions. Normality of the data were examined using the Kolmogorov-Smimov test. A non-parametric Mann–Whitney U test was used for both comparisons due to non-normality of the data. All statistical analyses were performed on EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (The R Foundation for Statistical Computing) [18]. The P value less than 0.05 was considered statistically significant.
Results
Metabolic rate and swimming speed
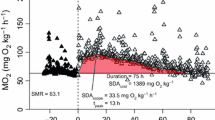
The feeding rate for each time measuring metabolic rate, and daily metabolic rate fluctuation in fed and unfed fish are shown in Figs. 1 and 2, respectively. In a fasting condition, there was no remarkable fluctuation in ḾO2 for all day with mean value 445 ± 44 mgO2 kg−0.80 h−1. There were little differences in feeding rate for each time. However, the fluctuation of post-feeding ḾO2 was varied among feeding times. Increment of ḾO2 after the first feeding was smaller than that of the others. The maximum values after the second and the third feeding were 829 ± 138 and 865 ± 61 mgO2 kg−0.80 h−1, respectively (1.9 ± 0.3 and 1.9 ± 0.1 times of fasting level, respectively). After reaching a peak, the post-feeding ḾO2 decreased gradually until a similar level to that of the pre-feeding level condition within 4, 4, and 9 h from the first, second, and the third feeding, respectively. However, post-feeding ḾO2 remained at higher values compared to a fasting condition for all day.
Changes of oxygen consumption rate. Filled diamond post-feeding oxygen consumption rate (n = 5, mean ± SEM); dashed line oxygen consumption rate under fasting condition (n = 5, mean ± SEM); arrowed lines feeding time. Asterisk indicates the significant difference between feeding and fasting condition
Change in post-feeding swimming speed similarly fluctuated as that of ḾO2 (Fig. 3). However, the swimming speed declined for 8 h after the third feeding of the peak value, and the values returned to pre-feeding levels at 04:00. Swimming speed under a fasting condition also continued to decline from sunset (18:50) to sunrise (05:00). After 04:00, swimming speed increased in both groups of fish. Additionally, a significant positive exponential relationship between both ḾO2 and swimming speed was detected (Fig. 4).
Growth performance, ADC, apparent nutrient retention, and energy partitioning
Growth performance, and proximate composition of carcass, ADC, and retention efficiency are shown in Tables 2 and 3, respectively. The final body weight increased 1.5 times the initial value in 2 weeks. Moreover, no fish died during the experimental period. ADCs of protein, lipid, and energy were over 90 %. However, PRE was as low as 12.3 %, while LRE and ERE showed comparatively higher values compared with PRE.
Energy budget and energy partitioning rate of juvenile chub mackerel are shown in Table 4 and Fig. 5, respectively. GE of the juveniles was 1474 kJ kg−1 day−1. Since the juveniles lost only 16.1 % of GE as FE and UE + ZE, they could use over 80 % of GE for metabolic activity. Energy partitioning rate for H i E + H j E and SME were approximately 35.9 and 23.5 %, respectively. On the other hand, the juveniles could use one quarter of GE for their RE.
Discussion
Daily fluctuation of metabolic rate and swimming speed
ḾO2 of fish was elevated after feeding due to digestive activity [19]. Since scombrid fish can breathe using ram-ventilation, they need to elevate their swimming speed with increasing oxygen demand [20–22]. In this study, the post-feeding ḾO2 and swimming speed were increased and showed a positive exponential relationship between them. However, the magnitude of both parameters was increased remarkably after the second and the third feeding compared to that of the first feeding. It was due to the increasing load of feed in the digestive organ from the first feeding onward.
ḾO2 under feeding conditions maintained higher values than that of the fasting group until next morning. These results might be related to the difference in swimming activity, which was higher until 04:00 under feeding conditions compared to that of fasting conditions. Several studies revealed that the increase in oxygen demand was covered by an increasie in swimming activity for some fishes including chub mackerel [23, 24].
Although the swimming speed of chub mackerel decreased gradually during the night time irrespective of feeding condition, the ḾO2 was stable at night time. It is assumed that juvenile chub mackerel shifted respiratory strategy from ram-ventilation to branchial ventilation associated with a decrement of swimming activity at night time. This is in order to maintain oxygen supply to the gills by the branchial pump system to compensate the decrement of ventilation volume associated with decreasing swimming speed. The same trend has considered for Atlantic mackerel (Scomber scombrus) blue runner (Caranx crysos), striped mullet (Mugil cephalus), and sharksucker (Echeneis Naucrates) [25, 26]. Since a fluorescent light had been turned on all day in this experiment to observe swimming behavior, the fish might had been more active than that of a condition with complete darkness [27]. However, since ḾO2 was stable for all day in spite of fluctuating swimming speed, it is thought that the lightning conditions did not have an influence on the energy metabolism of juvenile chub mackerel.
Growth performance, digestibility, and energy partitioning
Although juvenile chub mackerel ingested 2–3 times more energy in this study than juvenile red sea bream and juvenile yellowtail, the energy excretion through FE was only about half compared to the above juveniles [8, 28]. The FE of as low as 7.4 % of GE is due to the higher digestibility of experimental diet, which was composed of easily digestible EFM. However, the excreted energy through FE is also half when compared to PBT juvenile fed with the same feed [9]. Therefore, it is suggested that EFM in feed for juvenile chub mackerel may not be necessary, because this species has higher digestive and absorptive capacity than juvenile PBT.
The energy partitioning rate for UE + ZE of juvenile chub mackerel was about 1.5 times greater than that of PBT [9]. Although the ADC of protein in juvenile chub mackerel showed over 90 %, the PRE was only about 10 %, which indicates that the consumed protein was mostly used as an energy source. Since the production of metabolic waste from protein is ammonia, the energy partitioning rate for UE + ZE increased with increasing protein expenditure as energy. The same trend has been observed for juvenile red sea bream and juvenile tiger puffer [7].
Energy partitioning rate for H i E + H j E was about double compared to that of juvenile PBT, which is the same as for scombridae fish [9]. The duration before post-feeding ḾO2 increment with H i E + H j E returned to pre-feeding levels for juvenile chub mackerel (4–9 h) was two-fold longer than that of juvenile PBT (2–4 h), suggesting that the difference in pace of the metabolic cycle caused the difference of the energy partitioning rate. Moreover, ADC of juvenile chub mackerel showed 10 % higher values than that of juvenile PBT, which indicates that the difference was also caused by the difference of energy partitioning rate for H i E + H j E.
The energy partitioning rate for SME of ME in juvenile chub mackerel was 3–8 % higher than that of juvenile red sea bream and juvenile yellowtail [8, 28]. However, the value was about half compared to that of juvenile PBT, which is a closely related species [9]. This is attributed to the difference in respiratory activity between the species. Although PBT need to swim continuously to intake oxygen because of the obligatory ram-ventilator [22], chub mackerel can shift respiratory strategy from ram-ventilation to branchial ventilation to fit the situation as mentioned earlier. This respiratory strategy is one of the reasons of less than half metabolic rate per unit time in juvenile chub mackerel compared to that of juvenile PBT [9] under the same measuring conditions.
The energy partitioning rate for RE in juvenile chub mackerel was about 10 % lower than that of juvenile red sea bream and juvenile yellowtail [8, 28]. This is due to the lower retention efficiency of nutrients and energy [8, 28]. RE are met after being used in metabolic activity as excess energy [29]. Since juvenile chub mackerel need to consume more energy for their maintenance, they catabolize more nutrients compared with the above juveniles. The same characteristic of low retention efficiency and high energy expenditure for maintenance was revealed in juvenile PBT [9]. However, since GE of juvenile chub mackerel showed a 2–3 fold value greater than that of the above juveniles, they can consume more energy for RE and grow up fast compared to the above juveniles [8, 28].
In conclusion, the present study revealed that juvenile chub mackerel increase their swimming speed after feeding to meet the demand of increasing ḾO2. It is suggested that this species may change the respiratory strategy from ram-ventilation to branchial ventilation at night time. Since this species at this early growth stage uses much energy for maintenance, the retained energy is lower than that of juvenile red sea bream, juvenile yellowtail, and juvenile tiger puffer. Moreover, the lower retention of protein in spite of higher digestibility indicates that protein is being preferred as the energy source. This information will help to design a proper feed formula for juvenile chub mackerel to contribute towards the establishment of sustainable aquaculture. In the future, further studies are necessary to determine the influence of diet formula, feeding level, and growth stage on energy partitioning in chub mackerel.
References
Hernandez JJC, Ortega ATS (2000) Synopsis of biological data on the chub mackerel (Scomber japonicus Houttuyn, 1782). FAO Fish Synop 157
Sever TM, Bayhan B, Bilecenoglu M, Mavili S (2006) Diet composition of the juvenile chub mackerel (Scomber japonicus) in the Aegean Sea (Izmir Bay, Turkey). J App Ichthyol 22:145–148
Murata O, Yamamoto S, Ishibashi R, Oka Y, Yoneshima H, Kato K, Miyashita S, Kumai H (2005) Egg development and growth of larval and juvenile cultured chub mackerel Scomber japonicus (Perciformes: Scombridae) in a captive spawning experiment. Aquacult Sci 53:319–324
Shiraishi T, Ohta K, Yamaguchi A, Yoda M, Chuda H, Matsuyama M (2005) Reproductive parameter of the chub mackerel Scomber japonicus estimated from human chorionic gonadotropin-induced final oocyte maturation and ovulation in captivity. Fish Sci 71:531–542
Tsuda Y, Yamamoto S, Yamaguchi H, Ohnishi T, Sakamoto W, Murata O (2014) Vertical movement of spawning cultured chub mackerel (Scomber japonicus) in a net-cage. Aquaculture 422–423:136–140
Murata O, Yamamoto S, Ishibashi R, Oka Y, Yoneshima H, Kato K, Miyashita S, Kumai H (2005) Egg development and growth of larval and juvenile cultured chub mackerel Scomber japonicus (Perciformes: Scombridae) in a captive spawning experiment. Aquacult Sci 53(3):319–324
Takii K, Konishi K, Ukawa M, Nakamura M, Kumai H (1997) Influence of feeding rates on digestion and energy flow in tiger puffer and red sea bream. Fish Sci 63:355–360
Masumoto T, Ruchimat T, Hosokawa H, Shimeno S (1997) Energy requirement and energy partitioning in juvenile yellowtail. B Mar Sci Fish Kochi Univ 17:79–87 (In Japanese)
Ohnishi T, Biswas A, Kaminaka K, Nakao T, Nakajima M, Sakakibara N, Takii K (2014) Energy partitioning in cultured juvenile Pacific Bluefin tuna, Thunnus orientalis (Temminck & Schlegel, 1844). Aquacult Res. doi:10.1111/are.12658
Takii K, Seoka M, Izumi M, Hosokawa H, Shimose S, Ukawa M, Kohbara J (2007) Apparent digestibility coefficient and energy partition of juvenile Pacific bluefin tuna, Thunnus orientalis and Chub mackerel, Scomber japonicas. Aquacult Sci 55:571–577
Halver JE (1957) Nutrition of salmonid fishes. J Nutr 62:225–243
AOAC (1984) Official methods of analysis of Association of Official Analytical Chemists, 14th edn. Washington
Hodge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrates. In: Whistler RL, Wolform ML (eds) Methods in carbohydrate chemistry, vol 1. Academic Press, New York, pp 388–389
Fukuhara A, Sukahara H (1966) On the acid digestion method for the determination of chronic oxide as an index substance in the study of digestibility of fish feed. Nippon Suisan Gakkaishi 32:502–506
NRC (2011) Nutrient requirements of fish and shrimp. The National Academies Press, Washington, D.C.
Beamish FWH, Trippel EA (1990) Heat increment: a static or dynamic dimension in bioenergetics models? T Am Fish Soc 119:649–661
Cho CY, Kaushik SJ (1990) Nutritional energetics in fish: energy and protein utilization in rainbow trout (Salmo gairdneri). World Rev Nutr Diet 61:132–172
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 179:1–56
Freadman MA (1979) Swimming energetics of striped bass (Morone Saxatilis) and bluefish (pomatomus Saltatrix): gill ventilation and swimming metabolism. J Exp Biol 83:217–230
Wengner NC, Sepulveda CA, Aalbers SA, Graham JB (2012) Structural adaptations for ram ventilation: gill fusions in scombrids and billfishes. J Morphol 274(1):108–120
Fitzgibbon QP, Seymour RS, Ellis D, Buchanan J (2007) The energetic consequence of specific dynamic action in southern bluefin tuna Thunnus maccoyii. J Exp Biol 210:290–298
Sepulveda C, Dickson KA (2000) Maximum sustainable speeds and cost of swimming in juvenile kawakawa tuna Ruthynnus Affinis and chub mackerel Scomber Japonics. J Exp Biol 203:3089–3101
Blank JM, Farwell CJ, Morrissette JM, Schallert RJ, Block BA (2007) Influence of swimming speed on metabolic rates of juvenile pacific bluefin tuna and yellowfin tuna. Physiol Biochem Zool 80:167–177
Steffensen JF, Lomholt JP (1983) Energetic cost of active branchial ventilation in the sharksucker, Echeneis Naucrates. J Exp Biol 103:185–192
Roberts JL (1975) Active branchial and ram ventilation in fishes. Biol Bull 148:85–105
Anras MLB, Lagardère JP, Lafaye JY (1997) Diel activity rhythm of seabass tracked in a natural environment: group effects on swimming patterns and amplitudes. Can J Fisheries Aquat Sci 54(1):162–168
Takii K, Akira T, Seoka M, Kitamura S, Kurifuji K (2008) Feeding protocols with artificial diet effect on growth performance and energy partition of red sea bream, Pagrus major. Aquacult Res 56:237–243 (In Japanese with English abstract)
Mourente G, Tocher DR (2003) An approach to study the nutritional requirement of the bluefin tuna (Thunnus thynnus thynnus, L.). In: Bridges CR, García A, Gordin H (eds) Domestication of the bluefin tuna Thunnus thynnus thynnus. CIHEAM, Zaragoza, pp 143–150
Acknowledgments
We would like to thank the staff of the Shirahama Fisheries Laboratories, Kinki University, for their kind support in rearing chub mackerel. We also thank Dr. Yuichi Tsuda for comments and editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohnishi, T., Biswas, A., Kaminaka, K. et al. Energy partitioning in cultured juvenile chub mackerel (Scomber japonicas) fed with diet composed of enzyme treated fish meal. Fish Sci 82, 473–480 (2016). https://doi.org/10.1007/s12562-016-0975-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-0975-y