Abstract
This study was conducted to investigate the mechanism of green liver symptom induction and the effect of dietary taurine supplementation on growth performance in juvenile red sea bream fed non-fishmeal diets based on soy protein concentrate (SPC). Juvenile fish (initial BW 72 g) were fed for 20 weeks on SPC diets supplemented with taurine at levels of 0, 1.0, and 2.0%. In the taurine-unsupplemented SPC diet group, specific growth rate (SGR) and feed conversion ratio (FCR) were significantly inferior (P < 0.001), and incidence of green liver was observed in 70% of fish. In this group, hepatopancreatic and plasma taurine concentrations were lowest (P < 0.05), hepatopancreatic content of bile pigments was highest (P < 0.05), and osmotic tolerance of erythrocytes was inferior (P < 0.05) among the dietary treatment groups. Serum osmolality of all treatment groups was at similar levels. These physiological abnormalities as well as SGR and FCR were improved by dietary taurine supplementation. These results indicate that the mechanism for induction of green liver symptom is bile pigment overproduction due to increased hemolysis because erythrocytes become osmotically fragile due to dietary taurine deficiency. Taurine supplementation of SPC diets is essential for maintaining normal physiological condition and growth performance in juvenile red sea bream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish harvested from aquaculture are increasingly important as a source of good quality animal protein for human consumption [1]. Cultured marine fish species are mainly of high economic value and are generally carnivorous. Hence, formulated feeds for these fish species contain high levels of fish meal (FM) as the protein source [2]. In recent years, however, the demand for FM has been heavy worldwide, and thus the development of feeds containing lesser amounts of FM is needed for the sustainable development of the aquaculture industry [2]. Previous studies have demonstrated that FM in fish feed can be partially replaced with alternative protein sources, while excessive replacement of FM results in inferior growth performance together with physiological abnormalities [3]. In red sea bream Pagrus major [4] and yellowtail Seriola quinqueradiata [5], important mariculture species in Japan, fish fed lesser amounts of FM diets including larger amounts of alternative proteins experienced inferior growth performances and physiological abnormalities, such as anemia and so-called green liver syndrome in which the liver has a green discoloration. Green pigment in the liver originates from biliverdin, which is a bile pigment produced by heme metabolism [6]. In fish, biliverdin is excreted into the bile as is or converted to bilirubin, and bilirubin conjugates mainly with taurine to elevate the polarity prior to excretion [7].
Taurine (aminoethylsulfonic acid) is one of the sulfur amino acids and has diverse and important physiological roles [8, 9]. Generally, mammals produce taurine via metabolism of methionine and cystine. However, taurine is a dietary essential nutrient for kittens [10] and infants [11, 12], since the activity of taurine synthesis enzymes in kittens and infants is inadequate. Previous studies have demonstrated that the capacity for taurine synthesis is different among fish species, and the ability is markedly lower in carnivorous fish species [13, 14], while taurine is the most abundant extract component in fish tissues [15].
Fish meal contains approximately 4 mg/g of taurine, but plant protein sources do not contain taurine [16]. Hence, we deduced that green liver syndrome in yellowtail and red sea bream fed diets with lesser amounts of FM might be caused by an abnormality in the excretion and production of bile pigments due to dietary taurine deficiency. In juvenile yellowtail [17–19] and yearling red sea bream [20, 21] fed low or non-FM diets based on soy protein concentrate (SPC), green liver syndrome was observed in a large percentage as was inferior growth performance, and these conditions were improved by dietary taurine supplementation. In red sea bream, however, it is well known that green liver syndrome is observed frequently during periods of low water temperature [22]. In addition, green liver syndrome has been observed in the mature stage of fish (H. Murata, pers. comm., 2000). Thus, previous studies on yearling red sea bream do not exclude the effects of low water temperature and maturation of fish on the appearance of green liver syndrome.
To develop high quality diets with lesser amounts of FM, it is important to clarify the roles of taurine in the physiological condition and growth performance of fish. Therefore, this study was conducted to investigate the mechanism for induction of green liver syndrome and the effect of dietary taurine supplementation on improving growth performance in the juvenile red sea bream fed non-FM diets based on SPC under warm water conditions, to exclude the influence of maturity and low water temperature on the occurrence of green liver syndrome.
Materials and methods
Experimental diets
Non-FM diets containing 55% SPC (Danpro A, Aarhus Olie, Denmark), 1.27% l-lysine hydrochloride, and 0.86% dl-methionine were supplemented with taurine at levels of 0% (0TAU), 1.0% (1.0TAU), and 2.0% (2.0TAU) (Table 1). The diets were made into steam dry pellets and were stored at –20°C until use. Proximate composition, methionine and cystine contents of all the experimental diets were similar. Taurine content of the diets increased proportionally with dietary taurine supplementation, and the content (mg/g diet) was 0.01 in 0TAU, 9.79 in 1.0TAU, and 20.8 in 2.0TAU.
Fish and feeding conditions
Juveniles of red sea bream were obtained from Ehime Prefectural Fish Farming Center (Uwajima, Ehime, Japan). Fish with an initial mean body weight of 72 g were allotted to duplicate 500 l tanks for each dietary treatment, with each tank being assigned 15 fish. The fish were fed the experimental diet to satiation twice a day, 6 days week−1 for 20 weeks. The tanks were supplied with sand-filtered seawater at a flow rate of 9.8 l min−1. The water temperature during the feeding trial was in the range of 17.4–25.7°C. After the water temperature fell below 18°C, the water was warmed gradually using an aquatron (Marine Catcher SCW-20, Ryomei Kogyo, Aichi, Japan) and maintained at about 22°C for 5 weeks prior to sampling at the end of the feeding trial, to prevent the incidence of green liver due to low water temperature [22].
Sampling and analytical methods
Five fish from each tank were sampled at the end of the feeding trial. Fish were starved for 48 h prior to sampling and anesthetized with 0.4 ml/l 2-phenoxyethanol at the time of sampling. Anatomical observations were made to check for the presence of green liver syndrome. Hematological and hemochemical variables [19], hepatopancreatic and plasma taurine concentrations [23], hepatopancreatic and biliary bile pigment concentrations [24], serum osmolality, and osmotic tolerance of erythrocytes (EC50 value) [18] were measured. In addition, histological examinations of the hepatopancreas and spleen of three fish from each tank were also conducted at the end of the feeding trial. Hepatopancreas samples were stained with hematoxylin-eosin to look for the stagnation of bile and occlusion of bile duct in the tissue. Spleen samples were stained with Berlin blue to check for hemosiderin deposition in the tissue, which indicates an increase in hemolysis [25].
Statistical analysis
Data are presented as means of two replicate tanks in each treatment group. All data were subjected to one-way ANOVA using 4 Steps Excel SQC (OMS, Saitama, Japan), and when appropriate, the statistical significance of the differences among treatment groups was analyzed using a Tukey-Kramer test and assessed at a 5% level of probability [26].
Results
Growth and feed performance
Palatability of the experimental diets was not influenced by dietary treatments, but the feeding activity of the 0TAU group that received the taurine-unsupplemented SPC diet declined slightly with prolonged experimental period. Fish in the 1.0TAU and 2.0TAU groups that received the taurine-supplemented SPC diets fed actively throughout the course of this study. Mortalities of all the treatment groups were 3.4% (Table 2). Specific growth rate of the 0TAU group was the lowest and feed conversion ratio of this group was the highest among the treatment groups. Daily feed intake of the 0TAU group was the lowest among the treatment groups. In contrast, these parameters were significantly improved by dietary taurine supplementation and were not significantly different between the 1.0TAU and 2.0TAU groups.
Hepatopancreatic and plasma taurine concentrations
Hepatopancreatic taurine content of the 0TAU group was the lowest among the treatment groups (Table 3). The taurine content was significantly increased with dietary taurine supplementation, and the taurine contents were significantly different in the different groups. Plasma taurine concentration of the 0TAU group was the lowest among the treatment groups. The taurine concentration significantly increased with dietary taurine supplementation, and the concentration of the 2.0TAU group tended to be higher compared to the 1.0TAU group, although the difference was not significant.
Incidence of green liver and bile pigment concentration
Incidence of green liver of the 0TAU group was 70%, and the syndrome was not observed in the 1.0TAU and 2.0TAU groups (Table 4). Hepatopancreatic biliverdin and bilirubin contents of the 0TAU group were the highest, and thus the total bile pigment content of this group was the highest among the treatment groups. The biliverdin, bilirubin, and total bile pigment contents decreased with dietary taurine supplementation, and the contents of these substances between the 1.0TAU and 2.0TAU groups were not significantly different.
Biliary biliverdin concentrations of the treatment groups were at similar levels and were not significantly different from each other (Table 5), although the hepatopancreatic biliverdin content of the 0TAU group was the highest among the treatment groups (Table 4). Taurobilirubin concentration of the 0TAU group was the highest among the treatment groups. Bilirubin concentration of the 0TAU group was significantly higher than that of the 2.0TAU group. Bilirubin monoglucuronide and bilirubin diglucuronide concentrations of the treatment groups were not significantly different from each other, although the concentrations of the 0TAU group tended to be higher compared to the 1.0TAU and 2.0TAU groups.
Hematological and hemochemical variables
Red blood cell count (RBC) of the 0TAU group was the lowest among the treatment groups (Table 6). Hemoglobin concentration (Hb), hematocrit value (Ht), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH) of the 0TAU group tended to be low compared to the 1.0TAU and 2.0TAU groups, although the differences were not significant. Mean corpuscular volumes (MCV) of the treatment groups were at similar levels and did not differ significantly.
The activities of the plasma enzymes, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), γ-glutamyl transpeptitase (GGT), and alkaline phosphatase (ALP) were at similar levels between treatment groups and were not significantly different from each other (Table 6).
Serum osmolality and EC50 value
Serum osmolality of all the treatment groups showed similar levels regardless of the dietary taurine supplementation (Table 7). EC50 value decreased with the dietary taurine supplementation, and the value of the 0TAU group was significantly higher than that of the 2.0TAU group (Table 7).
Histological studies
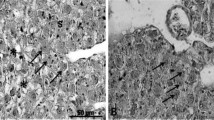
In the hepatopancreatic tissue, neither stagnation of bile nor biliary obstruction was observed in any of the treatment groups (Fig. 1a–c). In the spleen tissue, hemosiderin deposits, which indicate increased hemolysis, were not observed in any of the treatment groups (Fig. 2a–c).
Photomicrographs of histological sections of spleen in juvenile red sea bream fed non-FM diets based on SPC supplemented with taurine at levels of a 0%, b 1.0%, and c 2.0%. Berlin blue stain. Hemosiderin deposits, which stain blue and are an index of an increase in hemolysis, were not observed in any of the treatment groups
Discussion
In the present study, dietary taurine supplementation prevented green liver syndrome and markedly improved growth performance of juvenile red sea bream fed the SPC diets supplemented with methionine and lysine. Here, we examine the mechanism of induction of taurine deficiency-dependent green liver symptom in juvenile red sea bream fed non-FM diets based on SPC compared with the cases of yearling red sea bream [20, 21] and juvenile yellowtail [17–19]. Furthermore, we discuss the effect of taurine supplementation in alternative protein diets on improving the growth performance of red sea bream.
Mechanism for induction of taurine deficiency-dependent green liver symptom
In fish, green liver syndrome is observed in the mature stage (H. Murata, pers. comm., 2000). In addition, it is well-known that green liver syndrome of red sea bream is frequently observed during periods of low water temperatures. In the rearing experiment of juvenile red sea bream, incidence of green liver syndrome was high under the low water-temperature conditions of about 10°C, but the incidence was remarkably reduced by warming the water to more than 18°C for 5 weeks [22]. In the present study, the influences of both maturation and low water temperature on the occurrence of green liver were excluded by using immature juvenile red sea bream and maintaining the fish at about 22°C for 5 weeks prior to the sampling at the end of the experiment. As a result, in fish fed the taurine-unsupplemented SPC diet, the incidence of green liver syndrome was high. In these fish, the hepatopancreatic and plasma taurine concentrations were the lowest and the hepatopancreatic bile pigment contents were the highest among the treatment groups. In contrast, in fish fed the taurine-supplemented SPC diets, incidence of green liver syndrome was not observed, as the taurine concentrations of tissues significantly increased together with a significant decrease in hepatopancreatic bile pigment contents with dietary taurine supplementation. Hepatic tissues of fish fed the taurine-unsupplemented SPC diet were not markedly affected, as shown in the activities of hepatic function enzymes in plasma. Therefore, green liver syndrome of juvenile red sea bream fed the taurine-unsupplemented SPC diet is concluded to be caused by dietary taurine deficiency. Green liver syndrome appears to be due to an increase in hepatopancreatic biliverdin content, which is a bile pigment and is green in color [6]. An increase in hepatopancreatic bile pigment contents is caused by (1) a decrease in bile pigment excretion from the hepatopancreas into bile and (2) an increase in bile pigment production.
As for bile pigment excretion, neither hepatobiliary obstruction nor bile pigment stagnation in the hepatopancreas occurred in any of the treatment groups as shown in the results of the histological study. In red sea bream fed the taurine-supplemented SPC diets, bile pigments were excreted from the hepatopancreas into the bile mainly as biliverdin, which has a high polarity [7], as observed with yearling red sea bream fed the FM diet [20]. On the other hand, in red sea bream fed the taurine-unsupplemented SPC diet, bile pigments were excreted from the hepatopancreas into the bile as biliverdin, and in addition hepatopancreatic biliverdin that exceeded the capacity of excretion was reduced to bilirubin, which has a low polarity [7]. Then, bilirubin conjugated with taurine and/or glucuronic acid, which are synthesized in vivo to elevate polarity, and was excreted into the bile. Moreover, in the present study, biliary bile pigment concentration increased with the increase in hepatopancreatic bile pigment contents, and pronounced atrophy of the gallbladder was not observed in the treatment groups. These results indicate that bile pigment excretion in juvenile red sea bream is not markedly affected by dietary taurine deficiency as in the case of yearling red sea bream [20, 21]. This differs from the case of yellowtail [17–19]. In yellowtail, the ability of taurine synthesis is lower compared with red sea bream [13, 14], and bile pigments are excreted from the liver into the bile mainly as taurobilirubin [7, 17, 19]. Hence, in yellowtail fed taurine-unsupplemented SPC diets, bile pigment excretion is markedly reduced because bilirubin could not conjugate with taurine due to dietary taurine deficiency [17–19].
Concerning bile pigment production, in fish fed the taurine-unsupplemented SPC diet, Hb and Ht were lower, RBC was significantly lower, and osmotic tolerance of erythrocytes was inferior as shown in the EC50 value compared with fish fed the taurine-supplemented SPC diets. These results indicate that hemolysis progresses in fish fed the taurine-unsupplemented SPC diet because the erythrocytes become osmotically fragile due to dietary taurine deficiency. A previous study demonstrated that in juvenile yellowtail fed taurine-unsupplemented SPC diet, hemolysis increased due to a decrease in osmotic tolerance in erythrocytes, and n-3 highly unsaturated fatty acids (n-3 HUFA) content in the biomembrane of erythrocytes was lower [18]. Hence, it is considered that the same phenomenon occurred in the red sea bream as in the case of yellowtail. In contrast, osmotic tolerance of the erythrocytes together with n-3 HUFA content in the biomembrane of erythrocytes increased, and hemolysis was prevented by taurine supplementation to the SPC diets for juvenile yellowtail [18]. Taurine has a biomembrane stabilization role [8, 9]. In addition, docosahexaenoic acid, one of the n-3 HUFA, affects the fluidity and rigidity of the cell membrane structure [27]. Previous studies have also suggested that stability of erythrocytes is related to the n-3 HUFA content in the biomembrane of erythrocytes [28–30]. Digestive absorption of lipid is promoted with bile acid, and bile acid conjugates with glycine and/or taurine prior to secretion [31]. Particularly in fish, bile acid conjugates with taurine [31, 32]. Hence, erythrocytes of fish fed taurine-deficient diets might become osmotically fragile because the n-3 HUFA content of erythrocytes decreases with a decrease in digestive absorption of lipid via a decrease in bile acid secretion.
In the present study, hemosiderin deposition, which indicates increased hemolysis [25], was not observed in the spleen tissue of juvenile red sea bream fed a non-FM diet based on SPC, although a marked hemosiderin deposition was observed in the spleen tissue of yearling red sea bream [21] and juvenile yellowtail [17] fed such diets. The difference in the results between juvenile and yearling red sea bream might be influenced by the stage of maturation and water temperature, since the rearing periods of the studies were similar in juvenile (week 20) and in yearling (week 22) fish. The difference in the results between red sea bream and yellowtail might be reflected in the difference in their taurine synthesis ability [13, 14] and in the requirement for taurine to maintain the physiological conditions as mentioned above.
On the other hand, serum osmolality of all the treatment groups was at similar levels, although the plasma taurine concentration significantly increased with taurine supplementation to SPC diets. Hence, it is considered that the dietary taurine content does not influence serum osmolality in juvenile red sea bream as in the case of yearling fish [21], although taurine plays an important role in osmoregulation of fish [8, 9, 33], and serum osmolality of juvenile yellowtail is highly correlated with the plasma taurine concentration [18].
From these results, the mechanism for induction of taurine deficiency-dependent green liver symptom in red sea bream fed non-FM diets based on SPC is as follows: in red sea bream fed the taurine-unsupplemented SPC diets, hemolysis increases gradually because the erythrocytes become osmotically fragile due to dietary taurine deficiency, hepatopancreatic biliverdin content increases, and this induces the green liver symptom. On the other hand, in yellowtail fed taurine-unsupplemented SPC diets, severe green liver symptoms occur by both a decrease in bile pigment excretion and an increase in hemolytic bile pigment production due to taurine deficiency [17–19]. Therefore, the influence of taurine deficiency on physiological condition is different among fish species, since the capacity for taurine synthesis and the need for taurine for bile pigment excretion and osmoregulation are species-specific.
Effect of taurine supplementation in alternative protein diets on improving growth performance
We previously reported that growth performance of juvenile red sea bream fed low-FM diets based on SPC was improved by dietary methionine and lysine supplementation, but the performance was slightly inferior compared with fish fed the FM diet [34]. In addition, growth performance of red sea bream fed low-FM diets based on SPC supplemented with methionine and lysine was improved by dietary taurine supplementation to a level comparable to the fish fed an FM diet [20]. In the present study, in juvenile red sea bream fed a non-FM diet based on SPC supplemented with methionine and lysine, tissue taurine concentration was lower and growth performance was inferior. In contrast, tissue taurine concentration was increased and growth performance was significantly improved with taurine supplementation to the SPC diets. Therefore, inferior growth performance of red sea bream fed the taurine-unsupplemented SPC diet is concluded to be caused by dietary taurine deficiency. The inferior growth performance due to dietary taurine deficiency is considered to be a result of the following: Feeding activity of juvenile red sea bream fed the taurine-unsupplemented SPC diet declined with prolonged experimental period, and this resulted in decreased energy intake. In addition, the supply of oxygen to the cells is insufficient due to increased hemolysis, and this means a decrease in energy production, since absorbed macronutrients are oxidized during the process of metabolism in the cells [35, 36]. An increase in hemolysis might induce an increase in energy consumption for increases in both bile pigment excretion and hematopoietic function. Furthermore, digestion and adsorption of dietary lipids and n-3 HUFA are considered to decrease with decreased secretions of bile acids, since the amount of bile acids conjugated with taurine is insufficient. Accordingly, one of the causes for inferior growth performance of juvenile red sea bream fed the taurine-unsupplemented SPC diets might be the insufficient energy supply and the increase in energy consumption for recovering abnormal physiological condition due to dietary taurine deficiency.
In mammals, herbivores can synthesize taurine in vivo, but most omnivores and carnivores cannot generally synthesize sufficient taurine in vivo [8]. Hence, the supply of taurine in omnivores and carnivores is dependent on feeds. Kittens [10] and infants [11, 12] require taurine as an essential nutrient. In fish species, the capacity for taurine synthesis is species-specific, and in highly carnivorous species such as bluefin tuna Thunns thynus, yellowtail, Japanese flounder Paralichthys olivaceus, and red sea bream it is markedly low or negligible [13, 14]. In addition, the taurine requirement of yellowtail [37] and Japanese flounder [38, 39] juveniles fed FM-based diets is more than 10 mg/g diet and about 15 mg/g diet, respectively, and for juvenile red sea bream fed a casein-based diet, it is 5 mg/g diet [40]. It is reported that dietary taurine supplementation improved growth performance of glass eel Anguilla anguilla [41] fed casein-based diets, and of cobia Rachycentron canadum [42], European sea bass Dicentrarchus labrax [43], and common dentex Dentex dentex [44] fed diets where FM was partially replaced with alternative protein sources. Therefore, in carnivorous fish species, taurine supplementation is essential in diets where FM is partially or totally replaced by plant protein sources. Furthermore, recent studies have reported that growth performance of rainbow trout fed plant protein–based diets was improved by dietary taurine supplementation regardless of methionine supplementation [45, 46], although this fish species has a comparatively high ability to synthesize taurine [13, 14].
The results of the present and previous studies demonstrate that, compared with fish fed FM diet, one of the causes for the inferior growth performance in fish fed alternative protein diets supplemented with essential amino acids is dietary taurine deficiency. Taurine supplementation improves the nutritive value of alternative protein diets supplemented with essential amino acids. This indicates that the paradox of rearing fish by feeding them on other fish can be overcome. In other words, the production of fish will become possible by feeding them diets based on plant protein sources and thereby providing good quality animal protein for human consumption. These results are useful for the development of a sustainable aquaculture industry. Taurine has various physiological roles, however, elucidation of the roles in fish species has been limited and fragmented. Hence, further studies on the physiological roles and requirement for taurine in fish species are necessary to develop high quality diets with reduced amounts of FM.
References
Subasinghe R, Soto D, Jia J (2009) Global aquaculture and its role in sustainable development. Rev Aquac 1:2–9
Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285:146–158
Watanabe T (2002) Strategies for further development of aquatic feeds. Fish Sci 68:242–252
Goto T, Takagi S, Ichiki T, Sakai T, Endo M, Yoshida T, Ukawa M, Murata H (2001) Studies on the green liver in cultured red sea bream fed low level and non-fish meal diets. Relationship between hepatic taurine and biliverdin levels. Fish Sci 67:58–63
Watanabe T, Aoki H, Shimamoto K, Hadzuma H, Maita M, Yamagata Y, Kiron V, Satoh S (1998) A trial to culture yellowtail with non-fishmeal diets. Fish Sci 64:505–512
Kikuchi G, Yoshida T, Tsunoo H, Noguchi M, Uesugi T (1987) Disintegration of heme and bile pigment. In: Yamakawa T (ed) Blood, vol 1 (in Japanese). Tokyo Kagaku Dojin, Tokyo, pp 419–460
Sakai T, Tabata N, Watanabe K (1990) Bile pigments in the bile of marine fish: yellowtail, red sea bream, and flounder. Agric Biol Chem 54:2047–2053
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 2:101–163
Yamaguchi K (1985) The metabolism and nutrition of the sulfur-amino acids mainly on taurine (in Japanese). Kagaku To Seibutsu 23:299–308
Knopf K, Sturman JA, Armstrong M, Hayes KC (1978) Taurine: an essential nutrient for the cat. J Nutr 108:773–778
Chesney RW (1988) Taurine: is it required for infant nutrition? J Nutr 118:6–10
Sturman JA, Messing JM, Rossi SS, Hofmann AF, Neuringer M (1991) Tissue taurine content, activity of taurine synthesis enzymes and conjugated bile acid composition of taurine-deprived and taurine-supplemented rhesus monkey infants at 6 and 12 mo of age. J Nutr 121:854–862
Goto T, Tiba K, Sakurada Y, Takagi S (2001) Determination of hepatic cysteinesulfinate decarboxylase activities in fish by means of OPA-prelabeling and reverse-phase high-performance liquid chromatographic separation. Fish Sci 67:553–555
Yokoyama M, Takeuchi T, Park GS, Nakazoe J (2001) Hepatic cysteinesulphinate decarboxylase activity in fish. Aquac Res 32:216–220
Hujita M (1988) Nitrogenous compounds of vertebrates. In: Sakaguchi M (ed) Extractive components of fish and shellfish (in Japanese). Koseisha Koseikaku, Tokyo, pp 25–43
Yamamoto T, Akimoto A, Kishi S, Unuma T, Akiyama T (1998) Apparent and true availabilities of amino acids from several protein sources for fingerling rainbow trout, common carp and red sea bream. Fish Sci 64:448–458
Takagi S, Murata H, Goto T, Ichiki T, Munasinghe DMS, Endo M, Matsumoto T, Sakurai A, Hatate H, Yoshida T, Sakai T, Yamashita H, Ukawa M, Kuramoto T (2005) The green liver syndrome is caused by taurine deficiency in yellowtail Seriola quinqueradiata fed diets without fishmeal. Aquac Sci 53:279–290
Takagi S, Murata H, Goto T, Hayashi M, Hatate H, Endo M, Yamashita H, Ukawa M (2006) Hemolytic suppression roles of taurine in yellowtail Seriola quinqueradiata fed non-fishmeal diets based on soybean protein. Fish Sci 72:546–555
Takagi S, Murata H, Goto T, Endo M, Yamashita H, Ukawa M (2008) Taurine is an essential nutrient for yellowtail Seriola quinqueradiata fed non-fish meal diets based on soy protein concentrate. Aquaculture 280:198–205
Takagi S, Murata H, Goto T, Ichiki T, Endo M, Hatate H, Yoshida T, Sakai T, Yamashita H, Ukawa M (2006) Efficacy of taurine supplementation for preventing green liver syndrome and improving growth performance in yearling red sea bream Pagrus major fed low-fishmeal diet. Fish Sci 72:1191–1199
Takagi S, Murata H, Goto T, Endo M, Yamashita H, Miyatake H, Ukawa M (2010) Necessity of dietary taurine supplementation for preventing green liver symptom and improving growth performance in yearling red sea bream Pagrus major fed nonfishmeal diets based on soy protein concentrate. Fish Sci 76:119–130
Sakaguchi H, Hamaguchi A (1979) Physiological studies on cultured red sea bream-IV. Prevention of green liver and changes in plasma constituents and enzymatic activities on winter culturing in warm water (in Japanese with English abstract). Nippon Suisan Gakkaishi 45:1371–1373
Sakai T, Nagasawa T (1992) Simple, rapid and sensitive determination of plasma taurine by high-performance liquid chromatography using pre-column derivative formation with fluorescamine. J Chromatogr 576:155–157
Sakai T, Tabata N (1988) Analysis of bile pigments in the bile of yellowtail and eel by high performance liquid chromatography. Biosci Biotechnol Biochem 52:2967–2968
Takashima F (1982) Volume VI. Vascular system, pt. 3 spleen. In: Hibiya T (ed) An atlas of fish histology: normal and pathological features (in Japanese). Kodansha Scientific, Tokyo, pp 64–65
Yanai H (2000) 4 Steps Excel SQC (in Japanese). OMS, Saitama
Glencross BD (2009) Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aquac 1:71–124
Kiron V, Takeuchi T, Watanabe T (1994) The osmotic fragility of erythrocytes in rainbow trout under different dietary fatty acid status. Fish Sci 60:93–95
Mabile L, Piolot A, Boulet L, Fortin LJ, Doyle N, Rodriguez C, Davignon J, Blache D, Lussier-Cacan S (2001) Moderate intake of n-3 fatty acids is associated with stable erythrocyte resistance to oxidative stress in hypertriglyceridemic subjects. Am J Clin Nutr 74:449–456
Van den Berg JJM, De Fouw NJ, Kuypers FN, Roelofsen B, Houtsmuller UMT, Op den Kamp JAF (1991) Increased n-3 polyunsaturated fatty acid content of red blood cells from fish oil-fed rabbits increases in vitro lipid peroxidation, but decreases hemolysis. Free Rad Biol Med 2:393–399
Haslewood GAD (1967) Bile salt evolution. J Lipid Res 8:535–550
Goto T, Ui T, Une M, Kuramoto T, Kihira K, Hoshita T (1996) Bile salt composition and distribution of the D-cysteinolic acid conjugated bile salts in fish. Fish Sci 62:606–609
Assem H, Hanke W (1983) The significance of the amino acids during osmotic adjustment in teleost fish-I. Changes in the euryhaline Sarotherodon mossambicus. Comp Biochem Physiol 74A:531–536
Takagi S, Shimeno S, Hosokawa H, Ukawa M (2001) Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream Pagrus major. Fish Sci 67:1088–1096
Swyer PR (1991) Assumptions used in measurements of energy metabolism. J Nutr 121:1891–1896
Tauson A-H, Elnif J, Hansen NE (1994) Energy metabolism and nutrient oxidation in the pregnant mink (Mustela vison) as a model for other carnivores. J Nutr 124:2609S–2613S
Matsunari H, Takeuchi T, Takahashi M, Mushiake K (2005) Effect of dietary taurine supplementation on growth performance of yellowtail juveniles Seriola quinqueradiata. Fish Sci 71:1131–1135
Kim SK, Takeuchi T, Akimoto A, Furuita H, Yamamoto T, Yokoyama M, Murata Y (2005) Effect of taurine supplemented practical diet on growth performance and taurine contents in whole body and tissues of juvenile Japanese flounder Paralichthys olivaceus. Fish Sci 71:627–632
Park GS, Takeuchi T, Yokoyama M, Seikai T (2002) Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus. Fish Sci 68:824–829
Matsunari H, Yamamoto T, Kim SK, Goto T, Takeuchi T (2008) Optimal dietary taurine level in casein-based diet for juvenile red sea bream Pagrus major. Fish Sci 74:347–353
Sakaguchi M, Murata M, Daikoku T, Arai S (1988) Effect of dietary taurine on whole body and tissue taurine levels of guppy and eel. Nippon Suisan Gakkaishi 54:1647–1652
Lunger AN, McLean E, Gaylord TG, Kuhn D, Craig SR (2007) Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 271:401–410
Martinez JB, Chatzifotis S, Divanach P, Takeuchi T (2004) Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fish Sci 70:74–79
Chatzifotis S, Polemitou I, Divanach P, Antonopoulou E (2008) Effect of dietary taurine supplementation on growth performance and bile salt activated lipase activity of common dentex, Dentex dentex, fed a fish meal/soy protein concentrate-based diet. Aquaculture 275:201–208
Gaylord TG, Teague AM, Barrows FT (2006) Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss). J World Aquac Soc 37:509–517
Gaylord TG, Barrows FT, Teague AM, Johansen KA, Overturf KE, Shepherd B (2007) Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 269:514–524
Acknowledgments
This study was financially supported by the Ministry of Agriculture, Forestry, and Fisheries, Government of Japan. We are grateful to Dr. S. Shimeno and Dr. H. Hosokawa of the Laboratory of Fish Nutrition, Faculty of Agriculture, Kochi University, and Dr. V. Kiron of the Department of Fisheries and Natural Science, Bodø University College, for their valuable advice. We thank Dr. A. Hosokawa of Faculty of Marine Science, Tokyo University of Marine Science and Technology, for technical support for photomicrography. Thanks are due to the staff of the Ehime Prefectural Fisheries Experimental Station and Ehime Prefectural Fish Farming Center for their kind assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Deceased: M. Ukawa.
Rights and permissions
About this article
Cite this article
Takagi, S., Murata, H., Goto, T. et al. Role of taurine deficiency in inducing green liver symptom and effect of dietary taurine supplementation in improving growth in juvenile red sea bream Pagrus major fed non-fishmeal diets based on soy protein concentrate. Fish Sci 77, 235–244 (2011). https://doi.org/10.1007/s12562-011-0322-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0322-2