Abstract
The object of this study was to improve the isolation procedure of hyaluronan and to compare characteristics of hyaluronan from the eyeball of bigeye tuna Thunnus obesus with other sources. General sources of hyaluronan are from Streptococcus zooepidemicus and rooster comb. Hyaluronan can be also obtained from the vitreous of fish eyes. Pure hyaluronan of higher molecular weight was obtained by the following improved extraction procedure: the frozen vitreous of a tuna eyeball was used to avoid contamination with blood, muscle tissue, and other factors; extracting was carried out over a long time period under cold conditions; cetylpyridinium chloride was used in order to separate mucopolysaccharides containing hyaluronan in the initial procedure without the process of removing fat and protein by reagents. The hyaluronan obtained was characterized by gel permeation chromatography, dynamic light scattering measurements, and viscometry. The characteristics of hyaluronan from tuna eyeballs were similar to those from other sources. However, the viscosity was lower. The possible reason could be ascribed to the wide distribution of molecular size in the vitreous humor of fish eye.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
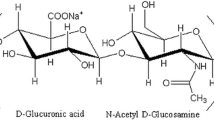
Hyaluronan is a biological substance that has been found in various sources, such as rooster combs [1], umbilical cords [2], zones of maturing chondrocytes [3], and bovine submaxillary glands [4]. In 1934, Karl Meyer and his assistant, John Palmer, described a procedure for isolating a novel glycosaminoglycan from the vitreous of bovine eyes [5]. The chemical structure of hyaluronan is a natural linear polymer composed of disaccharide repeating unit [(1 → 4)-O-(β-d-glucopyranosyluronic)-(1 → 3)-O-(2-acetamido-2-deoxy-β-d-glucopyranosyl)] [6, 7].
Due to the very high hyaluronan molecular weight in the synovial fluid, hyaluronan fulfills its function; for example, hyaluronan provides frictionless functioning of the joints. However, in the case of patients suffering from rheumatic diseases during joint inflammation, hyaluronan is degraded by the action of free radicals. The synovial fluid loses its lubricating properties, which leads to increased wear of the joints and results in arthritic pain [8].
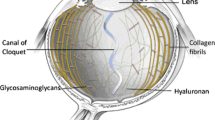
The inner space of the eyeball occupied by the vitreous between the lens and the retina is free of cells, suspended matter, and blood vessels, and it blocks or scatters light. Although the details of how this is achieved are not known, the outline is. The vitreous is a stable bio-gel, which contains hyaluronan. Even high molar mass hyaluronan does not form the gel found in the vitreous, and rigidity in this tissue is increased by the incorporation of a very sparse network of thin collagen fibrils [9]. The vitreous is a fiber-reinforced composite material, introducing as little solid material as possible into the light path because the amount of non-aqueous components is reduced to a minimum, compatible with the mechanical stability. The thin collagen fibrils are held apart in bunches or sprays by bridges and ties of anionic glycosaminoglycans, probably chondroitin sulfate. However, there have been no biochemical reports to indicate the presence of these macromolecules in vitreous.
In recent years, bovine spongiform encephalopathy (BSE) and avian influenza have been serious problems. These diseases are not only negative image, but also are possibily infectious. For example, a cow brain extract is used for the culture to propagate Streptococcus hyaluronan products. When we use skin cream containing hyaluronan, the risk of infection with BSE is low. However, when we use these products made with bovine extracts for injection and ingestion, the risk is 10–50 times as high compared with applying it to skin [10, 11]. Especially products that will be kept in the human body for a long time carry the danger of infection. Therefore, we hope to find a new resource for hyaluronan outside of animal husbandry. Also, hyaluronan from different sources has different characteristics [12]. The source of hyaluronan should be considered because of differences in the amounts and types of contaminants.
The previous extraction method of hyaluronan from the vitreous of bigeye tuna was time consuming, and the yield and molecular weight of the extracted hyaluronan were low [13]. The extraction procedure was the same as the extraction from urine. But on extraction from the vitreous, fat and proteins that surround the eyeball were mixed in the extract. To remove these, a long reaction time for defatting by using acetone and enzymatic digestion is required. Therefore, hyaluronan degradation might take place. To obtain pure hyaluronan with higher molecular weight, the extraction procedure was improved as follows: frozen vitreous of eyeballs was used to avoid contamination with blood, muscle, tissue, and other contaminants; extraction was carried out under cold conditions using a longer time period; cetylpyridinium chloride (CPC) was used in order to separate the mucopolysaccharide containing hyaluronan in the initial procedure before removing fat and protein by reagents. Although CPC could combine with not only hyaluronan but also the other glycosaminoglycans (i.e., chondroitin-sulfate, heparin-sulfate, dermatan-sulfate, etc.), hyaluronan could be separated from CPC complexes by dissolving it in 0.4 M NaCl, which was the NaCl-critical concentration [14].
This study aimed to establish the isolation method for fish eyeballs and to characterize hyaluronan from the vitreous of bigeye tuna by comparison with the production of other resources. Due to the low viscosity, the distribution of hyaluronan molecular size was observed by using dynamic light scattering techniques.
Materials and methods
Materials
Frozen eyeballs of bigeye tuna Thunnus obesus (Seikou Suisan Co., Shizuoka, Japan) stored at −18°C were used. Hyaluronan from rooster comb and Streptococcus zooepidemicus (Wako Pure Chemical Industries Ltd., Tokyo, Japan) were used as references. Reagents, such as sodium chloride, acetic acid, ethanol, potassium acetate, trichloroacetic acid (Kokusan Chemical, Tokyo, Japan), hexadecylpyridinium chloride monohydrate, 2-amino-2-hydroxymethyl-1,3-propanediol, pyridine, formic cid, and alcian blue 8GX (Wako Pure Chemical Industries, Tokyo, Japan), were used for hyaluronan extraction. Mycolysin, protease from Streptomyces griseus (EC 3.4.24.31 Sigma-Aldrich, St. Louis, MO), were used. Dialysis membrane with ca. 5-nm pore size (Wako Chemicals USA, Inc., VA, USA) was used for desalting of the solution by dialyses.
Isolation of hyaluronan
The eyeball of bigeye tuna was dissected, and the vitreous was taken out in the frozen state. The vitreous was thawed and then filtered at 4°C. The filtrate was treated with 3% CPC to precipitate polysaccharide and was centrifuged at 2.22 × 103 g for 15 min at 4°C. The obtained precipitate was re-suspended by adding 0.4 M NaCl to dissociate the hyaluronan-CPC complex and centrifuged at 2.22 × 103 g for 15 min at 4°C. The obtained precipitate was re-suspended by 0.4 M NaCl, and this process was repeated three times. The supernatant was mixed with 10% potassium acetate-95% ethanol solution and later centrifuged. The obtained precipitate was re-suspended with 0.1 M Tris–HCl (pH 7.7) containing mycolysin and held for 24 h at 37°C. Later, the mixture was heated at 80°C for 15 min, and 30% trichloroacetic acid was added to twice the volume of re-suspended solution. After stirring, the solution was centrifuged, and then the supernatant was obtained. After adding acetone, the supernatant was centrifuged at 2.22 × 103 g for 15 min at 4°C. The obtained precipitate was re-suspended by distilled water and dialyzed for 2 days against distilled water. Finally, the sample was dried under a vacuum and was recovered in a freeze-dried state.
Audit test
Identification of hyaluronan was carried out by cellulose acetate membrane electrophoresis [15] (Shimadzu Scientific Instruments Ltd., model MI-I, Tokyo, Japan). A cellulose acetate membrane filter was used (SELECA-V; Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Electrophoresis solution was 0.1 M pyridine–0.47 M formic acids. Stain solution was 0.1% acetic acid–0.1 M alcian blue 8 GX, and bleaching was done with 0.1% acetic acid. The sample concentration was 1.0 mg/0.8 cm3 H2O.
The electrophoresis solution was put in both an electrophoresis bath and an acetate film, which was wet with electrophoresis solution and set on the bridge on the bath. Before the sample was set, the equipment was pre-run for 10 min to equalize out. The samples were set by a 0.2 μm3 capillary tube on the film at regular intervals. After pre-running at 3.0 mA for 1.5 h, the film was stained with alcian blue for 10 min and destained in 0.1% acetic acid for 1 min. After drying the film, mobility of the hyaluronan was compared between bigeye tuna and the references.
Viscosity measurement
The hyaluronan obtained was dissolved overnight in aqueous 0.5 M NaCl at 4°C, and this solution was clarified by filtration through a 0.45-μm nylon filter (Millipore Corp., Bedford, MA). Intrinsic viscosity [η] was obtained with an Ubbelhode capillary viscometer at 20 ± 0.02 °C with a flow time of 796.8 s for 0.5 M NaCl. The intrinsic viscosity was determined by using the Huggins equation.
where c is the hyaluronan concentration, η sp/c the reduced viscosity, η sp the specific viscosity, and k′ the Huggins constant.
Gel permeation chromatography (GPC)
Hyaluronan molecular weight distribution was measured by using gel permeation chromatography (HLC-8120 GPC, TOSOH, Tokyo, Japan). Pulluran (Showa Denko K·K., Yokohama, Japan) was used as the standard polysaccharide. The value of \( \overline{M}_{\text{w}} \) was calculated by using a calibration curve generated with eight monodisperse pullurans. The molecular weight for both samples being tested and the hyaluronan reference were calculated by using the above-described equation. The built-in refractive index detector detected elution components.
Two columns connected in a series (G4000PWXL + G6000PWXL) were kept at 35°C during measurements. These columns were equilibrated with a 0.5 M NaCl solution at a flow rate of 1.0 cm3/min. Hyaluronan was dissolved in 0.5 M NaCl (4°C, 24 h) and filtered through a 0.45-μm filter prior to injecting it into the column.
Dynamic light scattering (DLS) measurements
In a dynamic light scattering experiment, a laser beam is scattered by a small volume of the sample: a photo multiplier at an angle with respect to the incident beam collects light. Dynamic light scattering experiments were performed with the dynamic light scattering spectrophotometer (DLS-7000, Otsuka Electronics Co., Ltd., Osaka, Japan) in the homodyne mode using a 488 nm Ar laser (75 mW). The solvent was 0.5 M NaCl. The intensity autocorrelation function was measured at 90° and analyzed to obtain the particle distribution. The sample cells were 10 cm3 cylindrical ampules immersed in an index-matching bath with toluene at 20°C.
In addition, the frozen vitreous humor was divided into 27 regions. Parts adjacent to the retina, in the vicinity of the lens, and in the middle region were divided into nine parts, respectively (Fig. 1). Samples from each region were thawed and centrifuged at 2.22 ×103 g for 15 min at 4°C. The supernatant samples were collected as liquid vitreous. Freezing and thawing had no apparent optical effect on tissues like the lens and vitreous [16].
The 27 regions for the vitreous humor from tuna eye. A, adjacent to the retina; B, middle parts between the retina A and lens; C the vicinities of lens 1, 2, and 3 were the regions on the tail fin side; 4, 5, and 6 were the middle parts of the vitreous, which were in three from the mouth side to tail fin; 7, 8, and 9 were regions on the mouth side; 1, 4, and 7 were the topside; 2, 5, and 8 were the middle parts of the vitreous humor and were divided into three from top to bottom; 3, 6, and 9 were on the lower side
Each liquid vitreous sample was used for the dynamic light scattering measurement.
Results and discussion
Improvement of isolation method
Figure 2 shows the result of cellulose acetate membrane electrophoresis. This figure showed that hyaluronan in this study moved the same distance as the reference hyaluronan. Also, this purifying method is commonly used to purify glycosaminoglycans from various tissues and sources. Therefore, this new extraction procedure could yield pure hyaluronan and prevent hyaluronan from degradation during the purification procedure. Hyaluronan purified from bigeye tuna was not degraded during the purification process.
Our improved extraction methods could yield 10.5 mg hyaluronan from one tuna eyeball, and 0.42 g per 1 dm3 of vitreous humor, which was higher than the value of the previous method, 0.13 g per 1 dm3 of vitreous humor. In the previous methods, when the vitreous was taken out of the eyeball, the vitreous was contaminated with large amounts of blood and fat because frozen eyeballs were thawed completely prior to the extraction. This led to long operation times to remove these contaminants. However, our improved methods involved removing the vitreous from eyeballs that were still frozen. Therefore, vitreous with few contaminants could be obtained. This allowed for both shorter extraction and shorter purification times, and allowed us to avoid hyaluronan degradation by heat and chemicals during the operation. As a result, this improved extraction method for obtaining hyaluronan took about half the extraction time as previous methods. This quantity of hyaluronan from a dry source (42 mg/g) showed as high a value as that from a dry comb (50 mg/g) [17].
As shown in Table 1, the molecular weight of hyaluronan from tuna eyeball obtained by the improved method showed the same million orders (106) as those of hyaluronan from comb and Streptococcus. We therefore decided to use this improved method for the extraction of hyaluronan from bigeye tuna vitreous humor.
Molecular size and distribution
Hyaluronan from the vitreous of bigeye tuna was characterized by GPC, DLS, and viscometry. Figure 3 shows Huggins plots of hyaluronan of different origins. Huggins equations were used in determining their intrinsic viscosities. Resulting values are shown in Table 1. Intrinsic viscosity of hyaluronan from bigeye tuna was obviously lower than the others. Possibly, the lower intrinsic viscosity of bigeye tuna hyaluronan was influenced by some of factors, such as the effect of the molecular branch, the small size of containing molecules, and particle shape, etc. [18].
Figure 4 shows molecular weight distribution curves of hyaluronan from Streptococcus, tuna eyeball, and rooster comb. Average molecular weight, \( \overline{M}_{\text{w}} , \) and the polydispersity index, \( \overline{M}_{\text{w}} /\overline{M}_{\text{n}} , \) are shown in Table 1. Although hyaluronan from bigeye tuna had a lower molecular weight fraction affected by freeze-drying, the distribution of the higher molecular weight fraction was similar to the other sources. Consequently, \( \overline{M}_{\text{w}} /\overline{M}_{\text{n}} , \) of hyaluronan from tuna eyeball showed a larger value than from the other sources, which meant that hyaluronan from tuna eyeball had the polydispersity of molecular weight. Also \( \overline{M}_{\text{w}} \) of hyaluronan from tuna’s eyeball showed a lower value because of the polydispersity.
Figure 5 shows macromolecular size distribution panels of hyaluronan from tuna eyeball and other sources. The average molecular size of hyaluronan from rooster comb was 10.6 ± 2.8 nm, from Streptococcus was 4.9 ± 4.9 nm, and from tuna eyeball was 15.1 ± 9.3 nm. The molecular size distribution of hyaluronan from tuna eyeball was not narrow, but broad. Such a unique distribution could be one possible reason why the hyaluronan from tuna eyeball had a lower viscosity than the others. Profiles of these molecular size distribution curves were different from those of molecular weight distribution curves in Fig. 4. Also the average value of molecular size and the molecular weight of hyaluronan from tuna eyeball had the largest size and the lowest \( \overline{M}_{\text{w}} ,\) respectively, among the sources. These differences were caused by the difference of the supposed molecular shape. \( \overline{M}_{\text{w}} \) obtained by GPC was calculated assuming the same molecular shape as the standard substance, pulluran, whereas the molecular size obtained by DLS was calculated by supposing a molecule to be a sphere.
Figure 6 shows three size distribution panels for vitreous from tuna eyeball. The molecular sizes increased from the lens to the retina direction. These distribution patterns were obtained with short delay times (i.e., 10 μs), allowing the visualization of large particles, i.e., hyaluronan centered around 50 nm and collagen centered around 500 nm in diameter. The presence of these species has been demonstrated in bovine vitreous [19]. Early biochemical studies [20, 21] showed that the two main macromolecular components, collagen and hyaluronan, have distribution gradients within the bovine vitreous humor. However, the function of the gradient is not yet understood. Average sizes of hyaluronan from each region of the tuna eyeball are displayed graphically in Fig. 7. The molecular size of hyaluronan from tuna eyeball increased from the lens side to the retina side. Such a tendency was found in all fresh vitreous samples surveyed. This distribution gradient was larger than that of the bovine vitreous humor. It was supposed that this wide size distribution of hyaluronan in tuna eyeball was related to the lower intrinsic viscosity of tuna eyeball hyaluronan. We must leave these points for future study.
Size distribution pattern of macromolecules in liquid vitreous from tuna eyeball. The correlation diagram was obtained with a 10-μs delay time and was analyzed. Upper panel (a7), adjacent to retina; middle panel (b7), between retina and lens; lower panel (c7), the vicinity of the lens. Each panel corresponds to the regions in Fig. 1
In this study, we could obtain hyaluronan in high yield and high molecular weight from tuna eyeball by our improved extraction method. The tuna eyeball hyaluronan had a wider distribution of molecular weight than those from Streptococcus and rooster comb, and also the size depended on the position within the eyeball.
References
Boas NF (1949) Isolation of hyaluronic acid from the cock’s comb. J Biol Chem 181:573
Lago G, Oruna L, Cremata JA, Perez C, Coto G, Lauzan E, Kennedy JF (2005) Isolation, purification and characterization of hyaluronan from human umbilical cord residues. Carbohydr Polym 62:321–326
Sunwoo HH, Nakano T, Sim JS (1998) Isolation and characterization of proteoglycans from growing antlers of wapiti. Comp Biochem Physiol B Biochem Mol Biol 124:437–442
Devaraj N, Bhavanandan VP (1992) Purification of mucin glycoprotein by density gradient centrifugation in cesium trifluroacetate. Analy Biochem 206:142–146
Meyer K, Palmer JW (1934) The polysaccharide of the vitreous humor. J Biol Chem 107:629–634
Laurent TC (1970) Structure of hyaluronic acid. In: Balazs EA (ed) Chemistry and molecular biology of the intercellar matrix. Academic Press, New York
Meyer K (1958) Chemical structure of hyaluronic acid. Fed Proc 17:1075–1077
Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J (2006) Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7:659–668
Scott JE (1992) The chemical morphology of vitreous. EYE 6:553–555
Dittmar H (1998) Der aktuelle Stand der BSE-Verordmumgen: Verwendungsverbote fuer Risikomaterial in kosmetischen Mitteln. (The present state of BSE regulations: prohibition of use of hazardous material in cosmetics). Parfuem Kosmet 79:14–16 (in German)
Rimpler M (1996) BSE-Rinderwahnsinn und seine Bedeutung fuer die Kosmetik. (BSE-bovine madness and its impact on cosmetics). Parfuem Kosmet 77:404–408 (in German)
Shiedrin A, Bigelow R, Christopher W, Yang L, Miller RJ, Arbabi S, Maier RV (2004) Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 5:2122–2127
Mizuno H, Iso N, Saito T, Ogawa H, Sawairi H, Saito M (1991) Characterization of hyaluronic acid of yellowfin tuna eyeball. Nippon Suisan Gakkaishi 57:517–519
Matsumura G, Salegui MD, Herp A, Pigman W (1963) The preparation of hyaluronic acid from bovine synovial fluid. Biochimi Biophys Act 69:574–576
Bettelheim FA, Paunovic M (1979) Light scattering of normal human lens. I. Application of random density and orientation fluctuation theory. Biophys J 26:85–99
Kohn J (1957) A cellulose acetate supporting medium for zone electrophoresis. Clin Chem Acta 2:297
Swann DA (1968) Studies on hyaluronic acid: I. The preparation and properties of rooster comb hyaluronic acid. Biochim Biophys Acta 156:17–30
Matsumura G (1971) Hyaluronic acid. Tanpakushitsu Kakusan Koso (Protein Nucleic Acid and Enzyme) 16:233–238 (in Japanese)
Ansari RR, Suh KI, Dunker S, kitaya N, Sebag J (2001) Quatitative molecular characterization of bovine vitreous and lens with non-invasive dynamic light scattering. Eye Res 73:859–866
Balazs EA (1960) Physiology of the vitreous body. In: Schepens (ed) Importance of the vitreous body in retina surgery with special emphasis on reoperations. St Louis, Mosby
Balazs EA, Denlinger JL (1984) The vitreous. In: Davson J (ed) The eye. Academic Press, New York, pp 535–589
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amagai, I., Tashiro, Y. & Ogawa, H. Improvement of the extraction procedure for hyaluronan from fish eyeball and the molecular characterization. Fish Sci 75, 805–810 (2009). https://doi.org/10.1007/s12562-009-0092-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-009-0092-2