Abstract
Raspberries have lately caused several human norovirus (HuNoV) outbreaks in Europe. In this study, we developed and evaluated for HuNoV reverse transcription (RT)-PCR detection in frozen raspberries extraction methods that have equal sensitivity but are less time-consuming than widely used methods based on polyethylene glycol (PEG) precipitation and chloroform–butanol purification. One method was applied to stored frozen raspberries linked to previous HuNoV outbreaks and berries on sale. In the virus elution-based Method 1, sparkling water eluted viruses most efficiently from the berries. Method 2, based on direct nucleic acid extraction with minor PEG supplement, yielded the highest number of positive findings (4 out of 9) at low virus concentration level of 100 genome copies HuNoV genogroup II per 25 g raspberries. Both methods showed approximately equal sensitivity to a method including PEG precipitation and chloroform–butanol purification. Two naturally contaminated berry samples linked to HuNoV outbreaks in 2006 and 2009 were still positive for HuNoV genogroup I, but all berry products purchased from a local store remained negative for HuNoV. In conclusion, this study presents two efficient and rapid methods which can be used in urgent HuNoV outbreak investigations, since the results of the virus analysis are available in a few hours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human norovirus (HuNoV) has been one of the most significant causes of food-related illness in developed countries, inducing gastroenteritis outbreaks through the contamination of water and various foodstuffs (Mathijs et al. 2012). In recent years, frozen raspberries in particular have caused numerous HuNoV episodes in European countries (Cotterelle et al. 2005; European Commission 2014, 2015; Falkenhorst et al. 2005; Hjertqvist et al. 2006; Le Guyader et al. 2004; Maunula et al. 2009; Sarvikivi et al. 2012). Lately, several published studies have increased our knowledge of how HuNoVs contaminate food items during food handling (Mokhtari and Jaykus 2009; Rönnqvist et al. 2014; Stals et al. 2013; Tuladhar et al. 2013; Verhaelen et al. 2013), harvesting and irrigation (Kokkinos et al. 2012; Maunula et al. 2013).
HuNoVs, members of the Caliciviridae family, are currently classified into six genogroups (Gs) (White 2014), and the strains infectious to humans belong to GI, GII and GIV (Zheng et al. 2006). Recent reviews have proposed that water-related outbreaks are more often caused by GI noroviruses and food-related outbreaks as well as person-to-person infections by GII noroviruses (Bitler et al. 2013; Mathijs et al. 2012; Matthews et al. 2012). The authors explain this difference by GI noroviruses’ greater tolerance of environmental stress in water. HuNoVs can survive cold temperatures for years and can also withstand a temperature of 60 °C for 30 min (Carter 2005; Dolin et al. 1972). Consequently, the consumption of unheated foodstuffs such as berries, vegetables and shellfish poses the highest risk of food-related norovirus infection.
A literature review by Stals et al. (2012), covering the past decade, has identified several methods for detecting noroviruses in various high-risk foodstuffs such as berries, salads and cold cuts. The methods are based mainly on ultrafiltration, ultracentrifugation, cationic separation and polyethylene glycol (PEG) precipitation, and they yield virus recovery efficiencies ranging from 3 to 72% (Stals et al. 2012). High variability in the recovery of HuNoV with commonly used detection methods was one of the issues that led to the need for a standardized method. In 2013, ISO published the first technical specification for HuNoV detection in foods (ISO/TS 15216) and the part of the specification concerning quantitative detection was published as ISO standard 15216-1:2017 in 2017. The method is the achievement of the efforts of several experts in the field. The application for soft fruit, however, involves numerous steps such as time-consuming virus concentration, and it often requires two working days to obtain results, especially if the analysis involves several samples.
Because viral contamination levels in foods are usually low and the presence of only a few virus particles can lead to disease (Teunis et al. 2008), highly sensitive detection methods are needed. Furthermore, to prevent the spread of an outbreak, the results of virus analysis are needed quickly, which also emphasizes the need for a rapid method. The objective of this study was to create and present simple and rapid methods for detecting HuNoV in raspberries and to evaluate them using berries artificially inoculated with known quantities of HuNoV GII. To determine the capability to detect HuNoV also in naturally contaminated berries, we applied the fastest method, which was used with minor modifications in an outbreak situation in 2009 (Maunula et al. 2009), to a selection of naturally contaminated stored frozen berry samples linked to suspected HuNoV outbreaks in 2006–2013. A small-scale screening study was also implemented with this method to find out the incidence of HuNoV in frozen raspberries on sale in local grocery shops.
Materials and Methods
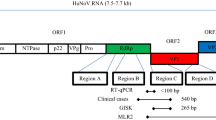
A total of 170 raspberry samples were used in the study according to the scheme shown in Fig. 1.
HuNoV and Inoculation of Berries for Elution Study and for Method Evaluation
An anonymous faecal sample containing HuNoV strain GII.4-2006b and obtained from Huslab, Helsinki, Finland was used in this study. Processing of the virus-containing faecal sample and the inoculation procedure of raspberries are described by Summa et al. (2012a). Briefly, the endpoint dilution polymerase chain reaction (PCR) method defined the virus concentration for this strain as 1.7 × 108 genome copies (gc)/ml in a 10% stool suspension in phosphate-buffered saline (PBS) as the stock solution. Spiking solutions containing virus loads from 104 to 101 gc in tenfold dilutions were prepared from the stock solution. Fresh Finnish raspberries, all belonging to the same batch, were obtained from a wholesaler of berries. 25 g portions of raspberries were spiked by spreading 100 μl of spiking solutions as small droplets onto the berries, which were then kept in a fume chamber for up to 2 h until the droplets had dried before freezing the samples at − 20 °C. These berry portions were used in HuNoV elution study and for method evaluation (Fig. 1). 100 μl portions of spiking solution were frozen as a spiking control for each virus load for the purpose of recovery efficiency evaluation.
Description of the Untreated Samples
HuNoV analysis was also performed on archived frozen berries linked to nine suspected HuNoV outbreaks in Finland and one outbreak in Sweden in 2006–2013. The berries had been kept stored at − 20 °C (altogether 11 samples, later called ‘epidemic samples’). Virus analysis made with an in-house PEG precipitation-based method and real-time reverse transcription (RT)-PCR during the course of the outbreak investigation had revealed that seven of these samples contained HuNoV GI or GII genome or both; four remained negative. In addition, 39 packages of frozen raspberries or mixed berries containing raspberries were purchased from several local grocery shops in southern Finland in 2010, 2014 and 2017 (later called ‘screening samples’) and analysed for HuNoV GI and GII. More detailed information about ‘screening samples’ is presented in Fig. 1. All ‘epidemic’ and ‘screening’ samples were stored at − 20 °C, and 25–30 g portions were analysed in 2014 with the exception of 20 samples purchased in 2017.
HuNoV Elution Study
Five elution fluids for berries were tested. The fluids were (1) untreated tap water, (2) salt solution (1.04 mM NaHCO3, 0.59 mM K2CO3, 0.25 mM CaCl2, 0.37 mM MgCl2) prepared in the laboratory, (3) commercial sparkling mineral water (water, carbon dioxide and salts corresponding to the salt solution in (2), Vichy, Rainbow, Finland, stored at room temperature), (4) sparkling water prepared in the laboratory (untreated tap water was turned into sparkling water with SodaStream© equipment powered by a CO2 cylinder according to the manufacturer’ instructions, no other supplements were added to the water) and (5) TGBE buffer (pH 9.5) (100 mM Tris, 50 mM glycine, 1% beef extract; beef extract, Becton–Dickinson, USA). The elution test, which was repeated twice with each elution fluid, included three 25 g raspberry samples each contaminated with 104 gc of HuNoV GII.4, i.e. six samples for every fluid. Briefly, after melting at room temperature for 1 h, the berries were washed in a Stomacher® bag (Seward Ltd, UK) with 30 ml of one of the five elution fluids for 10 min in a shaker. The elution fluid was collected and decanted into a 50 ml tube that contained 1 ml of chloroform–butanol (CB) (1:1) solution. The tubes were then shaken vigorously by hand for 1 min and centrifuged at 15,000×g for 30 min at room temperature. After centrifugation, the water phase was moved to another 50 ml tube that contained 10 g of guanidine thiocyanate (GITC, Sigma-Aldrich, USA). The procedure was completed as described for Method 1 for RNA extraction.
Virus Extraction
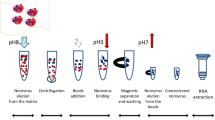
Two of the methods presented in Fig. 2 served to extract viruses in frozen raspberries spiked with 104, 103, 102 or 101 gc of HuNoV GII.4. Three samples were analysed simultaneously for each dilution, and analysis was repeated three times for each triplicate. To scale the results of the two methods, a third method based on PEG precipitation and CB purification (Butot et al. 2007; Dubois et al. 2002) described in detail in Fig. 2 and also by Summa et al. (2012a) was applied for each dilution in two (104 and 103 gc) or nine (102 gc) samples. Briefly, the melted berries were washed with TGBE buffer (pH 9.5), which was then centrifuged for 15 min. The pH of the supernatant was adjusted to 7.2 and it was then incubated at room temperature with 1000 units of pectinase (≥ 3800 units/ml, Pectinex, Sigma, USA) for 30 min. Then the supernatant was incubated at 4 °C with PEG and NaCl solution (50% (w/v) PEG 8000, Sigma-Aldrich, USA and 1.5 M NaCl) for 2 h. After 30 min centrifugation, the resuspended pellet was treated with CB mixture. Before the virus analyses, all berry samples, including also ‘epidemic’ and ‘screening samples’, were allowed to melt in a fume chamber at room temperature for 1–2 h. A commonly used process control, mengovirus (a kind gift from Dr A. Bosch, University of Barcelona, Spain) as a load of 105 PCR-units, was added to all the berry samples immediately after melting. RNA extraction took place directly after virus extraction.
Method 1
Method 1 took about 2.5 h to complete from a frozen berry sample to the nucleic acid with 1 h melting time. Melted berries were first quickly (1–5 min) washed in a Stomacher® bag with 27 ml of sparkling water (prepared with SodaStream© equipment) which was then supplemented with 3 ml of 10 × TGBE buffer (pH 11) (1 M Tris, 500 mM glycine, 5% beef extract; beef extract, Becton–Dickinson, USA) for the elution step. After centrifugation in a 50 ml tube, the supernatant was combined with 10 g of GITC powder and 3 ml PEG/NaCl solution in another 50 ml tube. The tube was then incubated in a water bath (at about 55 °C) until the GITC had completely dissolved before beginning the lysis step of RNA extraction.
Method 2
Method 2 consisted of only three steps: melting the berries, combining the juice from the berries with PEG/NaCl solution and RNA extraction. Completion of the procedure from melting the berries to extracted RNA took about 1.5 h, including 1 h melting time. First, 1 ml of juice released from the defrosted berries was collected in a tube where 250 μl of PEG/NaCl solution was added. After a 1 min manual shaking of the tube, 2 ml lysis buffer was inserted into the tube to perform the lysis step of RNA extraction. Method 2 was also tested without PEG/NaCl; in this case the RNA was extracted directly from the juice from the melting raspberries. Method 2 with PEG/NaCl supplement was used in the HuNoV investigations of the ‘epidemic’ and ‘screening’ berry samples.
RNA Extraction
For Method 2, the RNA extraction was performed using a miniMAG (MM) RNA extraction system and a NucliSENS Magnetic Extraction Reagents kit (bioMérieux, France) according to the manufacturers’ instructions. For Method 1 some modifications were made. The lysis step began by adding 12 ml of ethanol (Etax A 96.1 vol%, Altia Oyj, Finland) and 100 μl of sodium dodecyl sulphate (SDS, solution of 10% (w/v), ICN Biomedicals, Inc., USA) to the 50 ml tube containing the supernatant, PEG/NaCl and GITC, and then incubation continued at room temperature for 10 min. After incubation, 100 μl of the MM magnetic beads were added to the tube, which was briefly manually shaken and then incubated at 37 °C in a rotator for 10–15 min. The beads were then separated from the supernatant in a magnetic rack and moved to the MM tubes with wash buffer 1. After this, the MM procedure was continued according to the manufacturers’ instructions. The viral RNA was eluted in 50 µl of elution buffer and then frozen at − 20 °C. Each extraction group comprised, in addition to the berry samples, a corresponding HuNoV spiking control (104–101 gc in 100 µl) and at least one blank sample to control for cross-contamination.
Viral Nucleic Acid Detection
Virus detection targeting the polymerase–capsid junction was completed using one-step TaqMan real-time RT-PCR as described by Summa et al. (2012a) and a QuantiTect Probe RT-PCR kit (Qiagen, USA). All PCR reactions containing 15 µl of Master Mix which included primers and a FAM-labelled probe and 5 µl of extracted RNA from the berry samples were performed as duplicates for the neat samples and their tenfold dilutions. In addition to the berry samples, each PCR run included the spiking control, a standard and one to two blank samples. For each 25 g berry sample, the minimum detectable quantity of viruses was 10 gc. All ‘screening’ and ‘epidemic samples’ were analysed for HuNoV GI and GII separately as described by Summa et al. (2012b) using primers and probe COG2R/QNIF2/QNIFS, respectively, for GII and QNIF4/QNIF3/JJV1P, respectively, for GI.
Mengovirus served as an internal process control to estimate the success of the virus extraction for each analysis during this study. The same protocol as for HuNoV GII, but using primers and probe Mengo110/Mengo209/Mengo147, respectively, was used to detect mengovirus in the master mix as described by Summa et al. (2012a).
Murine norovirus (MuNoV) (obtained from Herbert W. Virgin at the Washington University School of Medicine, St. Luis, MO, USA) served as an external control in evaluating the degree of PCR inhibition in each sample. The master mix formula for MuNoV was the same as used for HuNoV GII with the primers (MNVfor and MNVrev) and the probe (MNV) described by Hewitt et al. (2009). One MuNoV reaction contained 15 µl of Master Mix, which included primers and probe, 5 µl of RNA from the berry samples and 1 µl (104 PCR-units) of MuNoV RNA. As a control, each run included a reaction consisting of 15 µl of mastermix, 5 µl of water and 1 µl of MuNoV RNA.
Determination of Virus Recovery Efficiency and Inhibition of the PCR Reaction
Qualitative virus analysis was used for the ‘epidemic’ and ‘screening samples’. Quantitative analysis of the samples of elution fluid tests and the evaluation of methods were based on a standard curve generated by sequential tenfold dilutions of the viral RNA of the HuNoV GII.4 strain used in the study. The results of each PCR run were quantified by plotting against the standard curve. The virus recovery efficiency of the higher virus loads was explored. The recovery percentage was calculated by dividing the number of gc in the sample by the number of gc in the spiking control and multiplying by 100.
As noted in “RNA Extraction”, MuNoV served as an external control to evaluate the degree of inhibition of the berry samples in the PCR runs by comparing the quantification cycle (C q) value of the MuNoV RNA in the berry samples against the corresponding value of the controls (for details, see “RNA Extraction” and “Evaluation of the Virus Extraction Methods”). In this study, the amplification efficiencies of MuNoV and HuNoV GII.4 were sufficient to enable reliable estimations of the effect of PCR inhibitors in HuNoV PCR assays.
Results
Selection of Elution Fluid for Virus Extraction
Five fluids (untreated tap water, salt solution, sparkling water, sparkling mineral water and TGBE buffer) were tested for their suitability to elute viruses from 25 g portions of raspberries spiked with 104 gc HuNoV GII.4. HuNoV detection in all six samples was possible only with the sparkling water elution (Table 1). Water, salt solution and TGBE failed to detect HuNoV GII.4 in several raspberry samples, although mengovirus, the process control, was detectable in all samples. MuNoV as an external control showed less than one log difference (ΔC q < 3 cycles) in levels of PCR inhibitors between the elution fluids. Based on these results, sparkling water without minerals was selected for elution of the viruses from the berries in Method 1.
Evaluation of the Virus Extraction Methods
Two rapid methods were evaluated using frozen raspberries artificially contaminated with HuNoV loads of 104, 103, 102 and 101 gc per 25 g sample. We scaled the results of the two methods to results of a common method based on PEG precipitation and CB purification (for details, see “Virus Extraction”).
As Table 2 shows, both rapid methods successfully detected HuNoV GII.4 in all nine berry samples with a virus load of 104 gc. Method 1 yielded positive results in nine samples and one sample (of nine) with virus loads of 103 and 102 gc, respectively. For Method 2, the PEG/NaCl supplement increased the positive results from six to nine (of nine) with a virus load of 103 gc and from zero to four (of nine) with a virus load of 102 gc. Thus, Methods 1 and 2 appeared to work at least as well as the PEG precipitation method (PEGP), which successfully detected HuNoV GII.4 in all berry samples spiked with virus loads of 104 and 103 gc, and one with 102 gc. Neither Method 2 nor PEGP was capable of detecting HuNoV GII.4 in berry samples with lower levels of contamination. The positive signal of mengovirus as a process control in the neat sample or its tenfold dilution was a condition for the acceptance of each result of the HuNoV analysis.
The highest virus recovery efficiencies for a virus load of 104 gc were obtained by Method 2 and they varied between 10 and 81% with an overall mean of 32% (standard deviation, SD 0.16). The recovery efficiencies showed less variation with Method 1; however, these were slightly lower than those of Method 2, with a mean recovery of 9% (SD 0.05). Efficiencies of Methods 1 and 2 were comparable to that of PEGP, which had an efficiency of 24% (SD 0.02).
The PCR inhibition level, revealed by MuNoV, in Method 2 was lower and had less variability between the samples when the PEG/NaCl supplement was used in the virus extraction protocol than when the supplement was not used (Table 2). PCR inhibitors affected virus detection more severely (even over one log) when berries inoculated with lower amounts of the virus were analysed. When the amplification was performed with 1:10 dilutions of RNA extracted from the berry samples, only minor PCR inhibition was seen for each method (Table 2).
Screening of Frozen Berries
Method 2, which proved to be the most sensitive and quickest method, was used for screening the frozen raspberries or berry mixes. The method was used to detect HuNoV in 11 stored berry samples that were linked to suspected HuNoV outbreaks and of which seven had earlier been verified as positive for HuNoV genome. In the current analysis, two of the berry samples kept frozen for 5 and 8 years (outbreaks in 2006 and 2009, respectively) still contained a detectable amount of HuNoV GI genome (Table 3). All 39 frozen berry packages purchased from local stores in 2010, 2014 and 2017 tested negative for HuNoVs GI and GII.
Discussion
Numerous rapid methods for HuNoV detection have been introduced in recent years for different kinds of foods (Baert et al. 2008; Fumian et al. 2009; Lee et al. 2013; Morillo et al. 2012; Perrin et al. 2015; Rzezutka et al. 2005; Schwab et al. 2000). However, some of them require special equipment such as ultracentrifugation or filtration devices and only a few of them are suitable for frozen raspberries, which have been recognized as an important source of HuNoV outbreaks in Europe (Cotterelle et al. 2005; European Commission 2014, 2015; Falkenhorst et al. 2005; Hjertqvist et al. 2006; Le Guyader et al. 2004; Maunula et al. 2009; Sarvikivi et al. 2012). Raspberries are a challenging food matrix for virus detection because the low-pH juice they release contains compounds that inhibit the PCR reaction. This study demonstrated that Method 2, which is suitable for food that releases liquid when melted, was found to be more sensitive, quicker and more efficient with artificially contaminated frozen raspberries than Method 1. It was capable of detecting HuNoV genomes in naturally contaminated berries stored frozen for up to 8 years. Sparkling water was shown to elute viruses most efficiently and was therefore chosen to be used in Method 1, which is suitable also for fresh foods and frozen non-juicy food matrices. Benefits of these rapid methods are that most laboratories can perform them easily and the results of the whole virus analysis are available within one working day.
Butot et al. (2014) divided various virus extraction methods into two groups: elution-concentration of virus particles and direct viral RNA extraction, which usually involves washing the food sample directly with a GITC-based lysis buffer. Method 1 presented in this study is a combination of these as it has an elution step but no concentration step. Method 2, in contrast, involves direct RNA extraction, as does a method originally presented by Schwab et al. (2000) for deli meat, which proved successful with foods other than oysters or berries suspected of causing viral gastroenteritis outbreaks (Anderson et al. 2001; Boxman et al. 2007). Baert et al. (2008) and, recently, Perrin et al. (2015) have described applications of direct viral RNA extraction for artificially contaminated frozen raspberries. These methods entail eluting the virus from berries by washing them with GITC and phenol or with GITC-based buffers; in our Method 2, melted juice from the berry sample is directly added to the GITC-based lysis buffer. To our knowledge, no direct RNA extraction methods other than Method 2 have been successfully used and published for naturally contaminated frozen raspberries.
Different buffers and equipment have been tested for the elution of viruses from a variety of food matrices in recent studies (Cheong et al. 2009; de Abreu Corrêa and Miagostovich 2013; Dubois et al. 2006; Park et al. 2010). Data in many of these studies suggested that the best buffer was Tris base and glycine for the elution step, which is one of the most critical points of virus extraction, as incomplete elution will compromise the entire method. In this study, we wanted to test simple and easily available fluids against the widely used TGBE buffer. In our previous study (data not shown) we discovered that gas bubbles formed when carbonate-based buffers came into contact with the surface of the raspberries. Reports indicate that the use of sonication, which in our experiments (data not shown) also created bubbles on the surface of the food, increases virus recoveries when combined with ultrafiltration (Jones et al. 2009). Other studies have also examined the use of vacuum-induced bubbles in inactivation tests for HuNoV surrogates in lettuce (Fraisse et al. 2011). All of these findings evoked an idea about bubbles enhancing the release of virus particles from the surface of the food, making the elution more efficient. In our tests, the two different elution fluids inducing bubbling eluted more HuNoV GII.4 particles from the berries than did the other liquids tested.
Certain components such as organic compounds, fats, sugars and Ca2+, which are known to inhibit the PCR reaction and to be released from food matrices during processing, are a significant challenge to HuNoV detection in foods (Wilson 1997). Raspberry analyses, especially, are easily harmed by PCR inhibitors which, however, have rather efficiently been removed with CB treatment (Butot et al. 2007; Dubois et al. 2002; Summa et al. 2012a). When developing the two methods presented in this study, we tested Method 2 also with CB treatment. However, when analysing naturally contaminated raspberries, Method 2 produced a weak positive HuNoV signal only when performed without this treatment (data with CB treatment not shown), perhaps partly because of a loss of virus particles when separating the water phase from the organic phase when the treatment was used. In addition, concentrating viruses using PEG/NaCl has proved an efficient way to extract viruses in various kinds of foods (Baert et al. 2008; Boxman et al. 2007; Butot et al. 2007; Guévremont et al. 2006; Kim et al. 2008; Rutjes et al. 2006; Stals et al. 2011a). PEG polymer is widely used in numerous chemical, biological and industrial applications for various purposes. In this study, we decided to use PEG as a supplement without precipitating the viruses, because it was found to reduce the effect of PCR inhibitors released from raspberries.
Method 2, without the PEG/NaCl supplement, served to detect HuNoV in naturally contaminated raspberries during an outbreak situation in 2009 (Maunula et al. 2009). In this study, the capability of this method, supplemented with PEG/NaCl, was demonstrated since it detected HuNoV genome in two archived naturally contaminated berry samples from suspected HuNoV outbreaks in 2006 and 2009. The results also show that the HuNoV genome is quite stable for several years at least in high contaminations in frozen berries. The five originally HuNoV-positive berry samples which now tested negative had produced only a weak positive signal in tests when performed during the outbreak 3–5 years earlier, which may explain negative results. All our retail frozen raspberries tested negative for HuNoV, whereas other research groups in Europe (Baert et al. 2011; De Keuckelaere et al. 2015; Stals et al. 2011b) have screened berry batches from raspberry processing companies and found HuNoV-positive signals in some of them. However, there were no reported HuNoV outbreaks related to frozen raspberries in Finland during the sampling periods (personal communication, Finnish Food Safety Authority Evira); thus the Finnish epidemic data and other recent studies (Bouwknegt et al. 2015; Maunula et al. 2013) are in line with our negative results. On the other hand, because the number of samples analysed was not high, the conclusions that can be drawn based on these results are limited. Another limitation of this study is that the sensitivity of the methods has been tested only for HuNoV GII and not for GI. However, as mentioned above, GI HuNoV could be detected in naturally contaminated berries using Method 2, which is a promising result.
The rapid methods presented here would be most valuable when used to detect sources of sudden bursts of outbreaks and when the results are needed quickly. The current virus analysis, however, comprises the PCR step, which is often vulnerable to PCR inhibitors. Further improvement is needed to increase the sensitivity of the genome detection without the need to dilute the samples for PCR reaction. Some promising results have already been achieved using an additional purification step for extracted nucleic acid with the PCR inhibitor removal kit and/or digital PCR, which is less influenced by inhibitors (Coudray-Meunier et al. 2015; Fraisse et al. 2017). These findings may offer solutions to overcome the current deficiencies in virus analysis.
References
Anderson, A. D., Garrett, V. D., Sobel, J., Monroe, S. S., Frankhauser, R. L., Schwab, K. J., et al. (2001). Multistate outbreak of norwalk-like virus gastroenteritis associated with a common carter. American Journal of Epidemiology, 154(11), 1013–1019.
Baert, L., Mattison, K., Loisy-Hamon, F., Harlow, J., Martyres, A., Lebeau, B., et al. (2011). Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? International Journal of Food Microbiology, 151, 261–269.
Baert, L., Uyttendaele, M., & Debevere, J. (2008). Evaluation of viral extraction methods on a broad range of ready-to-eat foods with conventional and real-time RT-PCR for norovirus GII detection. International Journal of Food Microbiology, 123, 101–108.
Bitler, E., Matthews, J., Dickey, B., Eisenberg, J., & Leon, J. (2013). Norovirus outbreaks: A systematic review of commonly implicated transmission routes and vehicles. Epidemiology and Infection, 141, 1563–1571.
Bouwknegt, M., Verhaelen, K., Rzezutka, A., Kozyra, I., Maunula, L., von Bonsdorff, C.-H., et al. (2015). Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains. International Journal of Food Microbiology, 198, 50–58.
Boxman, I., Tilburg, J., te Loeke, N., Vennema, H., de Boer, E., & Koopmans, M. (2007). An efficient and rapid method for recovery of norovirus from food associated with outbreaks of gastroenteritis. Journal of Food Protection, 70(2), 504–508.
Butot, S., Putallaz, T., & Sanchez, G. (2007). Procedure for rapid concentration and detection of enteric viruses from berries and vegetables. Applied and Environmental Microbiology, 73(1), 186–192.
Butot, S., Zuber, S., & Baert, L. (2014). Sample preparation prior to molecular amplification: Complexities and opportunities. Current Opinion on Virology, 4, 66–70.
Carter, M. J. (2005). Enterically infecting viruses: Pathogenicity, transmission and significance for food and waterborne infection. Journal of Applied Microbiology, 98, 1354–1380.
Cheong, S., Lee, C., Choi, W. C., Lee, C.-H., & Kim, S.-J. (2009). Concentration method for the detection of enteric viruses from large volumes of foods. Journal of Food Protection, 72(9), 2001–2005.
Cotterelle, B., Drougard, C., Rolland, J., Becamel, M., Boudon, M., Piende, S., et al. (2005). Outbreak of norovirus infection associated with the consumption of frozen raspberries, France, March 2005. Eurosurveillance, 10(17), 2690.
Coudray-Meunier, C., Fraisse, A., Martin-Latil, S., Guillier, L., Delannoy, S., Fach, P., et al. (2015). A comparative study of digital RT-PCR and RT-qPCR for quantification of hepatitis A virus and norovirus in lettuce and water samples. International Journal of Food Microbiology, 201, 17–26.
de Abreu Corrêa, A., & Miagostovich, M. P. (2013). Optimization of an adsorption-elution method with a negatively charged membrane to recover norovirus from lettuce. Food and Environmental Virology, 5, 144–149.
De Keuckelaere, A., Li, D., Deliens, B., Stals, A., & Uyttendaele, M. (2015). Batch testing for noroviruses in frozen raspberries. International Journal of Food Microbiology, 192, 43–50.
Dolin, R., Blacklow, N., DuPont, H., Buscho, R., Wyatt, R., Kasel, J., et al. (1972). Biological properties of norwalk agent of acute infectious nonbacterial gastroenteritis. Proceedings of the Society for Experimental Biology and Medicine, 140, 578–583.
Dubois, E., Agier, C., Traore, O., Hennechart, C., Merle, G., Cruciere, C., et al. (2002). Modified concentration method for the detection of enteric viruses on fruits and vegetables by reverse transcriptase-polymerase chain reaction or cell culture. Journal of Food Protection, 65(12), 1962–1969.
Dubois, E., Hennechart, C., Deboosere, N., Merle, G., Legeay, O., Burger, C., et al. (2006). Intra-laboratory validation of a concentration method adapted for the enumeration of infectious F-specific RNA coliphage, enterovirus, and hepatitis A virus from inoculated leaves of salad vegetables. International Journal of Food Microbiology, 108, 164–171.
European Commission (2014). RASFF, the rapid alert system for food and feed, 2013 Annual Report.
European Commission (2015). RASFF, the rapid Alert system for food and feed, 2014 Annual Report.
Falkenhorst, G., Krusell, L., Lisby, M., Madsen, S., Böttinger, B., & Mølbak, K. (2005). Imported frozen raspberries cause a series of norovirus outbreaks in Denmark, 2005. Eurosurveillance, 10(38), 2795.
Fraisse, A., Coudray-Meunier, C., Martin-Latil, S., Hennechart-Collette, C., Delannoy, S., Fach, P., et al. (2017). Digital RT-PCR method for hepatitis A virus and norovirus quantification in soft berries. International Journal of Food Microbiology, 243, 36–45.
Fraisse, A., Temmam, S., Deboosere, N., Guillier, L., Delobel, A., Maris, P., et al. (2011). Comparison of chlorine and peroxyacetic-based disinfectant to inactivate feline calicivirus, murine norovirus and hepatitis A VIRUS on lettuce. International Journal of Food Microbiology, 151, 98–104.
Fumian, T., Leite, J., Marin, V., & Miagostovich, M. (2009). A rapid procedure for detecting noroviruses from cheese and fresh lettuce. Journal of Virological Methods, 155, 39–43.
Guévremont, E., Brassard, J., Houde, A., Simard, C., & Trottier, Y.-L. (2006). Development of an extraction and concentration procedure and comparison of RT-PCR primer system for the detection of hepatitis A virus and norovirus GII in green onions. Journal of Virological Methods, 134, 130–135.
Hewitt, J., Rivera-Aban, M., & Greening, G. (2009). Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. Journal of Applied Microbiology, 107, 65–71.
Hjertqvist, M., Johansson, A., Svensson, N., Åbom, P.-E., Magnusson, C., Olsson, M., et al. (2006). Four outbreaks of norovirus gastroenteritis after consuming raspberries, Sweden, June–August 2006. Eurosurveillance, 11(36), 3038.
Jones, T. H., Brassard, J., Johns, M. W., & Gagne, M.-J. (2009). The effect of pre-treatment and sonication of centrifugal ultrafiltration devices on virus recovery. Journal of Virological Methods, 161, 199–204.
Kim, H.-Y., Kwak, I.-S., Hwang, I.-G., & Ko, G. P. (2008). Optimization of methods for detecting norovirus on various fruit. Journal of Virological Methods, 153, 104–110.
Kokkinos, P., Kozyra, I., Lazic, S., Bouwknegt, M., Rutjes, S., Willem, K., et al. (2012). Harmonised investigation of the occurrence of human enteric viruses in the leafy green vegetable supply chain in three European countries. Food and Environmental Virology, 4, 179–191.
Le Guyader, F. S., Mittelholzer, C., Haugarreau, L., Hedlund, K.-O., Alsterlund, R., Pommepuy, M., et al. (2004). Detection of noroviruses in raspberries associated with a gastroenteritis outbreak. International Journal of Food Microbiology, 97, 179–186.
Lee, H.-M., Kwon, J., Choi, J.-S., Lee, K.-H., Yang, S., Ko, S.-M., et al. (2013). Rapid detection of norovirus from fresh lettuce using immunomagnetic separation and a quantum dots assay. Journal of Food Protection, 76(4), 707–711.
Mathijs, E., Stals, A., Baert, L., Botteldoorn, N., Denayer, S., Mauroy, A., et al. (2012). A review of known and hypothetical transmission routes for noroviruses. Food and Environmental Virology, 4, 131–152.
Matthews, J., Dickey, B., Miller, R., Felzer, J., Dawson, B., Lee, A., et al. (2012). The epidemiology of published norovirus outbreaks: A systematic review of risk factors associated with attack rate and genogroup. Epidemiology and Infection, 140, 1161–1172.
Maunula, L., Kaupke, A., Vasickova, P., Söderberg, K., Kozyra, I., Lazic, S., et al. (2013). Tracking enteric viruses in the European berry fruit supply chain. International Journal of Food Microbiology, 167, 177–185.
Maunula, L., Roivainen, M., Keränen, M., Mäkelä, S., Söderberg, K., Summa, M., et al. (2009). Detection of human norovirus from frozen raspberries in a cluster of gastroenteritis outbreaks. Eurosurveillance, 14(49), 19435.
Mokhtari, A., & Jaykus, L.-A. (2009). Quantitative exposure model for the transmission of norovirus in retail food preparation. International Journal of Food Microbiology, 133, 38–47.
Morillo, S., Luchs, A., Cilli, A., & Timenetsky, M. (2012). Rapid detection of norovirus in naturally contaminated food: Foodborne gastroenteritis outbreak on a Cruise Ship in Brazil, 2010. Food and Environmental Virology, 4, 124–129.
Park, H., Kim, M., & Ko, G. (2010). Evaluation of various methods for recovering human norovirus and murine norovirus from vegetables and ham. Journal of Food Protection, 73(9), 1651–1657.
Perrin, A., Loutreul, J., Boudaud, N., Bertrand, I., & Ganzer, C. (2015). Rapid, simple and efficient method for detection of viral genomes on raspberries. Journal of Virological Methods, 224, 95–101.
Rönnqvist, M., Aho, E., Mikkelä, A., Ranta, J., Tuominen, P., Rättö, M., et al. (2014). Norovirus transmission between hands, gloves, utensils, and fresh produce during simulated food handling. Applied and Environmental Microbiology, 80(17), 5403–5410.
Rutjes, S. A., Lodder-Verschoor, F., van der Poel, W. H. M., van Duijnhoven, Y. T. H. P., & de Roda, Husman A. M. (2006). Detection of noroviruses in foods: A study on virus extraction procedures in foods implicated in outbreaks of human gastroenteritis. Journal of Food Protection, 69(8), 1949–1956.
Rzezutka, A., Alotaibi, M., D’Agostino, M., & Cook, N. (2005). A centrifugation-based method for extraction of norovirus from raspberries. Journal of Food Protection, 68(9), 1923–1925.
Sarvikivi, E., Roivainen, M., Maunula, L., Niskanen, T., Korhonen, T., Lappalainen, M., et al. (2012). Multiple norovirus outbreaks linked to imported frozen raspberries. Epidemiology and Infection, 140, 260–267.
Schwab, K., Neill, F., Fankhauser, R., Daniels, N., Monroe, S., Bergmire-Sweat, D., et al. (2000). Development of methods to detect “norwalk-like viruses” (NLVs) and hepatitis A virus in delicatessen foods: Application to a food-borne NLV outbreak. Applied and Environmental Microbiology, 66(1), 213–218.
Stals, A., Baert, L., Jasson, V., van Coillie, E., & Uyttendaele, M. (2011a). Screening of fruit products for norovirus and the difficulty of interpreting positive PCR results. Journal of Food Protection, 74(3), 425–431.
Stals, A., Baert, L., van Coillie, E., & Uyttendaele, M. (2011b). Evaluation of a norovirus detection methodology for soft red fruits. Food Microbiology, 28, 52–58.
Stals, A., Baert, L., van Coillie, E., & Uyttendaele, M. (2012). Extraction of food-borne viruses from food samples: A review. International Journal of Food Microbiology, 153, 1–9.
Stals, A., Uyttendaele, M., Baert, L., & van Coillie, E. (2013). Norovirus transfer between foods and food contact materials. Journal of Food Protection, 76(7), 1202–1209.
Summa, M., von Bonsdorff, C.-H., & Maunula, L. (2012a). Evaluation of four virus recovery methods for detecting noroviruses on fresh lettuce, sliced ham, and frozen raspberries. Journal of Virological Methods, 183, 154–160.
Summa, M., von Bonsdorff, C.-H., & Maunula, L. (2012b). Pet dogs—A transmission route for human noroviruses? Journal of Clinical Virology, 53, 244–247.
Teunis, P., Moe, C., Liu, P., Miller, S., Lindesmith, L., Baric, R., et al. (2008). Norwalk virus: How infectious is it? Journal of Medical Virology, 80, 1468–1476.
Tuladhar, E., Hazeleger, W., Koopmans, M., Zwietering, M., Duizer, E., & Beumer, R. (2013). Transfer of noroviruses between fingers and fomites and food products. International Journal of Food Microbiology, 167, 346–352.
Verhaelen, K., Bouwknegt, M., Carratala, A., Lodder-Verschoor, F., Diez-Valcarce, M., Rodriguez-Lazaro, D., et al. (2013). Virus transfer proportions between gloved fingertips, soft berries, and lettuce, an associated health risks. International Journal of Food Microbiology, 166, 419–425.
White, P. (2014). Evolution of norovirus. Clinical Microbiology & Infection, 20, 741–745.
Wilson, I. G. (1997). Inhibition and facilitation of nucleic acid amplification. Applied and Environmental Microbiology, 63(10), 3741–3751.
Zheng, D.-U., Ando, T., Fankhauser, R., Beard, R., Glass, R., & Monroe, S. (2006). Norovirus classification and proposed strain nomenclature. Virology, 346, 312–323.
Acknowledgements
We thank K. Söderberg for her valuable contribution during the method design and C.H. von Bonsdorff for his specialist advice when proceeding this study.
Funding
The work was funded by the Academy of Finland (Grant Number 1122782), the Walter Ehrström Foundation and the Finnish Foundation of Veterinary Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Summa, M., Maunula, L. Rapid Detection of Human Norovirus in Frozen Raspberries. Food Environ Virol 10, 51–60 (2018). https://doi.org/10.1007/s12560-017-9321-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9321-5