Abstract
A new virulent phage (Lcb) of Lactobacillus casei ATCC 393 was isolated from Chinese sauerkraut. It was specific to L. casei ATCC 393. Electron micrograph revealed that it had an icosahedral head (60.2 ± 0.8 nm in diameter) and a long tail (251 ± 2.6 nm). It belonged to the Siphoviridae family. The genome of phage Lcb was estimated to be approximately 40 kb and did not contain cohesive ends. One-step growth kinetics of its lytic development revealed latent and burst periods of 75 and 45 min, respectively, with a burst size of 16 PFU per infected cell. The phage was able to survive in a pH range between 4 and 11. However, a treatment of 70 °C for 30 min and 75 % ethanol or isopropanol for 20 min was observed to inactivate phage Lcb thoroughly. The presence of both Ca2+ and Mg2+ showed a little influence on phage adsorption, but they were indispensable to gain complete lysis and improve plaque formation. The adsorption kinetics were similar on viable or nonviable cells, and high adsorption rates maintained between 10 and 37 °C. The highest adsorption rate was at 30 °C. This study increased the knowledge on phages of L. casei. The characterization of phage Lcb is helpful to establish a basis for adopting effective strategies to control phage attack in industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactobacilli are widely used in many food fermentation processes, where they contribute to the flavor and texture of final products (Mariángeles et al. 2015). They also produce organic acids, and the resulting low pH prevents fermented products from degradation by spoilage microorganisms. In recent years, the industrial relevance of lactobacilli has been greatly enhanced by their increasing use as probiotics or as a biotechnological tool (Hultberg et al. 2007).
Lactobacillus casei (L. casei) is a remarkably adaptive species that may be isolated from raw and fermented dairy products, fresh and fermented products, and the reproductive and intestinal tracts of animals (Sturino and Klaenhammer 2006a, b; Wang et al. 2010; Pringsulaka et al. 2011) In industry, L. casei has been used as acid-producing starters in the fermentation of dairy products, processed meats and as specialty cultures for the intensification and acceleration of flavor development in certain bacterial-ripened cheese varieties (Cai et al. 2009). This bacterium is of interest because in addition to its organoleptic properties infermentation, some strains may have probiotic properties (Marranzino et al. 2012; Rochat et al. 2007). L. casei can tolerate the low pH of the stomach and colonize the gastrointestinal tract, with potential beneficial outcomes (Kawase et al. 2010; Galdeano et al. 2007). However, the increasing use of L. casei can lead to phage infections in industrial environments, with adverse effects on the final product (Jones et al. 2000).
Phage infection is still one of the ubiquitous causes of substandard dairy fermentation processes (Zago et al. 2015). In the dairy industry, large quantities of milk are transformed daily to produce fermented dairy products (Moineau et al. 2005; Brussow and Suarez 2006). Phage infection represents a significant risk to this industry; therefore, phage population must be kept under control and at a low level (Moineau and Levesque 2005). Considering the economic impact of phage infections on the dairy industry, the morphology, physiology, and genetics of LAB bacteriophages have been extensively studied (Brussow and Suarez 2006). As a matter of fact, phage infections are even more worrying when probiotic bacteria are the target. The manufacturing of certain types of probiotics involves the propagation of single strain as starter, and these strains often grow slow and, therefore, particularly vulnerable to phage. In recent years, probiotic lactobacilli have become increasingly important for infermented foods and nutraceuticals. Thus, we need to increase our knowledge of the ecology, phylogenesis, and genomics of the phages that infect lactobacilli (Brussow and Hendrix 2002; Desiere et al. 2001; Hendrix 2003). It can help elucidate phage–host interactions and the engineering of resistant strains which are more robust in fermentation (Barrangou and Horvath 2012; Sturino and Klaenhammer 2006a, b).
To date, forty-three phages that infect L. casei have been reported, 16 of which morphologically belong to the Myoviridae, 21 are Siphoviridae, and the other six have not been morphologically classified (Dieterle et al. 2014). Among these L. casei phages, A2 and phiAT3 are the best characterized. A2 was isolated in Spain from the whey of a failed homemade blue cheese product using L. casei ATCC 393, and phiAT3 was recovered following induction from L. casei 393 using mitomycin C (Garcia et al. 2003; Lo et al. 2005). And both of them are temperate and belong to the Siphoviridae family (Garcia et al. 2003; Lo et al. 2005). Among the other phages of L. casei, J-1 was isolated in 1965 in association with fermentation failures during the production of Yakult, a Japanese beverage fermented from skimmed milk and Chlorella extracts. Upon isolation, J-1 was shown to be a virulent phage in the L. casei Shirota strain used for manufacturing of Yakult. An L. casei strain resistant to J-1 infection was isolated, but after 2 years of use, a second phage designated PL-1 was isolated that infected the resistant strain (Dieterle et al. 2014). So far, virulent phage of L. casei ATCC 393 has not been reported.
Here, we report the isolation and characterization of a novel virulent phage (Lcb) of L. casei. The influence of physicochemical agents on the viability of phage and adsorption on cells was also investigated.
Materials and Methods
Bacterial Strains and Culture Conditions
The host strain L. casei ATCC 393 (L. casei 393) was kindly supplied by Prof. Jos Seegers (NIZO, Holland). MRS medium supplemented with 10 mM CaCl2 and 10 mM MgSO4 (MRS-Ca–Mg), only with 10 mM CaCl2 (MRS-Ca) (Ebrecht et al. 2010; Trucco et al. 2011; Mariángeles et al. 2012) and only with 10 mM MgSO4 (MRS-Mg) were used. Solid and soft agar media were MRS-Ca–Mg supplemented with 1.5 % and 0.5–0.6 % agar, respectively. Incubations were done at 30 °C without aeration, without shaking. Bacteria were stored as frozen stocks at −80 °C in the culture media supplemented with 30 % (v/v) glycerol. Phage Lcb was propagated on L. casei ATCC 393 in MRS broth. Phage stocks were stored at −80 °C with 15 % (v/v) glycerol.
Isolation, Propagation, and Purification of Lcb
Lactobacillus casei 393 was used as indicator strain to isolate phage from samples collected from Chinese sauerkraut. Sauerkraut juice was centrifuged at 3000×g for 10 min, and the supernatant filtered using a 0.45-μm pore size filter (Moscoso and Suárez 2000; Zago et al. 2013). Each sample filtrate (0.5 ml) and 0.1 ml (108 CFU/ml) of a fresh culture of the indicator strain were added to 10 ml of MRS-Ca–Mg broth and incubated at 30 °C. For each test, a control without sample was also incubated. The turbidity of each tube was regularly compared with the turbidity of control by visual examination. Once growth of the control became visible, both cultures were simultaneously transferred to fresh MRS-Ca–Mg broth. If no lysis occurred in the tube, three sequential subcultures were done to detect a possibly delayed lysis. The phage was purified by a five times cycle of resuspension, dilution, and replating of individual plaque of lysis. Phage enumeration (PFU/ml) was performed by double-layer plaque titration method. Large-scale culture of phage Lcb was done as follows: one liter of MRS-Ca–Mg broth was inoculated with L. casei ATCC 393 at 30 °C without aeration. When the optical density at 600 nm reached 0.3 (about 6.6 × 107 CFU/ml), phage Lcb was added at a multiplicity of infection (moi) of 0.1 and incubated for further 12 h.
The Determination of Host Specificity
The host specificity of phage Lcb was investigated using the spot test. The following lactic acid bacteria were used to determine the host specificity: L. casei ATCC393, Lactobacillus pentosus ATCC33152, Lactobacillus plantarum ATCC 149, Lactobacillus pentosus ATCC 8041, Lactobacillus brevis ATCC367, Lactococcus lactis MG1363, Lactococcus lactis PSM565, Lactococcus lactis NZ9000. One hundred microliters of each bacterial culture listed above was added to 3.5 ml of 0.5 % molten agar, and this mixture was overlaid onto MRS agar plate. Ten microliters of Lcb solution (109 PFU/ml) was spotted onto each agar plate. Plates were incubated at 30 °C for 20 h and were then examined for clear zones on the bacterial lawn.
Electron Microscopy
Phages were centrifugated at 35,000×g for 3.5 h and resuspended in SM buffer (0.58 % NaCl, 0.2 % MgSO4·7H2O, 1 mol/l pH 7.5 Tris–HCl, 2 % Gelatin). Purified phage particles were stained with 2 % (w/v) phosphotungstic acid (pH 7.2) and observed with a transmission electron microscope at an acceleration of 100 kV. Phage morphology and dimension were recorded (Danis-Wlodarczyk et al. 2015).
Phage DNA Extraction and Analysis
Lactobacillus casei ATCC 393 was cultivated overnight at 30 °C in MRS-Ca–Mg broth. Subsequently, 2 ml of the overnight culture was inoculated into 100 ml of fresh MRS-Ca–Mg broth. When OD600nm reached 0.2, phage Lcb was added at a moi of 0.1. The culture was incubated at 30 °C until complete lysis occured. Lysate obtained was centrifugated at 8000×g for 10 min to remove cell debris. The supernatant was filtered through the filter membrane. The filtrate was treated with DNase I (Sigma) and RNase A (Sigma) at a final concentration of 50 U/ml at 37 °C for 1 h. Phage particles were concentrated for 1 h in ice with 1 mol/l NaCl and 10 % (w/v) polyethylene glycol 8000 and then centrifuged (8000×g, 15 min) and resuspended in SM buffer. Phage DNA was obtained by phenol–chloroform–isoamyl alcohol extraction and isopropanol precipitation (Czajkowski et al. 2015).
The packaging site of phage Lcb was studied as follows (Quiberoni et al. 2004). Phage DNA aliquots ligated with T4 DNA ligase and not were digested with HindIII, EcoRI, BamHI, PstI, and SalI, and restriction digests were then treated for 10 min at 70 °C. DNA fragments generated were subjected to electrophoresis on a 1 % (w/v) agarose gel. The gel was stained with ethidium bromide and photographed under UV illumination. DNA standard fragments of HindIII/bacteriophage Lambda and 2000 bp DNA Ladder were used as markers to calculate the size of DNA fragments and estimate the genome size of phage.
One-Step Growth Curve
One-step growth curve of phage Lcb was studied as follows (Zhongjing and Breidt 2015; Mercanti et al. 2015): host strain cultures in mid-exponential phase (OD600nm = 0.5) were harvested by centrifugation and resuspended in 0.25 vol. fresh MRS-Ca–Mg broth. Phages were added approximately at a moi of 0.3 and allowed to adsorb for 10 min at 30 °C to achieve more than 95 % of adsorption. The culture was then centrifuged (6000×g, 10 min). The pelleted cells were resuspended in 10 ml of MRS-Ca–Mg broth. The dilutions were incubated at 30 °C. At 15 min intervals, aliquots from each dilution were collected for counting. The burst size was calculated using the following formula, where “titer after burst” is the phage titer after the initial burst and “phage added” is the phage titer added before adsorption: burst size = (titer after burst − titer at T0)/(phage added − titer at T0).
Influence of Physicochemical Agents on Phage Viability
To determine the thermal stability of phage Lcb, phage Lcb was suspended to give a final concentration of 106 PFU/ml in MRS-Ca–Mg broth (Capra et al. 2004). The mixture was incubated at 25, 37, 50, 60, 70, or 80 °C for 50 min, samples were collected every 10 min, and the PFU of Lcb was determined using serial dilution and the double-agar overlay method.
10, 25, 35, 50, 75, and 100 % v/v ethanol and isopropanol were selected to study the resistance of phage Lcb in MRS-Ca–Mg broth. Phage Lcb was suspended to give a final concentration of 106 PFU/ml. The mixture was incubated for 50 min, and samples were collected every 10 min. The surviving phages were immediately titred by the double-layer agar plate method.
Resistance to ultraviolet irradiation was studied according to Capra et al. (2004), modified as follows: phage Lcb was diluted to contain 106 PFU/ml with MRS-Ca–Mg broth (8 ml as final volume). The suspension was placed in a petri dish (90 mm in diameter and 1 mm in the depth of solution) and then was irradiated with ultraviolet light (20 W, 40 cm) for 50 min. The surviving phages were counted at 10 min intervals.
The influence of pH on the viability of the phage was assayed in MRS-Ca–Mg broth at different pH values ranging from 2 to 12. Phages were suspended in MRS-Ca–Mg broth previously adjusted to the desired pH values of 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 and incubated at 30 °C (Capra et al. 2006; Quiberoni et al. 2004). The phage–host cell mixtures were incubated at 30 °C for 30 min to allow phage adsorption, and phage count (PFU/ml) was determined as mentioned above. All assays were performed in triplicate. The results were expressed as the concentration (PFU/ml) of infectious bacteriophages and plotted against time.
Influence of Ca2+ and Mg2+ on Cell Lysis
The influence of Ca2+ and Mg2+ on cell lysis was investigated as follows (Quiberoni et al. 2004): Infected (moi = 0.5) L. casei ATCC 393 culture was incubated at 30 °C for 12 h in MRS-Ca–Mg, MRS-Mg, MRS-Ca, and MRS broth, respectively. Influence of Ca2+ and Mg2+ on the plaque formation was investigated using the double-layer agar plate method. Solid agar medium was MRS broth with 1.5 % agar, and soft agar media were MRS-Ca–Mg, MRS-Mg, MRS-Ca, and MRS with 0.5 % agar.
Influence of Ca2+ and Mg2+ on Phage Adsorption
The effect of Ca2+ and Mg2+ on phage adsorption was investigated by determination of adsorption kinetics of phage Lcb in MRS-Ca–Mg, MRS-Mg, MRS-Ca, and MRS broth (Suárez et al. 2009). Exponentially growing (OD600nm = 0.5) host strain cultures were infected by phage Lcb at a moi of 0.02, and the mixtures were incubated at 30 °C for 60 min. Samples were collected and centrifuged (13,000×g, 1 min) to precipitate the phage-adsorbed cells every 10 min. Then, the titres of unadsorbed phages in the supernatant were determined as described above. The results were expressed as a percentage of the initial phage counts.
Influence of Temperature on Phage Adsorption
The influence of temperature on the adsorption rates of phage Lcb on cells was determined as follows (Capra et al. 2006): phages were added approximately at a moi of 0.02, and the cultures were incubated at 0, 10, 20, 37, 45, and 50 °C, respectively, for 30 min in MRS-Ca–Mg broth. After centrifugation, the supernatants were assayed for unadsorbed phages, and the counts were compared with the titre of the control. The results were expressed as the percentage of adsorption.
Influence of Cell Viability on Phage Adsorption
Adsorption kinetics of phage Lcb on viable and nonviable cells were also determined to assay the ability of viable and nonviable cells to allow phage adsorption. Nonviable cells were obtained by keeping the cell suspension in boiling water for 5 min (Quiberoni et al. 2004). Nonviability of treated cells was checked by counting. Phages were added approximately at a moi of 0.02 and allowed to adsorb for 50 min at 30 °C. Every 10 min, the supernatants were assayed for unadsorbed phages by the double-layer agar plate method. The results were expressed as the percentage of adsorption.
Results
A virulent phage against the strain of L. casei 393 was isolated from Chinese sauerkraut and designated as Lcb. When the phage Lcb was incubated, clear plaques (0.8–1.2 mm in diameter) formed at 30 °C, and turbid and smaller ones formed at 37 °C. The phage was practically species-specific, attacking only the host strain. The other lactic acid bacteria used in the study were insensitive to this phage.
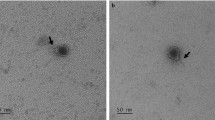
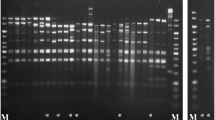
The phage Lcb has an isometric head of 60.2 ± 0.8 nm in diameter and a tail length of 251 ± 2.6 nm and a baseplate structure at the tip of the tail (Fig. 1). This is consistent with prior reports. Based on these properties, the phage should be assigned to the family Siphoviridae. These reflect the most frequently found morphologies among LAB phages (Villion and Moineau 2009). The restriction pattern of phage Lcb generated by digestion with HindIII is shown in Fig. 2. The mean size of phage Lcb genome was about 40 kb. When package site was investigated, heating of ligated (Fig. 2, lane 4) and not ligated (lane 3) restriction digests did not alter the restriction patterns. Other restriction enzymes gave similar results (data not shown). It indicated that the genome of phage Lcb did not contain cohesive ends, and it was packaged in a headful mechanism (pac-type).
Agarose gel electrophoresis of generated DNA fragments of phage Lcb by digestion with HindIII. Electrophoretogram showed the patterns of phage Lcb genome digestion by HindIII. The phage DNA digestion patterns produced by EcoRI, BamHI, PstI, and SalI showed the same result. Lane 1, HindIII/bacteriophage Lambda DNA marker. Lane 2, genomic DNA of phage Lcb. Lane 3, not ligated DNA of phage Lcb. Lane 4, ligated DNA of phage Lcb. Lane 5, 2000 bp DNA marker
Multiplication parameters of the lytic cycle of phage Lcb were determined from the one-step growth curve (Fig. 3). The latent period was about 75 min, followed by a relatively short burst period of 45 min, and the burst size was about 16 PFU per infected cell.
The effect of heat treatment on phage Lcb was determined by exposing phage to a range of temperatures for 50 min (Fig. 4a). The phage was observed to be heat-sensitive. The phage titre fell by approximately 4 log10 at 60 °C after treatment for 50 min. A fall of 4 log10 was obtained after 10 min at 70 °C, and complete inactivation was obtained after 30 min (a reduction of 6 log10). A treatment of 80 °C for 10 min was enough to inactivate the phages. Phage Lcb was irradiated with ultraviolet light for 50 min. The surviving phages were counted at 10 min intervals. As indicated in Fig. 4b, kinetics of inactivation followed the first-order reaction, and the reaction constant was 6.1 × 10−4 s−1. As indicated in Fig. 4c, d, little effect on the phages was observed in the presence of 10 and 25 % ethanol or isopropanol. 75 % ethanol or isopropanol produced a fast and complete inactivation of phages after 20 and 30 min of treatment, respectively (Fig. 4c, d). Both 100 % ethanol and isopropanol produced the fastest inactivation of the phages after 10 min of treatment (Fig. 4c, d). Phages were exposed to different pH values ranging from 2 to 12 at 30 °C for 30 min, prior to exposure to host cells. The phage maintained infectivity of approximately 6 log10 when challenged for 30 min at pH values of 4–11 (Fig. 4e). The titre of phage Lcb fell by 0.7 log10 at pH 11. At pH lower than 3 and higher than 12, the phages were inactivated completely. Phage Lcb was very sensitive to extreme pH values of 2, 3, and 12.
Influence of physicochemical agents on the viability of phage Lcb. a Thermal inactivation kinetics of phage Lcb at 25 °C (filled circle), 37 °C (filled square), 50 °C (filled triangle), 60 °C (open circle), 70 °C (open triangle), 80 °C (open square) in MRS-Ca–Mg broth. b Effect of ultraviolet irradiation on viability of phage Lcb. c Inactivation kinetics of phage Lcb with 10 % (filled circle), 25 % (filled square), 35 % (filled triangle), 50 % (open circle), 75 % (open triangle), 100 % (open square) of ethanol. d Inactivation kinetics of phage Lcb with 10 % (filled circle), 25 % (filled square), 35 % (filled triangle), 50 % (open circle), 75 % (open triangle), 100 % (open square) of isopropanol. e Effect of pH on viability of phage Lcb. For all the graphs, the results are shown as the mean ± standard deviation from three independent experiments
Cell lysis in MRS-Ca–Mg broth is faster than that in MRS-Ca or MRS-Mg broth which is faster than in MRS broth. Bigger plaques (0.8–1.2 mm) were formed on MRS-Ca–Mg, MRS-Ca, and MRS-Mg agar, and smaller ones (0.5–0.7 mm) were formed on MRS agar. The titre of phages was 1.5 × 106 PFU/ml in MRS broth, 4.1 × 107 PFU/ml in MRS-Mg broth, 6.3 × 107 PFU/ml in MRS-Ca broth, and 1.72 × 108 PFU/ml in MRS-Ca–Mg broth. These results indicated that Ca2+ or/and Mg2+ can promote clear plaque formation and improve phage titer. Adding both Ca2+ and Mg2+ was more effective than adding only Ca2+ or Mg2+ in improving phage titer.
The adsorption kinetics of phage Lcb on L. casei 393 are shown in Fig. 5a. It was observed that the adsorption of phage Lcb in MRS-Ca or MRS-Mg broth was faster than that in MRS broth. And all the adsorption rates in MRS, MRS-Ca, or MRS-Mg were achieved approximately 80 % after 60 min. By contrast, the adsorption in MRS-Ca–Mg broth was the fastest, and adsorption rate reached approximately 95 % after 60 min. Adsorption rates for phage Lcb at different temperatures on L. casei 393 are shown in Fig. 5b. It was observed that the adsorption process was influenced by the incubation temperature and that minimal rate of phage adsorption was attained at 0 °C (20 %). Adsorption rate reached 70–77 % at 10, 20, or 37 °C. In addition, it was found that the optimum temperature for phage Lcb was 30 °C, with an adsorption rate of 83.51 %. Figure 5c shows the phage adsorption kinetics on viable and nonviable cells. Phage particles were able to be adsorbed on nonviable cells. But a higher percentage of phage particles was adsorbed on viable cells compared with nonviable cells, this being significant at 50 min. At this time, only 38 % of phage particles were adsorbed on nonviable cells, while the adsorption percentage on viable cells was 91 %, approximately.
Influence of different parameters on the adsorption kinetics of phages Lcb on L. casei ATCC 393. a Adsorption kinetics of phage Lcb on cells of L. casei 393, in MRS-Ca–Mg (filled circle), MRS-Ca (filled square), MRS-Mg (filled triangle), and MRS (open circle) broth. b Influence of temperature on the adsorption of phage Lcb. c Adsorption kinetics of phage Lcb in MRS-Ca–Mg broth on viable (filled ellipse) and nonviable (filled square) cells of L. casei 393. For all the graphs, the results are shown as the mean ± standard deviation from three independent experiments
Discussion
Researches of lactic acid bacteria (LAB) phages have progressed significantly over the past decade. In this work, a novel virulent phage of L. casei ATCC 393 was isolated from Chinese sauerkraut. Further, its basic characteristics and the influence of environmental factors on it were studied, which supplied information and data for controlling phage infection on industrial strains and for understanding the interaction between the phage and host.
Examination by electron microscopy shows that phage Lcb has an isometric head of 60.2 ± 0.8 nm in diameter and a tail length of 251 ± 2.6 nm (Fig. 1). According to its morphology, phage Lcb belongs to the Siphoviridae family (morphotype B1) of International Committee on Taxonomy of Viruses (Villion and Moineau 2008). This reflects the most frequently found morphologies among LAB phages (Dieterle et al. 2014). But the sizes of head and tail are different from well-known L. casei phages, J-1 and PL-1. Next, we examined the host specificity of Lcb because the infection specificity of phages is generally species dependent. Lcb formed plaques only for L. casei 393, but could not form plaques against the other lactic acid bacteria tested in this study, suggesting that Lcb was specific to L. casei 393 and that might have not the common and conservative phage receptor among the other species. The mean size of the phage genome is about 40 kb. To date, the phage genomes of 24 Lactobacillus phages have been fully sequenced (http://www.ebi.ac.uk/genomes/phage.html), and their genetic complexity is clear with genome sizes ranging from 31 to 42 kb. The restriction pattern of phage Lcb turned out to be completely different from that of other L. casei phages (Shimizu-Kadota et al. 1983; Capra et al. 2004). From the restriction pattern, it was concluded that phage Lcb was packaged in a headful mechanism. The differences in the phenotypic and genotypic features of the phage Lcb demonstrate that it represents a novel L. casei phage.
The infection cycle of phage Lcb was investigated by performing one-step growth curve, and multiplication parameters of phage Lcb were determined. The latent period was about 75 min, which was shorter than most of those have been reported (Watanabe et al. 1982; Herrero et al. 1994), but similar to that observed for the group phage LL-H (Alatossava et al. 1991). The burst size was about 16 PFU per infected cell, which was also smaller than most of those have been reported (Eoghan et al. 2015).
To explore effective control measures to minimize the problems derived from phage attacks, it is significant to investigate the resistance of phage Lcb to physical and chemical agents. In this work, phage Lcb showed high sensitivity to thermal treatment. Phage Lcb was stable below 50 °C and almost completely inactive at 70 °C after 20 min, which was similar to PL-1 (Watanabe et al. 1970). It is consistent with that reported by Capra et al. (2004) that L. casei and L. paracasei phages were the most sensitive to temperature of all the phages studied. Regarding the sensitivity to ultraviolet irradiation, phage Lcb showed more sensitivity (the reaction constant of 6.1 × 10−4 s−1) than PL-1 (the reaction constant of 4.4 × 10−2 s−1). The results obtained from ethanol and isopropanol treatments showed that a treatment of 75 % ethanol for 20 min and 75 % isopropanol for 30 min was enough to achieve a complete loss of viability, but some other L. casei phages (Capra et al. 2004) showed high resistance to ethanol and isopropanol. Only ethanol 100 % was effective against these phages. Isopropanol showed to be extremely harmlessness and was not efficient to inactivate the phages (Ebrecht et al. 2010). Furthermore, the stability of phage Lcb at a pH ranging from 2 to 12 was examined. The results revealed that it was stable between pH 4 and 10. At pH lower than 3 and higher than 12, the phages were inactivated completely. Similar results were obtained from L. casei phages, J-1 and PL-1. (Capra et al. 2006). These results allow us to hypothesize that these extreme pH values could have a great contribution on phage inactivation.
According to Lu et al. (2003), requirement for Ca2+ or Mg2+ not only stabilized the coiled DNA inside the phage capsid, but also controlled the penetration of phage DNA into the cells. Therefore, Ca2+ or Mg2+ is essential for proliferation of phages. In this work, the effect of Ca2+ and Mg2+ on phage adsorption was investigated. The results showed that Ca2+ and Mg2+ were not indispensable for the completion of the lytic cycle, but they were indispensable to improve complete lysis and plaque formation. It is different from L. casei phage PL-1 that visible lysis plaques were produced only when Ca2+ or Mg2+ was added (Capra et al. 2006). For other lactobacilli phages, the requirement of Ca2+ for lytic cycle was variable (Lu et al. 2003; Quiberoni et al. 2004; Walakira et al. 2008).
The first step in the interaction between a lytic phage and its host is when the phages adsorb on the cell surface. However, the information available on the absorption of L. casei phage has been still scarce since it was only reported for phage PL-1 and J-1 (Capra et al. 2006). Here, we investigated the effect of some physical and chemical factors on phage Lcb adsorption. Firstly, Ca2+ and Mg2+ exhibited slight impact on its adsorption. And for phage Lcb, adsorption occurred even at 0 °C and was optimal at 30 °C. But PL-1/L. casei 393 maintained the adsorption rates along the temperature range studied evidencing its complete independence from temperature (Capra et al. 2006). System PL-1/L. paracasei A presented its maximum adsorption rate at 37 °C. Others (Watanabe and Takesue 1972) observed for cells of L. paracasei ATCC2709 incubated with PL-1 that the adsorption process was independent from temperature between 0 and 40 °C. The effect of temperature on both PL-1 and J-1 is different from that on phage Lcb. This also demonstrated that the effect of temperature on adsorption may depend on the phage/strain system. In addition, the ability of thermally killed cells allowing adsorption of phage Lcb was also observed. The results indicated that the adsorption of phage Lcb was independent on physiological state of cells, and the phage receptors were thermostable, and similar result was obtained for phages such as LL-H, BYM, YAB, and Ib3 (Quiberoni et al. 2004). However, for both PL-1 and J-1, significant differences were observed for adsorption rates in comparison with a control of untreated cells, which indicated that adsorption was dependent on the physiological state of cells and/or the phage receptors might have a thermosensitive nature.
In conclusion, phage Lcb is different from those previously reported L. casei phages in both phenotypic and genotypic features, which demonstrates that it represents a novel L. casei phage. Moreover, this study provided some knowledge on this novel L. casei phage, which can allow a better knowledge and give useful information to outline more effective treatments to reduce the phage infections in dairy industry. These results also make a better understanding of phage–host interactions. In the future, phage genomes can be exploited to develop genetic tools for industrial purposes.
References
Alatossava, T., Forsman, P., Mikkonen, M., & Vasala, A. (1991). Molecular biology of Lactobacillus phage LL-H. Finn Journal of Dairy Science, 49, 1–13.
Barrangou, R., & Horvath, P. (2012). CRISPR: new horizons in phage resistance and strain identification. Annual Review of Food Science and Technology, 3, 143–162.
Brussow, H., & Hendrix, R. W. (2002). Phage genomics: Small is beautiful. Cell, 108(1), 13–16.
Brussow, H., & Suarez, J. E. (2006). Lactobacillus phages. In R. Calendar & S. T. Abedon (Eds.), The bacteriophages (pp. 653–666). New York: Oxford University Press.
Cai, H., Thompson, R., Budinich, M. F., Broadbent, J. R., & Steele, J. L. (2009). Genome sequence and comparative genome analysis of Lactobacillus casei: Insights into their niche-associated evolution. Genome Biology Evolution, 1, 239–257.
Capra, M. L., Quiberoni, A., & Reinheimer, J. A. (2004). Thermal and chemical resistance of Lactobacillus casei and Lactobacillus paracasei bacteriophages. Letters in Applied Microbiology, 38(6), 499–504.
Capra, M. L., Quiberoni, A., & Reinheimer, J. (2006). Phages of Lactobacillus casei/paracasei: Response to environmental factors and interaction with collection and commercial strains. Journal of Applied Microbiology, 100(2), 334–342.
Casey, E., Mahony, J., Neve, H., Noben, J.-P., Dal Bello, F., & van Sinderen, D. (2015). Novel phage group infecting Lactobacillus delbrueckii subsp. lactis, as revealed by genomic and proteomic analysis of bacteriophage Ldl1. Applied and Environmental Microbiology, 81(4), 1319–1326.
Czajkowski, R., Ozymko, Z., de Jager, V., Siwinska, J., Smolarska, A., Ossowicki, A., et al. (2015). Genomic, proteomic and morphological characterization of two novel broad host lytic bacteriophages ΦPD10.3 and ΦPD23.1 infecting Pectinolytic Pectobacterium spp. and Dickeya spp. PLoS One, 10(3), e0119812.
Danis-Wlodarczyk, K., Olszak, T., Arabski, M., Wasik, S., Majkowska-Skrobek, G., Augustyniak, D., et al. (2015). Characterization of the newly isolated lytic bacteriophages KTN6 and KT28 and their efficacy against Pseudomonas aeruginosa biofilm. PLoS One, 10(5), e0127603.
Desiere, F., Mahanivong, C., Hillier, A. J., Chandry, P. S., Davidson, B. E., & Brüssow, H. (2001). Comparative genomics of lactococcal phages: Insight from the complete genome sequence of Lactococcus lactis phage BK5-T. Virology, 283(2), 240–252.
Dieterle, M. E., Bowman, C., Batthyany, C., Lanzarotti, E., Turjanski, A., Hatfull, G., & Piuri, M. (2014). Exposing the secrets of two well-known Lactobacillus casei phages, J-1 and PL-1, by genomic and structural analysis. Applied and Environmental Microbiology, 80(22), 7107–7121.
Ebrecht, A. C., Guglielmotti, D. M., Tremmel, G., Reinheimer, J. A., & Suárez, V. B. (2010). Temperate and virulent Lactobacillus delbrueckii bacteriophages: Comparison of their thermal and chemical resistance. Food Microbiology, 27(2010), 515–520.
Galdeano, C. M., de LeBlanc, A. D. M., Vinderola, G., Bonet, M. E., & Perdigon, G. (2007). Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clinical and Vaccine Immunology, 14(5), 485–492.
Garcia, P., Ladero, V., & Suarez, J. E. (2003). Analysis of the morphogenetic cluster and genome of the temperate Lactobacillus casei bacteriophage A2. Archives of Virology, 148(6), 1051–1070.
Hendrix, R. W. (2003). Bacteriophage genomics. Current Opinion in Microbiology, 6(5), 506–511.
Herrero, M., de los Reyes-Gavilán, C. G., Caso, J. L., & Suárez, J. E. (1994). Characterization of ø393-A2, a bacteriophage that infects Lactobacillus casei. Microbiology, 140(10), 2585–2590.
Hultberg, A., Tremblay, D. M., de Haard, H., Verrips, T., Moineau, S., Hammarström, L., & Marcotte, H. (2007). Lactobacilli expressing llama VHH fragments neutralise Lactococcus phages. BMC Biotechnology, 58(7), 1–7.
Jones, D. T., Shirley, M., Wu, X., & Keis, S. (2000). Bacteriophage infections in the industrial acetone butanol (AB) fermentation process. Journal of Molecular Microbiology and Biotechnology, 2(1), 21–26.
Kawase, M., He, F., Kubota, A., Harata, G., & Hiramatsu, M. (2010). Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Letters in Applied Microbiology, 51(2010), 6–10.
Lo, T. C., Shih, T. C., Lin, C. F., Chen, H. W., & Lin, T. H. (2005). Complete genomic sequence of the temperate bacteriophage PhiAT3 isolated from Lactobacillus casei ATCC 393. Virology, 339(1), 42–55.
Lu, Z., Breidt, F., Fleming, H. P., Altermann, E., & Klaenhammer, T. R. (2003). Isolation and characterization of a Lactobacillus plantarum bacteriophage, ΦJL-1, from a cucumber fermentation. International Journal of Food Microbiology, 84(2), 225–235.
Marcó, M. B., Garneau, J. E., Tremblay, D., Quiberoni, A., & Moineau, S. (2012). Characterization of two virulent phages of Lactobacillus plantarum. Applied and Environmental Microbiology, 78(24), 8719–8734.
Marcó, M. B., Garneau, J. E., Tremblay, D., Quiberoni, A., & Moineau, S. (2015). Characterization of two virulent phages of Lactobacillus plantarum. Applied and Environmental Microbiology, 78(24), 8719–8734.
Marranzino, G., Villena, J., Salva, S., & Alvarez, S. (2012). Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestine altract. Microbiology and Immunology, 56(11), 771–781.
Mercanti, D. J., Ackermann, H.-W., & Quiberoni, A. (2015). Characterization of two temperate Lactobacillus paracasei bacteriophages: Morphology, kinetics and adsorption. Intervirology, 58, 49–56.
Moineau, S., & Levesque, C. (2005). Control of bacteriophages in industrial fermentations. In E. Kutter & A. Sulakvelidze (Eds.), Bacteriophages: Biology and applications (pp. 285–296). Boca Raton: CRC Press.
Moscoso, M., & Suárez, J. E. (2000). Characterization of the DNA replication module of bacteriophage A2 and use of its origin of replication as a defense against infection during milk fermentation by Lactobacillus casei. Virology, 273(1), 101–111.
Pringsulaka, O., Patarasinpaiboon, N., Suwannasai, N., Atthakor, W., & Rangsiruji, A. (2011). Isolation and characterisation of a novel Podoviridae phage infecting Weissella cibaria N 22 from Nham, a Thai fermented pork sausage. Food Microbiology, 28(3), 518–525.
Quiberoni, A., Guglielmotti, D., Binetti, A., & Reinheimer, J. (2004). Characterization of three Lactobacillus delbrueckii subsp. bulgaricus phages and the physicochemical analysis of phage adsorption. Journal of Applied Microbiology, 96(2), 340–351.
Rochat, T., Bermudez-Humaran, L., Gratadoux, J.J., Fourage, C., Hoebler, C., Corthier, G., & Langella, P. (2007). Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependent catalase on DSS-induced colitis in mice. Microbial Cell Factories. http://www.biomedcentral.com/content/pdf/1472-6750-7-58.pdf. Accessed 17 September 2007.
Shimizu-Kadota, M., Sakurai, T., & Tsuchida, N. (1983). Prophage origin of a virulent phage appearing on fermentations of Lactobacillus casei S-1. Applied and Environmental Microbiology, 45(2), 669–674.
Sturino, J. M., & Klaenhammer, T. R. (2006a). Engineered bacteriophage-defence systems in bioprocessing. Nature Reviews Microbiology, 4(5), 395–404.
Sturino, J. M., & Klaenhammer, T. R. (2006b). Engineered bacteriophage-defence systems in bioprocessing. Nature Reviews Microbiology, 4(5), 395–404.
Suárez, V., Zago, M., Giraffa, G., Reinheimer, J., & Quiberoni, A. (2009). Evidence for the presence of restriction/modification systems in Lactobacillus delbrueckii. Journal of Dairy Research, 76(4), 433–440.
Trucco, V., Reinheimer, J., Quiberoni, A., & Sua ´rez., V. B. (2011). Adsorption of temperate phages of Lactobacillus delbrueckii strains and phage resistance linked to their cell diversity. Journal of Applied Microbiology, 110, 935–942.
Villion, M., & Moineau, S. (2008). Bacteriophages of lactobacillus. Frontiers in bioscience (Landmark edition), 14, 1661–1683.
Villion, M., & Moineau, S. (2009). Bacteriophages of lactobacillus. Front Bioscience, 14, 1661–1683.
Walakira, J. K., Carrias, A. A., Hossain, M. J., Jones, E., Terhune, J. S., & Liles, M. R. (2008). Identification and characterization of bacteriophages specific to the catfish pathogen, Edwardsiella ictaluri. Journal of Applied Microbiology, 105(6), 2133–2142.
Wang, S., Kong, J., Gao, C., Guo, T., & Liu, X. (2010). Isolation and characterization of a novel virulent phage (phiLdb) of Lactobacillus delbrueckii. International Journal of Food Microbiology, 137(1), 22–27.
Watanabe, K., Takesue, S. (1972). The requirement for calcium in infection with Lactobacillus phage. The Journal of general virology, l17, 19–30.
Watanabe, K., Takesue, S., Ishibashi, K., & Nakahara, S. (1982). A computer simulation of the adsorption of Lactobacillus phage PL-1 to host cells: Some factors affecting the process. Agricultural and Biological Chemistry, 46(3), 697–702.
Watanabe, K., Takesue, S, Jin-Nai, K. & Yoshikawa, T. (1970). Bacteriophage active against the lactic acid beverage-producing bacterium Lactobacillus casei. Applied microbiology, 20, 409–415.
Zago, M., Scaltriti, E., Rossetti, L., Guffanti, A., Armiento, A., Fornasari, M. E., & Giraffa, G. (2013). Characterization of the genome of the dairy Lactobacillus helveticus bacteriophage ΦAQ113. Applied and Environmental Microbiology, 79(15), 4712–4718.
Zago, M., Scaltriti, E., Rossetti, L., Guffanti, A., Armiento, A., Fornasari, M. E., et al. (2015). Characterization of the genome of the dairy Lactobacillus helveticus bacteriophage ΦAQ113. Applied and Environmental Microbiology, 81(4), 1319–1326.
Zhongjing, Lu, & Breidt, Fred. (2015). Escherichia coli O157:H7 bacteriophage Φ241 isolated from an industrial cucumber fermentation at high acidity and salinity. Frontiers in Microbiology, 6(67), 1–10.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (No. 31302127) and “Young Talents” Project of Northeast Agricultural University (14QC22). We would like to thank Prof. Jos Seegers for kindly providing the L. casei ATCC 393.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Lan, Y., Jiao, W. et al. Isolation and Characterization of a Novel Virulent Phage of Lactobacillus casei ATCC 393. Food Environ Virol 7, 333–341 (2015). https://doi.org/10.1007/s12560-015-9206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9206-4