Abstract
We compared the diet of Ommastrephes bartramii paralarvae with morphological changes in their beaks and proboscis (fused tentacles). The paralarvae were collected north of the Hawaiian Islands during 2001 and 2002 and ranged in mantle length (ML) from 1.1 to 13.2 mm. They fed on crustaceans, including copepods (copepodite stage) and amphipods. The rostral tips of upper and lower beaks began to protrude anteriorly at around 3–4 mm ML, and the smallest paralarva with identifiable prey in its digestive tract was 4.2 mm ML, which suggests that the paralarvae can masticate prey soon after the beaks protrude. The proboscis separated into two tentacles at 9.3–13.2 mm ML, but the newly formed tentacles were weakly developed even in the largest specimen, suggesting that tentacles do not operate functionally and that the arms are used to capture prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recruitment in fishes and squids is strongly affected by larval mortality [1, 2], which is high during a relatively short period in early development. Larval feeding success is a key factor for early growth and survival during this critical period [3, 4]. The processes influencing larval survival are complicated when animals undergo transitional stages between developmental stages [5, 6]. These transitions are often rapid and characterized by morphological, physiological, and behavioral changes [6, 7].
The neon flying squid Ommastrephes bartramii is a commercially and ecologically important ommastrephid species [8, 9] that occurs in temperate and subtropical parts of the Pacific, Indian, and Atlantic Oceans [10, 11]. The North Pacific population comprises two seasonal cohorts with different spawning periods (fall and winter–spring) [12].
Ommastrephid paralarvae hatchlings are unique among the decapod cephalopods because they have a fused proboscis, which separates to form two tentacles. Early authors suggested that the proboscis is used to capture prey [13], but recent morphological analysis suggest that it is not used for raptorial feeding [14]. O’Dor et al. [15] and Vidal and Haimovici [16] have suggested that it might be used in suspension feeding. Although the role of the proboscis in feeding is unclear, its division is presumably associated with a change in feeding behavior.
Another important feeding apparatus in cephalopods is the buccal mass, which contains a pair of chitinous beaks (upper and lower beaks) that masticate the prey before it is swallowed. As a result, the beaks presumably also play an important role during the critical period after yolk absorption, but little is known about their development.
Various physical and biological factors affect recruitment in squids, and starvation is considered to be one of the major causes of mortality in ommastrephid paralarvae [4, 17]. Newly hatched paralarvae have a small quantity of internal yolk and a high metabolic rate [18], so survival depends on their ability to successfully switch to active predation. The first prey of O. bartramii paralarvae is not known, so all attempts to rear the paralarvae in captivity to date have failed [19, 20]. Information about early feeding could improve captive experiments of the paralarvae and help us better understand the early life stages of this species.
In this paper, we compare the digestive tract contents of O. bartramii paralarvae collected near the Hawaiian Islands with ontogenetic changes in the proboscis and beaks, and propose a possible feeding scenario for the paralarvae.
Materials and methods
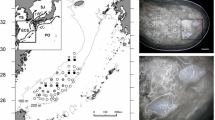
Surveys were carried out between 24°30′–34°00′N and 154°00′–163°37′W, north of the Hawaiian Islands during November to December in 2001 and 2002 aboard the R/V Shunyo-Maru. Paralarvae were collected with a larva net with a 2-m mouth diameter and 0.526-mm mesh. The net was towed horizontally at the surface for 15 min both day and night at a ship speed of 1.5–2.0 knots (0.8–1.0 ms−1). A total of 615 ommastrephid paralarvae was collected. The paralarvae were immediately removed from the catch and identified as O. bartramii based on morphological characteristics [21–23]. We measured the mantle length (ML) of each O. bartramii specimen to the nearest 0.1 mm, and then preserved them in 99.5% ethanol (in 2001) or 90% ethanol (in 2002) for later analyses on board.
In the laboratory, specimens were examined morphologically in detail and the pair of statoliths was extracted from each specimen. The formation of growth increments of O. bartramii has not been validated, but it was suggested that the increments deposited on a daily basis [24]. We have therefore assumed that the growth increments form daily. Paralarvae with identifiable prey in their digestive tracts were aged by counting statolith daily increments under a microscope equipped with a video image-analysis system (Zeiss KS-200ROTOC Statolith Daily Ring System, Version 2) containing a high-resolution and high-sensitivity color charge-coupled device (CCD) camera (SONY SXC-970MDXC-003) mounted on a light microscope (NIKON Labophoto FXA2; Nikon, Kanagawa, Japan). Details of the procedure used and interpretation of statoliths are given in Sakai et al. [20]. Counting statolith rings is the most accurate method for aging squids, but is time consuming and labor intensive. Because of our large sample size, O. bartramii paralarvae without identifiable food in their digestive tracts were aged based on their ML using the exponential model. Statoliths from 140 paralarvae collected during the 2001 surveys were used for counting rings. The model was described in Sakai et al. [25]
where ML is mantle length in millimeters, SST is the sea surface temperature where paralarvae was collected, and d is the age (days).
When morphological identification was difficult due to lost or damaged taxonomic features, DNA analysis was used (method described in Wakabayashi et al. [26]).
The numbers of paralarvae examined for each analysis are shown in Table 1. A total of 174 stomachs and caeca of specimens ranging from 2.0 to 13.2 mm ML was examined (contents of the esophagus and intestine were not examined). Under stereomicroscope, the stomach and caecum (referred to as “digestive tract” in this study) were extracted from each specimen, placed on a hole-slideglass, and dissected with fine dissecting needles. Contents of digestive tract were identified and counted using a stereomicroscope and light microscope. The digestive tracts of specimens with everted or seriously damaged mantles were not analyzed.
The methods used to measure the proboscis and proboscis division lengths are described in Shea [14]. In 2001, proboscis length and division length were measured to the nearest 1 μm under stereomicroscope, and the division of the proboscis was expressed as the division length divided by the proboscis length (%). In 2002, only the state of the proboscis division (undivided, partially divided, or completely divided) was recorded. In 6 of the better preserved 11 specimens with completely separated tentacles, relative lengths and widths of the tentacles were compared with those of the surrounding arms.
Upper and lower beaks were extracted from the buccal mass using the following procedure. The buccal mass was extracted from a paralarvae and soaked in a 10-ml-volume Petri dish containing 5 ml distilled water. Two or three drops of chloric acid solution or commercial bleach were added to dissolve the muscles covering the buccal mass. After several minutes, the upper and lower beaks were separated. As an index of beak development, protrusions of the rostral tips of the upper (n = 157) and lower beaks (n = 170) were measured to the nearest 0.1 μm using digital microscope (KEYYENCE VH-7000). The protrusion was defined as the ventral view of the distance from the anterior part of the shoulder to distal tip of the rostrum (Fig. 1a). In smaller paralarvae, the rostral tips of both beaks were often covered by a membrane-like tissue (Fig. 1b). These specimens were regarded as having no protrusion.
Results
Proboscis ontogeny
The proboscis started to divide at about 3-4 mm ML (Fig. 2). The smallest specimen with a partially divided proboscis was 3.1 mm ML (12 days old). Proboscis division was completed between 9.0 and 9.3 mm ML, and the youngest paralarva with two tentacles was 12.0 mm ML (28 days old). In the six specimens ranging from 10.7 to 13.2 mm ML, tentacles were weakly developed and 50–100% the length of arm III. The tentacles were about half the width of arm III and about the same length and width as arm IV.
Beak ontogeny
In all paralarvae smaller than 3 mm ML (<13 days old), the rostra of both beaks were transparent and usually embedded in the membrane-like tissue (Fig. 1b). The rostral tip of both beaks began to protrude anteriorly at about 3–4 mm ML (13–16 days old) (Fig. 3). The rostral tips of both beaks in all paralarvae larger than 4.3 mm ML protruded anteriorly.
Diet
A total of 174 individuals ranging from 2.0 to 13.2 mm ML (8–38 days old) was examined. The digestive tracts of 72 paralarvae (41%) were empty. In all paralarvae smaller than 4 mm ML, the digestive tracts were empty or contained a small amount of unidentified material that contained no identifiable chitin (Table 2).
Eleven paralarvae ranging from 4.2 to 13.2 mm ML ingested a total of 12 copepods (copepodite stage). In most of these cases, fragments of copepod swimming legs were found in the digestive tract (Fig. 4). The smallest paralarvae with copepod appendages was 4.2 mm ML (17 days old): it contained copepod swimming legs. In a 4.7-mm-ML paralarva (17 days old), branchipod fragments, possibly copepods or ostracods, were found. Many copepod fragments such as first antenna, first maxilla, maxilliped, cephalothorax, and swimming legs were found in a digestive tract from a 5.0-mm-ML paralarva (17 days old) (Fig. 4). A 10.5-mm-ML paralarva (30 days old) contained a mouth-part appendage (probably the second maxilla) of copepods, and a 12.2-mm-ML paralarva (36 days old) contained copepod urosome fragments, an anal segment, and furca. Amphipod fragments (e.g., pereon and pereopods) were found in digestive tracts of a 10.7-mm-ML paralarva (33 days old) and a 13.2-mm-ML paralarva (38 days old). The digestive tracts of six paralarvae ranging from 4.5 to 9.1 mm ML contained digested fragments of unidentified crustaceans. None of paralarvae contained microorganisms such as flagellates, dinoflagellates or ciliates.
Discussion
Information about the diet of ommastrephid paralarvae is limited, and the only studies on its diet are two reports about Sthenoteuthis oualaniensis [27] and Illex argentinus [16]. In the present study, paralarvae of O. bartramii fed on crustacean zooplankton such as copepods and amphipods (Table 2). Our results are similar to those of studies on the ommastrephids S. oualaniensis [27] and I. argentinus [16], which feed on copepods and amphipods. These suggest that ommastrephid paralarvae feed on small crustacean zooplankton after the onset of active predation.
Paralarvae of O. bartramii begin to absorb the internal yolk at about 1.4 mm ML, just after hatching (M. Sakai, unpublished data, 2004). The internal yolk disappears 3–8 days after hatching in other ommastrephids [20, 28, 29] and 4–7 days after hatching in O. bartramii ([30]; M. Sakai, unpublished data, 2004). These suggest that paralarvae of O. bartramii must begin feeding about 1 week after hatching; however, the smallest paralarva with copepods in its digestive tract was 17 days old (4.2 mm ML) in the present study. It has been proposed that ommastrephid paralarvae are suspension feeders on microorganisms such as flagellates, dinoflagellates, and ciliates [15, 16], which would explain the lack of hard parts in the digestive tracts of specimens younger than 17 days old. We did not observe any microorganisms in the digestive tracts. This could have been due to the preservation method used, since cells of microorganisms disintegrate rapidly in ethanol [31].
There are several possible feeding-mode scenarios after the internal yolk is exhausted in ommastrephid paralarvae: raptorial feeding with proboscis strikes, arm strikes, and tentacle-strikes and proboscis-based suspension feeding [14].
The rostral tips of the upper and lower beaks began to protrude at 3–4 mm ML (Fig. 3), and all paralarvae larger than 4.3 mm ML (>20-day-old) had protruding beaks, which is about the size of the smallest paralarva with copepods in its digestive tract (4.2 mm ML; 17 days old). Cephalopods have a small mouth with small and narrow esophagus, so prey must be cut into small pieces before it is swallowed [32]. This suggests that beak protrusion occurs concurrently with onset of prey capture, and that raptorial feeding may begin when the beaks become functional at 3–4.3 mm ML (about 13–20 days old). Therefore, beak protrusion might signal the beginning of raptorial feeding. Additionally, size at beak protrusion was concordant with onset of proboscis division (Figs. 2, 3). Shigeno et al. [33] suggested that prey capture begins after the proboscis divides in the ommastrephid Todarodes pacificus. O’Dor et al. [15] and Vidal and Haimovici [16] suggested that proboscis might facilitate suspension feeding. If the proboscis plays a role in suspension feeding, concordance with the beak protrusion and proboscis division might mean that ommastrephid paralarvae shift their feeding mode from suspension to raptorial feeding.
In paralarvae of I. argentinus, microorganisms and copepods are found together in the digestive tracts from 3.7 to 5.6 mm ML [16]. This suggests that, at the beginning of raptorial feeding, suspension feeding may still be important for ommastrephids. If the yolk is completely absorbed by 4–7 days after hatching and the paralarvae begin raptorial feeding at 13–20 days after hatching, the duration of suspension feeding as the lone feeding mode would be 6–16 days (4–20 days old).
There are two ways paralarvae can capture prey at the beginning of raptorial feeding: proboscis strikes and arm strikes. Morphological observations on the proboscis ontogeny of O. bartramii suggest that proboscis is not functional and very small paralarvae use their arms for prey capture [14]. Proboscis division in O. bartramii ended at around 9.0 mm ML (Fig. 2), which corresponds to about 30 days after hatching ([34]; present study). Tentacles were weakly developed even in the largest specimens (13.2 mm ML) in the present study. Therefore, tentacles are probably not useful for prey capture for a while after complete division of the proboscis. Vidal [35] also suggested that the tentacles of I. argentinus were probably not functional soon after the division of the proboscis. Although an ontogenetic shift of feeding behavior has not been observed in ommastrephid paralarvae, such a shift has been observed in larvae of the loliginid squid Loligo opalescens. Its larvae use their arms to capture prey up to 3–4 weeks old, and then changes gradually from arm strikes to tentacle strikes [36]. At the beginning of tentacle strikes, all of strikes were unsuccessful, because prey capture success by tentacle strikes is highly experience dependent [36]. In O. bartramii, tentacle strikes will occur in paralarvae larger than 13.2 mm ML, but prey capture using the tentacles may not be successful just after the tentacles separate.
As implications for the feeding scenario, we propose a succession of four feeding modes of O. bartramii paralarvae: yolk absorption, proboscis-based suspension feeding, raptorial feeding using the arms, and raptorial feeding using the tentacles (Fig. 5). This succession would involve three transition periods between feeding modes.
Such a feeding scenario may be common in other ommastrephid paralarvae, but the timing of each transition period will vary among species. These transition periods will likely be associated with morphological and/or behavioral changes. Each transition period will be critical for paralarval survival, but the transition from suspension feeding to raptorial feeding will likely have the highest mortality because it requires major changes in food type, morphology, and feeding behavior.
As suggested in loliginid hatchlings [4], low prey concentrations during the critical transition periods should expose the ommastrephid paralarvae to starvation caused by failure to capture active prey. Thus, the match/mismatch between paralarvae and their prey, especially in the early critical transition period(s), may generate variability in the paralarval survival rate and may be a major cause of recruitment fluctuation in O. bartramii.
References
Houde ED (1989) Subtleties and episodes in the early life of fish. J Fish Biol 35(Suppl A):29–38
Sakurai Y, Kiyofuji H, Saitoh S, Goto T, Hiyama Y (2000) Changes in inferred spawning areas of Todarodes pacificus (Cephalopoda: Ommastrephidae) due to changing environment conditions. ICES J Mar Sci 57:24–30
May RC (1974) Larval mortality in marine fishes and critical period concept. In: Blaxter JSH (ed) The early life history of fish. Springer, Berlin, pp 3–19
Vecchione M (1981) Aspects of the early life history of Loligo pealei (Cephalopoda: Myopsida). J Shellfish Res 1:171–180
Pechenik JA, Wendt DE, Jarret JN (1998) Metamorphosis is not a new beginning: larval experience influences juvenile performance. Bioscience 48:901–910
Hoey AS, McCormick MI (2004) Selective predation for low body condition at the larval–juvenile transition of a coral reef fish. Oecologia 139:23–29
McCormick MI, Makey L, Dufour V (2002) Comparative study of metamorphosis in tropical reef fishes. Mar Biol 141:841–853
Seki MP (1993) The role of neon flying squid, Ommastrephes bartramii, in the North Pacific pelagic food web. Bull Int North Pac Fish Comm 53:207–215
Bower JR, Ichii T (2005) The red flying squid (Ommastrephes bartramii): a review of recent research and the fishery in Japan. Fish Res 76:39–55
Roper CFE, Sweeney MJ, Nauen CE (1984) FAO species catalogue. Cephalopod of the world. An annotated and illustrated catalogue of species of interest to fisheries. FAO Fisheries Synopsis No. 125. FAO, Rome, pp 1–277
Murata M (1990) Oceanic resources of squids. Mar Behav Physiol 18:19–71
Yatsu A, Tanaka H, Mori J (1998) Population structure of the neon flying squid, Ommastrephes bartramii. the North Pacific Ocean. In: Okutani T (ed) Contributed papers to international symposium on large pelagic squids. Marine Fishery Resources Research Center, Tokyo, pp 31–48
Neaf A (1928) Fauna and flora of the Bay of Naples. Monograph No. 35—Cephalopoda: embryology. Part I, vol II. Smithsonian Institution Libraries, Washington (Translated from German by Boletzky SV)
Shea EK (2005) Ontogeny of the fused tentacles in three species of ommastrephid squids (Cephalopoda, Ommastrephidae). Invertebr Biol 124:25–38
O’Dor RK, Helm P, Balch N (1985) Can rhynchoteuthions suspension feed? (Mollusca: Cephalopoda). Vie Milieu 35:267–271
Vidal EAG, Haimovici M (1998) Feeding and the possible role of the proboscis and mucus cover in the ingestion of microorganisms by rhynchoteuthion paralarvae (Cephalopoda: Ommastrephidae). Bull Mar Sci 63:305–316
Okutani T, Watanabe T (1983) Stock assessment by larval surveys of the winter population of Todarodes pacificus Steenstrup (Cephalopoda: Ommastrephidae), with a review of early works. Biol Oceanogr 2:401–431
O’Dor RK, Foy EA, Helm PL, Balch N (1986) The locomotion and energetics of hatchling squid, Illex illecebrosus. Am Malacol Bull 41:55–60
Balch N, O’Dor RK, Helm P (1985) Laboratory rearing of rhynchoteuthions of the ommastrephid squid Illex illecebrosus (Mollusca: Cephalopoda). Vie Milieu 35:243–246
Sakai M, Burunetti N, Ivanovic M, Elena B, Nakamura K (2004) Interpretation of statolith microstructure in reared hatchling paralarvae of the squid Illex argentinus. Mar Freshwater Res 55:403–413
Harman RE, Young RE (1985) The larvae of ommastrephid squids (Cephalopoda, Teuthoidea) from Hawaiian waters. Vie Milieu 35:211–222
Young RE, Hirota J (1990) Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) paralarvae with evidence for spawning in Hawaiian waters. Pac Sci 44:71–80
Wakabayashi T, Saito K, Tsuchiya K, Segawa S (2002) Description of Eucleoteuthis luminosa (Sasaki, 1915) and Ornithoteuthis volatilis (Sasaki, 1915) paralarvae in the northwestern Pacific. Venus 60:237–260
Yatsu A, Midorikawa S, Shimada T, Uozumi Y (1997) Age and growth of the neon flying squid, Ommastrephes bartrami, in the North Pacific Ocean. Fish Res 29:257–270
Sakai M, Okamura H, Ichii T (2004) Mortality of Ommastrephes bartramii paralarvae of autumn cohort in northern waters of Hawaiian Islands. In: Report of the 2004 meeting on squid resources. Japan Sea National Fisheries Research Institute, Niigata, pp 35–48
Wakabayashi T, Suzuki N, Sakai M, Chow S (2006) Identification of ommastrephid squid paralarvae collected in northern Hawaiian waters and phylogenetic implications for the family Ommastrephidae using mtDNA analysis. Fish Sci 72:494–502
Vecchione M (1991) A method for examining the structure and contents of the digestive tract in paralarval squids. Bull Mar Sci 49:300–308
Watanabe K, Sakurai Y, Segawa S, Okutani T (1996) Development of the ommastrephid squid Todarodes pacificus, from fertilized egg to rhynchoteuthion paralarvae. Am Malacol Bull 13:73–88
Yatsu A, Tafur R, Maravi C (1999) Embryos and rhynchoteuthion paralarvae of the jumbo flying squid Dosidicus gigas (Cephalopoda) obtained through artificial fertilization from Peruvian waters. Fish Sci 65:904–908
Sakurai Y, Young RE, Hirota J, Mangold K, Vecchione M, Clarke MR, Bower JR (1995) Artificial fertilization and development through hatching in the oceanic squids Ommastrephes bartramii and Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae). Veliger 38:185–191
Ohman MD, Snyder RA (1991) Growth kinetics of the omnivorous oligotric ciliate Strombidium sp. Limnol Oceanogr 36:922–935
Hanlon RT, Messenger JB (1996) Cephalopod behaviour. Cambridge University Press, Cambridge
Shigeno S, Kidokoro H, Goto T, Tsuchiya K, Segawa S (2001) Early ontogeny of the Japanese common squid Todarodes pacificus (Cephalopoda, Ommastrephidae) with special reference to its characteristic morphology and ecological significance. Zool Sci 18:1011–1026
Bigelow KA, Landgraf KC (1993) Hatch dates and growth of Ommastrephes bartramii paralarvae from Hawaiian waters as determined from statolith analysis. In: Okutani T, O’Dor RK, Kubodera T (eds) Recent advances in cephalopod fisheries biology. Tokai University Press, Tokyo, pp 15–24
Vidal EAG (1994) Relative growth of paralarvae and juveniles of Illex argentinus (Castellanos, 1960) in southern Brazil. Antarct Sci 6:275–282
Chen DS, Van Dykhuizen G, Hodge J, Gilly WF (1996) Ontogeny of copepod predation in juvenile squid (Loligo opalescens). Biol Bull 190:69–81
Acknowledgments
We are grateful to J.R. Bower for critically reading the manuscript and for valuable comments. We thank the captain and crews of R/V Shunyo-Maru for help with sampling during cruises. We also wish to thank H. Miyata and K. Nonaka for their help in sample collection, and M. Senaga for her laboratory assistance. Thanks are also due to N. Suzuki for his technical assistance and helpful discussion on DNA analysis. We are also grateful to M. Nakamachi for providing information on microorganisms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchikawa, K., Sakai, M., Wakabayashi, T. et al. The relationship between paralarval feeding and morphological changes in the proboscis and beaks of the neon flying squid Ommastrephes bartramii . Fish Sci 75, 317–323 (2009). https://doi.org/10.1007/s12562-008-0036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-008-0036-2