Abstract
We compared profiles of serum insulin-like growth factor (IGF)-I levels during smoltification of masu salmon reared under different environments, hatcheries and growth histories. Masu salmon from the Kenichi River in Hokkaido showed a sharp increase in serum IGF-I from April to May, followed by a peak of gill Na+,K+-ATPase (NKA) activity. Fish at Kumaishi Hatchery had an IGF-I profile similar to that of the river fish, while the increase in gill NKA was lower. At Shimamaki Hatchery, interval feeding during winter appeared to suppress the spring IGF-I peak. At Kumaishi Hatchery, a difference in size during smoltification affected IGF-I levels at release, but the numbers of adults that returned to the release site were not significantly different. In the following year, three release groups differing in winter size and/or spring growth (Large-High, Large-Low and Small-High) were created. Large-High and Small-High fish showed a higher IGF-I peak than Large-Low fish in April, while smolt-to-adult return of Large-High fish was highest. These results suggest that in smolting masu salmon in freshwater, circulating IGF-I level alone is not a predictor of long-term survival in seawater. However, since growth history in freshwater affected the smolt-to-adult return, optimizing rearing conditions is a critical component of hatchery releases for masu salmon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Masu salmon Oncorhynchus masou is an important target species of coastal fisheries off northern Japan. However, as a resource, it has been unstable and decreasing since the 1970s [1, 2]. Hatchery programs are commonly used to enhance resources of anadromous salmonid species and has been successful for Japanese chum salmon O. keta populations [3]. A large amount of effort has also been invested in hatchery releases for masu salmon, although this has been far less successful [1, 2]. What makes hatchery releases for masu salmon difficult is the salmon's long residency in freshwater. In contrast to chum and pink salmon O. gorbuscha that migrate to the sea in their first spring, masu salmon reside in freshwater for more than 1 year and need to go through the smoltification process before entering the ocean. Smoltification is a transition process by which the river-resident form of juvenile salmon (parr) transforms to the sea-run form (smolt) in freshwater. This process involves a suite of morphological, behavioral, ecological and physiological changes adaptive to ocean life [4–7].
In order for hatcheries to be successful in enhancing masu salmon resources, it is important to identify smolt traits that are advantageous to post-release migration and ocean life, and such traits could be used to evaluate “smolt quality” of reared fish. Among several candidates proposed, seawater adaptability is the most widely used trait. The ability to maintain the internal osmotic pressure is essential for smolts to cope with the challenges of hypo-osmotic environment, and failure to do so results in immediate death or growth retardation in seawater [8]. Adaptation to seawater is achieved through coordination of the gills, kidney and intestine. The gills are important sites for extrusion of monovalent ions such as Na+, K+, Cl− [9]. Extruding ions from the gills relies primarily on the activity of an ion pump, Na+,K+-ATPase (NKA), that is located in the basolateral membrane of ionocytes [10–12]. NKA pumps out three Na+ from the cell and incorporates two K+ into the cell using the energy from hydrolyzing an ATP. This ion gradient is further utilized by other ion cotransporters, channels and exchangers to regulate ion concentrations inside the body [10–12]. However, seawater adaptability may not be enough to secure smolts to return as adults since other environmental factors such as presence of predators, food availability and water temperature also affect their survival.
Another important trait affecting survival of post-smolt salmon is their ability to grow in seawater. Several lines of evidence suggest that a majority of smolts die during the first year in the ocean, due to growth-dependent mortality such as predation and nutritional deficiency [13–15]. Maximizing potential for growth in the ocean should improve smolt survival. There is evidence that bigger fish have higher gill NKA activity [16, 17], and the size at release positively affected smolt-to-adult return (SAR) in Chinook salmon O. tshawytscha [18]. In masu salmon, large smolts (32.6 g) had better adult recovery than small smolts (14.8 g) [19]. Based on these findings, bigger smolts are generally preferred for hatchery release. However, some studies proposed that growth in spring rather than absolute size is more important for producing high quality smolts that return as adults [18, 20]. A rationale of this proposal is that enhanced spring growth promotes smolt-related traits, such as gill NKA activity and migratory behavior [17, 21]. However, such rearing protocols have not been assessed for masu salmon. Moreover, the mechanism by which growth affects seawater adaptability is not fully understood.
The growth hormone (GH)-insulin-like growth factor (IGF)-I system plays a major role in promoting animal growth. In this endocrine system, IGF-I is mainly produced by the liver in response to GH stimulation, is released into bloodstream as a hormone, and it mediates many of GH actions [22–24]. IGF-I is also produced by local tissues and acts through autocrine/paracrine pathways [23, 24]. In salmonids and other euryhaline fishes, the GH-IGF-I system also improves seawater adaptability by activating branchial NKA and inducing morphological development of the seawater-type ionocyte in concert with cortisol [25–29]. Circulating IGF-I often shows an increase during smoltification and is thought to activate NKA in the gills [21, 30–32]. Thus, circulating IGF-I is suggested to be a critical molecule linking growth with seawater adaptation.
We previously reported a profile of circulating IGF-I during smoltification of hatchery-reared masu salmon and found a positive relationship between IGF-I and gill NKA activity [32]. However, it is not known to what extent the IGF-I profile varies or how much it correlates with adult return. The aims of the present study were to compare circulating IGF-I profiles during smoltification of masu salmon reared under different conditions, and to examine if the level or profile of IGF-I could be used as an index of “smolt quality” that predicts adult return of hatchery-reared masu salmon.
Materials and methods
Fish
Naturally reared yearling masu salmon were caught monthly (n = 6–9) from February to May 2008 at the Kenichi River (42°N, 140°E; Futami-gun, Hokkaido, Japan) by electric shocker (Model 12, Smith-Root Inc., Vancouver, WA, USA) and immediately transferred to the South Branch of Salmon and Freshwater Fisheries Institute, Hokkaido Research Organization (Kumaishi Hatchery) located by the river. Age-matched hatchery fish were also sampled (n = 7–10) on the same day. Fish were anesthetized by 3.3 % 2-phenoxyethanol (Kanto Chemical, Tokyo, Japan) and measured for fork length (FL) and body weight (BW). Condition factor was calculated as follows: BW × 1000/FL3. Gill arches were excised and a block of gill filaments was immediately frozen on dry ice and stored at −80 °C until analyzed for NKA activity. Blood was withdrawn from the caudal vein by a syringe, allowed to clot overnight at 4 °C and centrifuged at 8050g for 10 min. Serum was collected and stored at −30 °C until use. The samplings were carried out in accordance with the guideline of Hokkaido University Animal Care and Use Committees.
In November 2010, underyearling masu salmon reared at Kumaishi Hatchery were sorted by size (>9.5 cm) and visually inspected to remove precociously maturing males and fish that would potentially be non-smolting the following spring. They were further divided into large (>11.5 cm) or small (9.5–11.5 cm) size categories. Fish from each group were marked by removing part of the adipose, dorsal or pelvic fin in order to identify the treatment of returning adults, and were reared separately in outdoor ponds (24.6 × 3.5 m) using river water. They were fed twice (November–February) or four times (March–June) a day on a commercial diet (Nippon Formula Feed Mfg, Kanagawa, Japan), with rations at 0.3–2.3 %/body weight/day. Fish were released to the Kenichi River on 13 May 2011 by opening the gates of the ponds. From January 2011 to May 2011, seven fish were sampled monthly.
In November 2011, underyearling masu salmon reared at Kumaishi Hatchery were sorted by size (>11.5 cm as large and 9.5–11.5 cm as small). They were further placed into one of three treatments: large size in winter and high feeding ration in spring (Large-High), large size in winter and low feeding ration in spring (Large-Low), and small size in winter and high feeding ration in spring (Small-High). We were unable to create the Small-Low group due to the limitation of space and labor, since each group consisted of approximately 30,000 fish for release. Fish from each group were marked by removing part of the adipose, dorsal or pelvic fin in order to identify the treatment of returning adults and those reared separately in outdoor ponds (24.6 × 3.5 m) using river water. The Large-High and Small-High groups were fed twice (November–February) or four times (March–May) a day on a commercial diet (Nippon Formula Feed Mfg), with rations at 0.3–2.3 %/body weight/day. The Large-Low group received a restricted feeding ration at 0.2–1.9 % depending on growth status. All fish were released to the Kenichi River on 10 May 2012 by opening the gates of the ponds.
Yearling masu salmon were also reared at Shimamaki Hatchery (42°N, 140°E; Shimamaki-gun, Hokkaido, Japan) during the smoltification period. Fish were maintained in river water from the Chihase River in outdoor ponds. In 2012, fish were fed every 3 or 4 days on a commercial diet (Nippon Formula Feed Mfg), with rations at 0.3–0.4 % (%/body weight/day as average) during January–early March, 1.0 % during mid March–April and 1.3 % in early May. In 2013, fish were fed daily with rations at 0.3–0.4 % during January–early March, 1.2 % during mid March–April and 1.3 % in early May. Fish were sampled as described above, the tail was cut and blood was withdrawn from the caudal vein using a plain glass tube. Blood was allowed to clot overnight at 4 °C and centrifuged at 8050g for 10 min. Serum was collected and stored at −30 °C until use.
Time-resolved fluoroimmunoassay (TR-FIA) for IGF-I
Prior to the assay, serum IGF-I was extracted with an acid–ethanol as described in Shimizu et al. [33]. IGF-I was quantified by TR-FIA based on the method described in Small and Peterson [34], using recombinant salmon/trout IGF-I (GroPep, Adelaide, SA, Australia) as astandard and labeling with europium, and anti-barramundi IGF-I (GroPep) as a primary antiserum.
NKA activity assay
Gill NKA activity was measured according to Quabius et al. [35] with minor modifications. This method is based on the ability of NKA to hydrolyze ATP to give ADP and inorganic phosphorus with or without the presence of ouabain at 37 °C for 10 min. Liberated inorganic phosphorus reacted with ammonium molybdate was quantified by measuring absorbance at 630 nm using a spectrophotometer (Corona Electronic, Ibaraki, Japan). Protein concentration was measured using a BCA (bicinchoninic acid) Protein Assay Kit (Thermo Scientific, IL). The activity was expressed as Pi (µmol) per protein (mg) per period (h).
Smolt-to-adult return (SAR)
From September through October in 2012 and in 2013, spawning adult masu salmon that returned to the Kenichi River near the hatchery drainage were caught daily using a net, identified for treatment and counted. SAR was calculated as follows: SAR = number of tagged adults returned × 100/number of tagged smolts released.
Statistical analysis
Values from precociously maturing males were not included in the analysis since they disturb IGF-I-growth relationships [36]. Results of the experiments were first analyzed by two-way ANOVA by including month and treatment as factors. When significant effects were found, the data were further examined by one-way ANOVA (between treatments within a month or between months within a treatment), followed by the Fisher’s protected least significant difference (PLSD) test using the JMP program (SAS Institute Inc., Cary, NC). Differences among groups were considered to be significant at p < 0.05. Simple regression analysis was also conducted using JMP program and the relations were considered to be significant at p < 0.05.
SARs of groups in the same year were compared by the χ 2 test using Stat View (SAS Institute). Differences among groups were considered to be significant at p < 0.05.
Results
Water temperature
Changes in water temperature at Kumaishi Hatchery using Kenichi River water and Shimamaki Hatchery using Chihase River water are shown in Fig. 1. Overall, water temperatures during January and February were similar in both hatcheries, at <4 °C. Water temperature increased from March to May at Kumaishi Hatchery but stayed relatively low at Shimamaki Hatchery.
River and hatchery in 2008
FL, BW and the condition factor of naturally reared and hatchery reared yearling masu salmon were similar in February and March (Fig. 2a–c). The condition factor was higher in naturally reared fish from April and their FL and BW were also higher in May (Fig. 2c). Gill NKA activity was relatively low during February and April in naturally reared fish, but showed a sharp increase in May (Fig. 2d). In contrast, gill NKA activity in hatchery fish gradually and consistently increased from February to May. Serum IGF-I in wild fish was low in February and March and increased significantly from April through May, whereas hatchery fish showed a consistent increase in IGF-I from February to May (Fig. 2e). In both fish, serum IGF-I level positively correlated with body size (r 2 = 0.45–0.71) and gill NKA activity (r 2 = 0.27–0.36).
Changes in FL (a), BW (b), condition factor (c), gill NKA activity (d) and serum IGF-I levels (e) during smoltification of naturally reared and hatchery reared masu salmon in 2008. Fish were caught monthly from the Kenichi River or reared at Kumaishi Hatchery. Values are expressed as mean ± SE (n = 6–10). Symbols sharing the same letters are not significantly different from each other
Kumaishi Hatchery in 2011 and 2012
In 2011, FL and BW were maintained at low levels in small-in-winter fish compared to large-in-winter fish throughout the rearing period (January to May), except for BW in April (Fig. 3a–c). The condition factor was similar between treatments from January to May, but became lower in large-in-winter fish in May (Fig. 3c). Gill NKA activity in both groups were low during January and March, but small-in-winter fish showed a sharp increase in April (Fig. 3d). In contrast, large-in-winter fish showed a gradual increase in gill NKA from March to May. Serum IGF-I in large-in-winter fish gradually increased from January to April, but tended to decrease in May (Fig. 3e). In contrast, small-in-winter fish showed a consistent increase in serum IGF-I from January to May and IGF-I levels in May were higher than those in large-in-winter fish. In both groups, serum IGF-I level positively correlated with body size (r 2 = 0.39–0.73) and gill NKA activity (r 2 = 0.26–0.47).
Changes in FL (a), BW (b), condition factor (c), gill NKA activity (d) and serum IGF-I levels (e) during smoltification of masu salmon reared at Kumaishi Hatchery in 2011. Fish were divided by size in winter (Large: >11.5 cm, Small: 9.5–11.5 cm) and reared separately. Values are expressed as mean ± SE (n = 6–7). Symbols sharing the same letters are not significantly different from each other
In 2012, FL and BW were lower in the Small-High group from January to April, but became insignificant in May (Fig. 4a–c). The condition factor was graded by treatment throughout the experimental period, it being highest in Large-High fish and lowest in Large-Low fish (Fig. 4c). In all groups, gill NKA activity gradually increased from February to April and showed a sharp increase in May (Fig. 4d). There were no significant differences in gill NKA among the three groups during April and May. Serum IGF-I in Large-High and Small-High fish consistently increased from February to April, while that in Large-Low was significantly lower than in the two other groups during March and April (Fig. 4e). In May, IGF-I levels in all groups became as low as those seen in winter. Serum IGF-I level showed no significant relationship with body size or gill NKA activity.
Changes in FL (a), BW (b), condition factor (c), gill NKA activity (d) and serum IGF-I levels (e) during smoltification of masu salmon reared at Kumaishi Hatchery in 2012. Fish were divided by size in winter (Large: >11.5 cm, Small: 9.5–11.5 cm) and fed at different rations to create high and low growth in spring (High and Low). Values are expressed as mean ± SE (n = 6–7). Symbols sharing the same letters are not significantly different from each other
Shimamaki Hatchery in 2012 and 2013
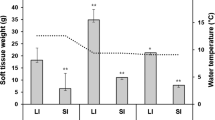
In both years, FL and BW were kept constant from January to April and increased in May (Fig. 5a, b). The condition factor was high in 2013 fish from February to April (Fig. 5c). It decreased in 2013 fish in May, whereas it increased in 2012 fish. In 2012, serum IGF-I levels stayed low from February to April but sharply increased in May (Fig. 5d). In 2013, serum IGF-I significantly increased from February to March and further increased from April to May.
Changes in FL (a), BW (b), condition factor (c) and serum IGF-I levels (d) during smoltification of masu salmon reared at Shimamaki Hatchery in 2012 and 2013. Fish were fed every 3 or 4 days during early spring in 2012, whereas they were fed daily in 2013. Values are expressed as mean ± SE (n = 6–7). Symbols sharing the same letters are not significantly different from each other
Smolt-to-adult return (SAR) at Kumaishi
Spawning adult masu salmon released from the hatchery in 2011 and 2012 were caught 16 months after release at the Kenichi River in 2012 and 2013, respectively. For the 2011-release fish caught in 2012, there was no significant difference in SAR between groups (Table 1). On the other hand, for the 2012-release fish caught in 2013, a significantly higher SAR was seen for Large-High fish (Table 1).
Discussion
The final goal of our study is to establish indices of “smolt quality” that enable us to evaluate survival of released fish in seawater and ultimately their return as adults. Gill NKA activity is routinely used as an index of the smolt quality since the ability to adapt to hypo-osmotic environment is essential for smolts to survive in the ocean. Indeed, gill NKA activity in spring had a positive relationship with SAR of Chinook salmon released to the Deschutes River, Oregon, USA [18]. However, seawater adaptability does not always guarantee long-term survival. Several studies suggest that high growth rate is the key to surviving predation pressure and nutritional deficiency during the first year in the ocean [13–15]. Thus, potential for growth after adapting to seawater is an important trait that affects, and in turn predicts, a long-term survival. Zydlewski and Zydlewski [37] compared growth rates of individually tagged Atlantic salmon Salmo salar smolts with varying degrees of gill NKA and suggested that gill NKA activity of smolt in freshwater did not predict long-term growth in seawater. Therefore, there is a need to establish indices that predict long-term growth potential in seawater. Circulating IGF-I is a candidate for this purpose since it is generally positively correlated with individual growth rate in fish, including masu salmon [36, 38–41]. In addition, circulating IGF-I levels in Chinook salmon smolts just before release have been shown to relate to SAR [18].
A good number of studies have looked at profiles of circulating IGF-I during smoltification of salmonids including masu salmon [32, 42]. However, the present study is the first to report the IGF-I profile in naturally reared smolting masu salmon. This data is relevant for the hatchery programs, since one of the major goals of the programs is to create hatchery fish that are physiologically equivalent to wild fish. Mizuno et al. [43] compared metabolic capability between naturally reared and hatchery reared masu salmon during smoltification and pointed out differences in some enzymes involved in carbohydrate metabolism, the citric acid cycle and the respiratory chain. In the present study, profiles of serum IGF-I as well as gill NKA activity of hatchery fish were compared to those of naturally reared fish. Fish from the Kenichi River showed a sharp increase in gill NKA activity that was preceded by a significant increase in circulating IGF-I. Gill NKA activity and circulating IGF-I in hatchery fish showed trends similar to those in naturally reared fish, although the magnitude of the changes was not as sharp as those in naturally reared fish. Given that both fish experienced the same water temperature profile, the difference should be attributed to other factors, such as nutritional status, physical rearing structure and rearing density. There may be a concern that sampling by electrofishing might cause a stress response and in turn affect measured IGF-I levels in naturally reared fish. Although this possibility cannot be completely ruled out, we think the effect of electrofishing was minimal, since this method has been proven to be less stressful compared to cast net in ayu Plecoglossus altivelis [44]. In the present study, food quality and availability are likely reasons for the difference, because the size of naturally reared fish was similar to that of hatchery fish during February and March, but the naturally reared fish became significantly larger and fatter in April through May. An increase in the condition factor in naturally reared fish in April might not be a typical response during smoltification, but it reflected an improvement of feeding status in the river. In both fish, serum IGF-I was positively correlated with body size and gill NKA activity. These results suggest that size or/and growth in spring is important for achieving high gill NKA activity and circulating IGF-I.
We next examined effect of winter size on gill NKA and serum IGF-I by sorting fish by fork length in November at Kumaishi Hatchery. Difference in size was retained throughout the smoltification period. Gill NKA activity was significantly higher in small-in-winter fish in April. In contrast, there was no difference in serum IGF-I levels between groups in April, but it was high in small-in-winter fish in May. These patterns of gill NKA and serum IGF-I in small-in-winter fish are in contrast to those found in the naturally reared fish where IGF-I increased prior to activation of gill NKA. Local IGF-I expressed by the gill may be involved in the activation of NKA [32]. However, our unpublished data on gill igf-1 mRNA levels in these fish showed that the relationship between gill igf-1 and NKA was weak, albeit significant (r 2 = 0.18; Shimomura et al., unpublished data). We assume that the increase in gill NKA in the small fish in April was secondary to the increase in fish size. Indeed, there was a positive relationship between fish size and gill NKA (r 2 = 0.38). Given that gill NKA activity is generally higher in large fish [16, 17], it is possible that IGF-I promoted growth of organs including the gill, which in turn resulted in higher NKA activity.
We further examined effects of winter size and spring growth on gill NKA and serum IGF-I in 2012. Profiles of gill NKA activity were similar among three groups, showing a sharp increase from April to May. In contrast, IGF-I levels in Large-Low fish were significantly low in early spring (March–April), which might reflect restricted feeding ration during that period. It is of note that although IGF-I in Large-High and Small-High fish consistently increased from February to April, it dropped to the basal levels in May. This IGF-I profile is similar to that of naturally reared fish in terms of an increase prior to the activation of gill NKA, but differs in terms of low values in May. Water temperature is known to affect the degree and/or timing of the spring IGF-I increase [45] and might also affect the IGF-I profile in the present study. However, there was little difference in rearing water temperature between 2011 and 2012 at Kumaishi Hatchery. Although a year-to-year variation of circulating IGF-I profile was also reported in Chinook salmon [18], the reason(s) for the variation is not known at present. It is also noteworthy that despite the depressed IGF-I level, gill NKA activity was high in May in all groups and there was no relationship between them, which is in contrast to our previous study [32]. This finding doesn’t discount the importance of IGF-I in regulating the development of seawater adaptability, but suggests that in masu salmon, circulating IGF-I does not correlate with gill NKA activity under certain conditions. Investigating this decoupling may be important to better understand the relative importance of circulating and local IGF-I in activating gill NKA.
Circulating IGF-I levels are affected by environmental factors such as feeding status, season, developmental stage, water temperature, photoperiod and stress. Larsen et al. [46, 47] examined the effect of low temperature and fasting during winter on the physiological status of smolting coho salmon O. kisutch. Their study suggested that winter fasting did not impair the condition or smoltification of hatchery reared fish [46, 47]. In the present study, a feeding protocol was tested at Shimamaki Hatchery in 2012 to give a compensatory increase of growth as well as IGF-I in spring by suppressing feeding frequency (fed every 3 or 4 days) during winter. As a result, growth and IGF-I were low during that period. From April to May when feeding frequency and rations were increased, FL, BW, condition factor and IGF-I increased. However, IGF-I values were relatively low compared to those in Kumaishi fish. In 2013, fish were fed daily at a slightly higher ration in early spring to test if an IGF-I profile resembling that in Kumaishi fish could be created by changing the feeding protocol. As a result, IGF-I levels started to increase in March and were further elevated in May, as seen at Kumaishi Hatchery. These changes might reflect IGF-I response to a combination of increased feeding frequency and ration. In spite of the low rearing water temperature, which could suppress the IGF-I peak, at Shimamaki Hatchery, the current result suggests IGF-I profiles can be manipulated by changing feeding frequency or/and ration, although more work needs to be done to prove this hypothesis.
The most reliable way to evaluate hatchery success is to look at SAR. In the present study, we estimated SAR of some rearing groups from Kumaishi Hatchery by catching spawning adults at the Kenichi River. In the 2011-release group, there were variations in growth history and profiles of serum IGF-I and gill NKA activity, but SARs were not significantly different between the groups. In contrast, despite little variations in gill NKA and serum IGF-I at the time of release in 2012, growth history had a significant effect on SAR, it being highest in Large-High fish. Beckman et al. [18] reported that the SARs of Chinook salmon released from three hatcheries on the Deschutes River, Oregon, USA were different. Their study also revealed that gill NKA activity and plasma IGF-I level at the time of release showed significant relationships to SAR [18], which is in contrast to our finding. We have no good explanation for the lack of the relation between IGF-I and SAR in masu salmon, but suspect that the ocean conditions and predation pressure had a stronger impact on survival than did the status of smolting at release. Ando et al. [48] reported that masu salmon released from Kumaishi Hatchery migrated to the Sea of Okhotsk, either along the Sea of Japan or on Pacific Ocean side of Hokkaido Island. Although data is limited, conditions in the Sea of Okhotsk may be critical to their survival (Iijima et al., unpublished data). As mentioned above, growth status in the early marine life is the key to survival for salmonids [13–15]. Filling a gap of information on the growth status of masu salmon from early ocean life through winter is necessary. Monitoring growth of outmigrating postsmolts using IGF-I has been conducted for coho and Atlantic salmon to better estimate their survival and stock recruitment [38, 49, 50], and introducing such approach to masu salmon is important. The present study, however, provided evidence that in masu salmon. growth history during freshwater residency affected long-term survival in the ocean.
In summary, the present study showed that profiles of circulating IGF-I during smoltification of masu salmon varied under different rearing conditions, depending mainly on growth history. Although serum IGF-I may be important for both development of seawater adaptability and growth enhancement, in masu salmon, serum IGF-I level alone was not a predictor of long-term survival after sea entry. However, differences in growth history had a significant effect on the adult returns. Thus, optimizing rearing conditions is a critical component for the success of hatchery release of masu salmon, and the development of physiological indices to evaluate the status of smolts is desired.
References
Miyakoshi Y (2006) Evaluation of stock enhancement programs and stock assessment for masu salmon in Hokkaido, Northern Japan. Sci Rep Hokkaido Fish Hatch 60:1–64 In Japanese with English abstract
Nagata M, Miyakoshi Y, Urabe H, Fujiwara M, Sasaki Y, Kasugai K, Torao M, Ando D, Kaeriyama M (2012) An overview of salmon enhancement and the need to manage and monitor natural spawning in Hokkaido, Japan. Environ Biol Fish 94:311–323
Miyakoshi Y, Nagata M, Kitada S, Kaeriyama M (2013) Historical and current hatchery programs and management of chum salmon in Hokkaido, northern Japan. Fish Sci 21:469–479
Hoar WS (1988) The physiology of smolting salmonids. In: Hoar WS et al (eds) Fish physiology. Academic Press Inc., San Diego, pp 275–343
Stefansson SO, Björnsson BT, Ebbesson LOE, McCormick SD (2008) Smoltification. In: Finn RN, Kapoor BG (eds) Fish larval physiology. Science Publishers, Enfield, pp 639–681
Björnsson BT, Stefansson SO, McCormick SD (2011) Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol 170:290–298
McCormick SD (2013) Smolt physiology and endocrinology. In: McCormick SD, Farrell AP, Brauner CJ (eds) Euryhaline fishes, vol 32., Fish physiologyAcademic Press, Oxford, pp 199–251
Folmar LC, Dickhoff WW, Mahnken CVW, Waknitz FW (1982) Stunting and parr-reversion during smoltification of coho salmon (Oncorhynchus kisutch). Aquaculture 28:91–104
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Evans DH (2008) Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol Regul Integr Comp Physiol 295:R704–R713
Hwang PP, Lee TH, Lin LY (2011) Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301:R28–R47
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184:257–268
Beamish RJ, Mahnken C (2001) A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog Oceanogr 49:423–437
Beamish RJ, Mahnken C, Neville CM (2004) Evidence that reduced early marine growth is associated with lower marine survival of coho salmon. Trans Am Fish Soc 133:26–33
Moss JH, Beauchamp DA, Cross AD, Myers KW, Farley EV, Murphy JM, Helle JH (2005) Evidence for size-selective mortality after the first summer of ocean growth by pink salmon. Trans Am Fish Soc 134:1313–1322
Shrimpton JM (1996) Relationship between size, gill corticosteroid receptors, Na+-K+ ATPase activity and smolting in juvenile coho salmon (Oncorhynchus kisutch) in autumn and spring. Aquaculture 147:127–140
Beckman BR, Larsen DA, Dickhoff WW (2003) Life history plasticity in chinook salmon: relation of size and growth rate to autumnal smolting. Aquaculture 222:149–165
Beckman BR, Dickhoff WW, Zaugg WS, Sharpe C, Hirtzel S, Schrock R, Larsen DA, Ewing RD, Palmisano A, Schreck CB, Mahnken CVW (1999) Growth, smoltification, and smolt-to-adult return of spring chinook salmon from hatcheries on the Deschutes River, Oregon. Trans Am Fish Soc 128(6):1125–1150
Miyakoshi Y, Nagata M, Kitada S (2001) Effect of smolt size on postrelease survival of hatchery-reared masu salmon Oncorhynchus masou. Fish Sci 67:134–137
Dickhoff WW, Beckman BR, Larsen DA, Mahnken CVW, Schreck CB, Sharpe C, Zaugg WS (1995) Quality assessment of hatchery-reared spring chinook salmon smolts in the Columbia River basin. Am Fish Soc Symp 15:292–302
Dickhoff WW, Beckman BR, Larsen DA, Duan C, Moriyama S (1997) The role of growth in endocrine regulation of salmon smoltification. Fish Physiol Biochem 17:231–236
Daughaday WH, Rotwein P (1989) Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10:68–91
Le Roith D, Bondy C, Yakar S, Liu JL, Butler A (2001) The somatomedin hypothesis: 2001. Endocr Rev 22:53–74
Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J (2009) The role of liver-derived insulin-like growth factor-I. Endocr Rev 30:494–535
Richman NH 3rd, Zaugg WS (1987) Effects of cortisol and growth hormone on osmoregulation in pre- and desmoltified coho salmon (Oncorhynchus kisutch). Gen Comp Endocrinol 65:189–198
Madsen SS (1990) The role of cortisol and growth hormone in seawater adaptation and development of hypoosmoregulatory mechanisms in sea trout parr (Salmo trutta trutta). Gen Comp Endocrinol 79:1–11
Prunet P, Pisam M, Claireaux JP, Boeuf G, Rambourg A (1994) Effects of growth hormone on gill chloride cells in juvenile Atlantic salmon (Salmo salar). Am J Physiol 266:R850–R857
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
Mancera JM, McCormick SD (2007) Role of prolactin, growth hormone, insulin-like growth factor I and cortisol in teleost osmoregulation. In: Baldisserotto B, Mancera JM, Kapoor BG (eds) Fish osmoregulation. Science Publishers, Enfield
Beckman BR, Larsen DA, Moriyama S, Lee-Pawlak B, Dickhoff WW (1998) Insulin-like growth factor-I and environmental modulation of growth during smoltification of spring chinook salmon (Oncorhynchus tshawystscha). Gen Comp Endocrinol 109:325–335
Reinecke M (2010) Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J Fish Biol 76:1233–1254
Shimomura T, Nakajima T, Horikoshi M, Iijima A, Urabe H, Mizuno S, Hiramatsu N, Hara A, Shimizu M (2012) Relationships between gill Na+, K+-ATPase activity and endocrine and local insulin-like growth factor-I levels during smoltification of masu salmon (Oncorhynchus masou). Gen Comp Endocrinol 178:427–435
Shimizu M, Swanson P, Fukada H, Hara A, Dickhoff WW (2000) Comparison of extraction methods and assay validation for salmon insulin-like growth factor-I using commercially available components. Gen Comp Endocrinol 119:26–36
Small BC, Peterson BC (2005) Establishment of a time-resolved fluoroimmunoassay for measuring plasma insulin-like growth factor I (IGF-I) in fish: effect of fasting on plasma concentrations and tissue mRNA expression of IGF-I and growth hormone (GH) in channel catfish (Ictalurus punctatus). Domest Anim Endocrinol 28:202–215
Quabius ES, Balm PHM, Bonga SEW (1997) Interrenal stress responsiveness of Tilapia (Oreochromis mossambicus) is impaired by dietary exposure to PCB 126. Gen Comp Endocrinol 108:472–482
Beckman BR, Shimizu M, Gadberry BA, Cooper KA (2004) Response of the somatotropic axis of juvenile coho salmon to alterations in plane of nutrition with an analysis of the relationships among growth rate and circulating IGF-I and 41 kDa IGFBP. Gen Comp Endocrinol 135:334–344
Zydlewski GB, Zydlewski J (2012) Gill Na+, K+-ATPase of Atlantic salmon smolts in freshwater is not a predictor of long-term growth in seawater. Aquaculture 362:121–126
Beckman BR, Fairgrieve W, Cooper KA, Mahnken CVW, Beamish RJ (2004) Evaluation of endocrine indices of growth in individual postsmolt coho salmon. Trans Am Fish Soc 133:1057–1067
Picha ME, Turano MJ, Tipsmark CK, Borski RJ (2008) Regulation of endocrine and paracrine sources of Igfs and Gh receptor during compensatory growth in hybrid striped bass (Morone chrysops X Morone saxatilis). J Endocrinol 199:81–94
Beckman BR (2011) Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol 170:233–252
Kawaguchi K, Kaneko N, Fukuda M, Nakano Y, Kimura S, Hara A, Shimizu M (2013) Responses of insulin-like growth factor (IGF)-I and two IGF-binding protein-1 subtypes to fasting and re-feeding, and their relationships with individual growth rates in yearling masu salmon (Oncorhynchus masou). Comp Biochem Physiol A 165:191–198
Azuma T, Noda S, Yada T, Ototake M, Nagoya H, Moriyama S, Yamada H, Nakanishi T, Iwata M (2002) Profiles in growth, smoltification, immune function and swimming performance of 1-year-old masu salmon Oncorhynchus masou masou reared in water flow. Fish Sci 68:1282–1294
Mizuno S, Urabe H, Aoyama T, Omori H, Iijima A, Kasugai K, Torao M, Misaka N, Koide N, Ueda H (2012) Changes in activity and transcript level of liver and gill metabolic enzymes during smoltification in wild and hatchery-reared masu salmon (Oncorhynchus masou). Aquaculture 362:109–120
Awata S, Tsuruta T, Yada T, Iguchi K (2013) Stress hormone responses in ayu Plecoglossus altivelis in reaction to different catching methods: comparisons between electrofishing and cast netting. Fish Sci 79:157–162
McCormick SD, Moriyama S, Björnsson BT (2000) Low temperature limits photoperiod control of smolting in Atlantic salmon through endocrine mechanisms. Am J Physiol Regul Integr Comp Physiol 278:R1352–R1361
Larsen DA, Beckman BR, Dickhoff WW (2001) The effect of low temperature and fasting during the winter on metabolic stores and endocrine physiology (insulin, insulin-like growth factor-I, and thyroxine) of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol 123:308–323
Larsen DA, Beckman BR, Dickhoff WW (2001) The effect of low temperature and fasting during the winter on growth and smoltification of coho salmon. N Am J Aquac 63:1–10
Ando D, Miyamoto M, Kasugai K, Miyakoshi Y, Nagata M (2005) Seasonal distribution of yearling masu salmon released from the Sea of Japan side of southwestern Hokkaido, Japan. N Am J Fish Manage 25:22–37
Stefansson SO, Haugland M, Bjornsson BT, McCormick SD, Holm M, Ebbesson LOE, Holst JC, Nilsen TO (2012) Growth, osmoregulation and endocrine changes in wild Atlantic salmon smolts and post-smolts during marine migration. Aquaculture 362:127–136
McCormick SD, Sheehan TF, Björnsson BT, Lipsky C, Kocik JF, Regish AM, O’Dea MF (2013) Physiological and endocrine changes in Atlantic salmon smolts during hatchery rearing, downstream migration, and ocean entry. Can J Fish Aquat Sci 70:105–118
Acknowledgments
We thank Yoshihide Sasaki and Shigeo Hirai, Shimamaki Fishery Cooperative for their excellent care of hatchery fish and collecting of samples. We also thank Tomoya Aoyama, Salmon and Freshwater Fisheries Research Institute, Hokkaido Research Organization, for his help in collecting fish in the rivers. This work was supported by a grant from the Northern Advancement Center for Science and Technology (Noastec).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneko, N., Iijima, A., Shimomura, T. et al. Profiles of circulating insulin-like growth factor-I during smoltification of masu salmon reared under different conditions. Fish Sci 81, 643–652 (2015). https://doi.org/10.1007/s12562-015-0870-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0870-y