Abstract
Purpose
The study of long-term radiation effects has become important due to the growing survival rates among cancer patients. Brachytherapy treatment planning systems do not account for radiation doses remote from the irradiated regions. The use of Monte Carlo (MC) simulation for the evaluation of scattered radiation doses received by other parts of the body of patients undergoing gynaecological brachytherapy has therefore become crucial to avert the causation of secondary cancers. This study compares two methods (MC simulation and TLD measurements) used to estimate scattered radiation doses received by other parts of the body (breast and thyroid) of patients undergoing gynaecological brachytherapy.
Method
100 patients undergoing HDR intracavitary brachytherapy were randomly selected and TLDs placed on their breast and thyroid region during treatment delivery. The patient treatment was modelled with SimpleGeo for the considered cancer types and simulated with MCNP using a predefined patient model based on the mean BMI of patients involved in this study.
Result
Average MC simulation doses were 2.65 ± 0.07 mSv and 8.62 ± 0.03mSv to thyroid and breast, respectively. The TLD measurements were 2.76 ± 0.14 mSv and 8.73 ± 0.09 mSv to the thyroid and breast, respectively. P-values of 0.64 for thyroid and 0.43 for breast (p > 0.05) were recorded.
Conclusion
P-values reveal an insignificant difference between measured and simulated doses. It is recommended that the verified MC models be used to develop an algorithm for organ dose estimation of other parts of the body (i.e. organs not considered by the treatment planning system).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Advances in brachytherapy (BT), over the years have increased the life expectancy of cancer patients [1, 2]. Regardless, life expectancy after BT is still a major concern due to certain long-term side effects and in some instances, the development of secondary cancers in other organs of patients after the procedure [3, 4].
One important advancement in the field of BT is the use of sophisticated treatment planning systems (TPS) to estimate dose distributions inside a patient’s body and calculate doses to the target and organs in proximity to the target being treated with high accuracy. However, the doses received by organs further away from the irradiated area are not accurately calculated by most commercial TPS [5,6,7]. This inaccuracy may lead to an underestimation of the dose by up to 40% [8] Additionally, dose calculations outside the irradiated area are often restricted by the limited anatomical coverage of the computed tomography (CT) simulation images used for the treatment planning during BT. Another cardinal point is that the doses outside the irradiated area vary with delivery techniques, treatment site, patient dimensions, and energy spectrum, hence making it challenging to estimate the doses [9].
Radiation dose to healthy tissues during gynaecological BT may lead to various side effects that may occur immediately after treatment or later [10, 11]. Studies in BT have shown a significant amount of dose to other organs of patients due to internal scatter within the patient’s body during BT [12,13,14,15,16,17]. The internally scattered radiation has the potential to contribute doses to any part of the patient’s body, either within or outside the irradiated area. Even though doses outside the irradiated region may be low compared to the doses within the irradiated area, they are of radiobiological interest. This is because they are administered to a considerable region of the healthy organs and tissues which may lead to new tumours or an increased chance of the occurrence of secondary cancers induced by ionizing radiation [18].
Monte Carlo N-Particle (MCNP) calculations for dose estimations in brachytherapy have become crucial and increased almost exponentially in the last decades due to the inability of the treatment planning systems to accurately account for doses outside the irradiated area [19,20,21,22]. Thermoluminiscence dosimeters (TLD) have also been available for nearly 100 years in the use of dosimetry for ionizing radiation [23].
This study therefore seeks to compare two methods (MC simulation and TLD measurements) used to estimate scattered radiation doses received by other parts of the body (breast and thyroid) of patients undergoing gynaecological brachytherapy using a cobalt-60 source. The MCNP computational simulation from this work can also be used to assess doses to other parts of the body during gynaecological brachytherapy in future studies. Parts of the body considered for this study were the breast and thyroid regions due to their sensitivities to radiation [24, 25].
2 Materials and methods
Ethical clearance (ECBAS 002/21–22) was sought from the College of Basic and Applied Sciences (CBAS) before the study commenced. Additionally, approval was also sought from the Cancer Center to use their Brachytherapy Unit for the study, and informed consent was obtained from all individual participants included in the study.
2.1 Thermoluminescence dosimeter calibration
Thermoluninescence dosimeters (TLDs) were used for dose measurements in this study as described in the work by Doudoo et al. (2023) [26]. Before the use of the TLDs (TLD-100 LiF, Thermofisher, Germany) for this study, a sensitivity test was performed to establish the dose as a function of the TLD reading. All the TLDs were irradiated to a dose of 2 Gy at depth of 5 cm within a solid water phantom (RW3; PTW Freiburg, Germany) using collimator settings of 10 cm × 10 cm and SSD of 100 cm with a 6 MV beam from a Synergy platform medical linear accelerator (Elekta, Sweden). TLDs with a sensitivity response falling within ± 3% of the given dose were considered for this study. All TLDs used in this study (for calibration and organ dosimetry) were read within 24 hours’ post-irradiation to allow them to settle to give a more reproducible result and to minimize fading effect.
Additionally, a dose rate dependence test was done where TLDs were irradiated with a known dose of 2 Gy for dose rates in the range 100–700 Gy/min for 6 MV energy. A graph of thermoluminescence (TL) count was plotted against dose rate and represented in Fig. 1. The results, displayed in Fig. 1 indicates a very weak trend towards a slightly increased yield with dose rate. This weak trend towards increased dose rate reveals the independence of the TLDs on dose rate.
2.2 Treatment planning and physical dosimetry
One hundred (100) gynaecological cancer (i.e., cervical, vagina, endometrial) patients undergoing HDR BT were intentionally selected based on their availability using a homogenous non-probability sampling method (purposive sampling) at the cancer facility. This was a prospective study within a 3-year period (2019 to 2023). Patients’ weight and height were collected from the hospital information system to obtain their body mass index (BMI). Information on the choice of applicators to use and their orientations within patients were determined by a Radiation Oncologist, and the applicators were inserted under local anaesthesia.
The patients were transferred to the computed tomography (CT) room for the acquisition of simulation images using the Siemens CT machine (Emotion 16, Siemens, Germany) to verify patient position and applicator position and also to be used for planning. The acquired diagnostic images were reconstructed to a 2 mm slice thickness and exported to the Oncentra treatment planning system (Monaco, Elekta, Sweden) to plan the dose delivery to the concerned target volume.
The Oncentra treatment planning system (OTPS) was used to create 3D conformal treatment plans for all the gynaecological patients involved in this study. An average dose of 7 Gy per fraction in 4 fractions were prescribed and evaluated quantitatively for the target volume as well as organs at risk (OARs). The high-risk clinical target volume (HR-CTV) was prescribed and defined as the area of gross residual disease at the time of brachytherapy. The parameters of the 3D treatment planning system were used for evaluation purposes and the planning modalities followed the standard protocols in use at the center.
Applicators for brachytherapy consisted of a 30° intrauterine and 20 mm small ovoid Fletcher System (Tandem and ovoid pair), vaginal cylinders as well as tandem and ring applicators (Nucletron BV, Netherlands).
The cumulative dose volume histogram (DVH) was computed for critical organs like the bladder, rectum and bowel (including sigmoid), and the dwell positions and the dwell times were adjusted to optimally minimize dose to these critical organs. Samples of HDR Brachytherapy treatment plans created for some of the patients of the study are shown in Fig. 2; where ‘A’ is a plan with vaginal applicator and ‘B’ is one with a tandem and ovoid.
The treatment plans were then exported to the HDR-BT Cobalt-60 Afterloader machine for treatment to commence. Calibrated Lithium Fluoride (LiF) thermoluminescence dosimeters (TLDs) [27] were placed on the thyroid and breast of the patient with the help of a masking tape. The Radiation Oncologist used his knowledge in anatomy to place the TLD at the front of the neck just below the base of the throat, which is where the thyroid gland is anatomically located. For the breast, TLDs were put on both breasts and average found during each insertion.
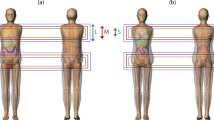
The dose measurements were taken by a team of Radiation therapy professionals and under the supervision of the Oncologist. The patient couch was positioned in the central part of the room to prevent scatter radiation influence from the walls if any. After dose delivery, the TLDs were read using a Harshaw TLD reader (6600 Plus, ThermoFisher, USA). This was repeated for all fractions for every patient (at most 4 fractions) and the average was documented in a data entry sheet. Figure 3 illustrates a schematic diagram of patient positioning and TLD positions during the HDR gynaecological cancer treatment.
The characterization of the LiF TLD makes it viable for the measurement of out-of-field radiation doses [28] as well as its use for the measurement of internal scatter radiation doses [29]. To ensure a high-quality performance of the TLDs, they were calibrated with a 6 MV photon energy. TLDs were exposed to different doses from 0 to 8 Gy of 6 MV photons. A graph of the Thermoluminescence (TL) signals was plotted against the various doses to produce a calibration curve as shown in Fig. 4. The calibration curve in this study had a linear behavior under 10 Gy which is in agreement with other studies [30, 31].
2.3 Modelling and monte carlo simulation
The patient, applicators, TLDs, the BT source and patient table were modelled using SimpelGeo, a three-dimensional (3D) modelling tool (Version 4.3.3, CERN, Switzerland). The geometry of the models was created by employing an interactive point, click, drag and drop technique using basic primitives and shapes in the constructive solid geometry (CSG) tree. The patient was modelled as a perspex whole body (not organs specific) phantom with an average BMI of 27.5 kg/m2 [26]. The applicators were modelled as an alloy of titanium (90%), aluminium (6%) and vanadium (4%). The TLDs were modelled as thin films of LiF.
The patient table was also modelled as steel with a carbon cover material. The BT source was modelled as a cobalt-60-point source with an average energy of 1.25 MeV. The models were outputted from the SimpelGeo tool to the MCNP simulation input file.
The energy tally of F6 (energy absorbed per mass per particle) was set on the TLDs. The input file was simulated to track 1.5 × 109 gamma particles. The models were validated with the TLD-measured organ doses as recommended by the American Association of Physicists in Medicine (AAPM) Task Group 158 [32]. Figure 4 is an illustration of the modelled patient in this study using the SimpleGeo software.
2.4 Statistical analysis
Measured and simulated doses for all the identified cancer sites were transferred from Microsoft Excel and analysed using the Statistical Package software for the Social Sciences, SPSS (Version 25, IBM, U.S.A). The paired T-test method was used to compare the means of the measured doses to that of the simulated doses to determine whether there was statistical evidence between the average values. A confidence interval of 95% (p < 0.05) was used to show significant relationships.
3 Results
Table 1 shows mean measured doses of the thyroid and breast for the various cancer types from the work by Doudoo et al. (2023) [26]. Table 2 shows mean simulated patient organ doses of the thyroid and breast for the various gynaecological cancer types involved in this work.
The measured doses provided in Table 1 recorded average doses of 2.76 ± 0.14 mSv and 8.73 ± 0.09 mSv at the thyroid and the breast, respectively. On the other hand, the simulated doses provided in Table 2 recorded average doses of 2.65 ± 0.07 mSv and 8.62 ± 0.03 mSv at the thyroid and breast, respectively.
Table 3 provides the comparison of statistical t-tests conducted for measured and simulated doses for the thyroid. Table 4 also provides the comparison of statistical t-tests conducted for measured and simulated doses for the breast. A p-value of 0.64 (p-value > 0.05) was obtained for the thyroid comparison whilst a p-value of 0.43 (p-value > 0.05) was obtained for the breast comparison.
Figure 1 is a graph of Thermoluminescence (TL) counts against dose rate. A schematic diagram to illustrate the BT setup and the placement of TLDs on a patient for this study is shown in Fig. 5. Figure 3 is a calibration curve of TLDs exposed to 6 MV photon beam and Fig. 4 is a modelled patient on the treatment table using the SimpleGeo software.
Lastly, Fig. 6 presents the comparison between measured and simulated doses for the organs considered for this study.
A B.
4 Discussion
This work was conducted in a clinical environment in a radiotherapy centre. It primarily compared TLD measurements and MC simulations of radiation doses received by other parts of the body during gynaecological brachytherapy. For this work, the breast and thyroid regions were studied due to the sensitivity of the organs (breast and thyroid) within the area to radiation as well as being outside the irradiated area during HDR gynaecological brachytherapy treatment with Cobalt-60 source.
The MC approach using MCNP6 was used and validated with TLD measured doses for the organs in the region studied. It must be noted that the breast and thyroid are organs situated just beneath the skin and since they are located only a few millimetres from the skin surface, one may estimate that the dose absorbed by the TLD (either simulated or measured) placed on the surface of the anatomical position is the dose absorbed by the organs [33, 34]. Therefore, the doses measured or simulated by the TLDs in this study during the various treatments provide reliable estimates of the absorbed organ doses. In addition, TLDs had successfully been used for the measurement of doses to organs out of the irradiated area since scatter radiation is dominant in these areas during brachytherapy and not the primary radiation [35]”.
It can be seen from Tables 1 and 2; Fig. 6 that endometrial cancers recorded a higher dose to the breast and thyroid compared with the other cancer types. Vaginal and cervical cancers were the second and third highest, respectively. The variation in the doses in these areas or organs could be attributed to their proximity to the cancer sites.
The location of the endometrium is anatomically proximal to the breast and thyroid regions as compared to the cervix and vagina. However, the same reasoning could not be made for the vagina and cervical as they are approximately (on average about 7.6 cm apart) located around the same place in the body. Hence, the variation in the doses could not primarily be as a result of the distance between them and the studied organs. This could be attributed to the dose distribution around the choice of applicator used for the cervix (Tandem and ovoid) as against that of the vagina (cylindrical) cases.
The percentage difference between the measured and simulated average doses for the thyroid and breast was 2.0% and 0.7%, respectively. The measured average dose at the thyroid was higher by a factor of 1.04 compared to the simulated average dose resulting in a percentage deviation of 4%. The measured average dose at the breast was higher by a factor of 1.01 compared to the simulated average dose resulting in a percentage deviation of 1.3%. The difference in the measured and simulated average doses could be attributed to possible sampling error from the placements of the TLDs during the process, the use of perspex phantom to represent the patient for the simulation and possible errors in the TLDs position in the model as compared with the physical position. However, the results of this study corroborate with several other studies [36,37,38,39] that also performed physical measurements against simulated measurements resulting in a percentage deviation of 2–5%. It must be noted that most of the other studies compared with this study focused on organs or areas that are a few centimetres from the irradiated area whereas this study focused on organs further away from the irradiated area.
It can also be seen from Tables 1 and 2 that the dose to the breast region was more than that to the thyroid region by an average factor of 3.16 and 3.26 for measured and simulated approaches, respectively. This could be attributed to the proximity of the organs from the irradiated area and the dominance of the influence of dose by the inverse square law in brachytherapy [40].
Additionally, both p-values (p-value of 0.64 for thyroid and 0.43 for breast) obtained from the paired t-test statistical analysis as shown in Tables 3 and 4 of this work were all greater than 0.05 indicating an insignificant difference between measured and simulated doses at the thyroid as well as measured and simulated doses at the breast. One can therefore conclude that there is no significant difference between the measured TLD doses and the doses from the MCNP computational simulation.
Therefore, the computational simulation approach provided by this study could be used in further studies to evaluate doses outside the irradiated area during gynaecological brachytherapy at the radiotherapy centre. Further studies on dose reduction techniques for organs or regions out of the irradiated area may be proposed using the computational model of this study for clinical approval. This will enhance the dose optimization of patients in radiotherapy as recommended by ICRP. Further studies are also recommended to verify the MC model with doses from a 3-D TPS planning system.
Lastly, as a potential limitation of this work, there is a possibility of an overestimation of the measured absorbed dose, since a higher energy (6MV) was used for calibrating the TLD for a low energy (HDR Cobalt-60 source) during this study and this has been corroborated by other studies [41]. To this end, an average energy correction factor of 1.005 obtained from Lee et al. (2015) [42] was used to correct the TLD readings calibrated with a 6MV photon energy relative to a Cobalt 60 beam for the brachytherapy treatments. Using the energy correction factor, a percentage deviation of 0.49% was noted for the TLD readings at the thyroid and breast regions.
The results showed less than 0.5% correction which compares well with other studies [43, 44]. This is an acceptable deviation for Radiation therapy applications where accuracy is expected to be less than a 3–5% deviation [45].
5 Conclusion
The observed percentage deviation between the simulated dose and measured doses at the thyroid and breast regions compared well with each other. This also compared well with that of other studies. The MC computational approach developed and validated by this study for assessing dose to organs out of the irradiated area is expected to be incorporated into the gynaecological brachytherapy treatment protocol of the facility to aid in assessing distal organ doses. This will go a long way to strengthen patient protection in radiotherapy at the radiotherapy centre.
Data availability
Not applicable.
Code availability
Not applicable.
References
Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. Int J Women’s Health. 2014;6:555–64. https://doi.org/10.2147/IJWH.S46247.
Mendez LC, Morton GC. High dose-rate brachytherapy in the treatment of prostate cancer. Translational Androl Urol. 2018;7(3):357–70. https://doi.org/10.21037/tau.2017.12.08.
American Cancer Society. Second Cancer Related to Treatment. (2020) (https://www.cancer.org/treatment/survivorship-during-and-after-treatment/long-term-health-concerns/second-cancers-in-adults/treatment-risks.html). [accessed 15 April, 2022].
Lee B, Ahn SH, Kim H, Son J, Sung J, Han Y, Huh SJ, Kim JS, Kim DW, Yoon M. Secondary cancer-incidence risk estimates for external radiotherapy and high-dose-rate brachytherapy in cervical cancer: phantom study. J Appl Clin Med Phys. 2016;17(5):124–32. https://doi.org/10.1120/jacmp.v17i5.6087.
Das IJ, Cheng CW, Watts RJ, Ahnesjo A, GibbonsJ, Li XA, TG-106 of the Therapy Physics Committee of the AAPM, Rosen II, Jessie Y, David S, Xin A, Stephen F. (2008). Accelerator beam data commissioning equipment and procedures: report of the. Medical Physics, 35 (9):4186–215.
Starkschall G, Steadham RE Jr, Popple RA, Ahman S &. (2000). Beam-commissioning methodology for a three-dimensional convolution/superposition photon dose algorithm. Journal of Applied Clinical Medical Physics,1(1):8–27.
Jessie Y, David S, Xin A, Stephen F. (2003). Accuracy and sources of error of out-of field dose calculations by a commercial treatment planning system for intensity modulated radiation therapy treatments. Journal of Applied Clinical Medical Physics, 14(2):4139.
Farhood B, Ghorbani M. Dose Calculation Accuracy of Radiotherapy Treatment Planning Systems in Out-of-Field Regions. J Biomed Phys Eng. 2019;9(2):133–136.
Fonseca GP, Johansen JG, Smith RL, Beaulieu L, Beddar S, Kertzscher G, Verhaegen F, Tanderup K. (2020). In vivo dosimetry in brachytherapy: Requirements and future directions for research, development, and clinical practice. Phys Imaging Radiat Oncol, 28;16:1–11. https://doi.org/10.1016/j.phro.2020.09.002
Paganetti H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys. 2012;103:652–61. https://doi.org/10.1097/HP.0b013e318261113d.
Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, Hall P, Langmark F, Pukkala E, Kaijser M, Andersson M, Fossa SD, Joensuu H, Boice JD, Kleinerman RA, Travis LB. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–43.
Ohno T, et al. Long-term survival and risk of second cancers after Brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:740–5.
Mazzone FA et.al. Long-term incidence of secondary bladder and rectal cancer in patients treated with brachytherapy for localized prostate cancer: a large-scale population-based analysis. Br J Urol. 2019;24(6):1006–13.
International Commission on Radiological Protection. (2005). Prevention of High Dose rate brachytherapy accidents. ICRP Publication 97 Ann ICRP, 35(2).
Liao Y, Dandekar V, Chu JC, Turian J, Bernard D, Kiel K. Reporting small bowel dose in cervix cancer high-dose-rate brachytherapy. Med Dosim. 2016;41(1):28–33. Epub 2015 Jul 30. PMID: 26235549.
Lee YC, Hsieh CC, Li CY, Chuang JP, Lee JC. Secondary cancers after radiation therapy for primary prostate or rectal cancer. J Gen Surg. 2016;40(4):895–905.
Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–8.
Taylor ML, Kron T. Consideration of the radiation dose delivered away from the treatment field to patients in radiotherapy. J Med Phys. 2011;36(2):59–71. https://doi.org/10.4103/0971-6203.79686.
Duggan DM. Improved radial dose function estimation using current version MCNP Monte-Carlo simulation: Model 6711 and ISC3500 125I brachytherapy sources. Appl Radiat Isot. 2004;61(6):1443–50. https://doi.org/10.1016/j.apradiso.2004.05.070.
Andreo P. (2018). Monte Carlo simulations in radiotherapy dosimetry. Radiat Oncol, 27;13(1):121. https://doi.org/10.1186/s13014-018-1065-3
Brualla L, Rodriguez M, Lallena AM. Monte Carlo systems used for treatment planning and dose verification. Strahlenther Onkol. 2017;193(4):243–59. https://doi.org/10.1007/s00066-016-1075-8.
Werner ChJ, Bull JS, Solomon CJ, Brown FB, McKinney GW, Rising ME, Dixon DA et al. (2018). MCNP Version 6.2 Release Notes, Los Alamos National Laboratory LA-UR-18-20808.
Kron T. Thermoluminiscence dosimetry and its applications in medicine. Part 2: history and applications. Australas Phys Eng Sci. 1995;18(1):1–25.
Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K. K Kodama Solid cancer incidence in atomic bomb survivors: 1958–1998. J Radiat Res, 168 (1) (2007), pp. 1–64, https://doi.org/10.1667/RR0763.1 PMID: 17722996.
ICRP Recommendations of the International Commission on Radiological Protection. (Users Edition) ICRP publication 103 (users Edition). Ann ICRP. 2007;37:2–4.
Doudoo CO, Gyekye PK, Emi-Reynolds G, Adu S, Kpeglo DO, Nii Adu Tagoe S, Agyiri K. Dose and secondary cancer-risk estimation of patients undergoing high dose rate intracavitary gynaecological brachytherapy. J Med Imaging Radiation Sci, 54(2):335–42. https://doi.org/10.1016/j.jmir.2023.03.031
D’Avino V, Caruso M, Arrichiello C, Ametrano G, La Verde G. Thermoluminescent dosimeters (TLDs-100) calibration for dose verification in photon and proton radiation therapy. Il Nuovo Cimento C. 2020;43(6):1–11.
Kry SF, Price M, Followill D, Mourtada F, Salehpour M. (2007). The use of LiF (TLD-100) as an out-of-field dosimeter. J Appl Med Phys, 8(4).
Majdaeen M et al. (2021).‘Skin dose measurement and estimating the Dosimetric Effect of Applicator Misplacement in Gynecological Brachytherapy: a patient and Phantom Study’. Pp. 917–29.
Chen R, Leung P, L. Non-linear dose dependence and dose-rate dependence of optically stimulated luminescence and thermoluminescence. Radiat Meas. 2001;33(5):475–81.
Montano-Garcia C, Gamboa-deBuen I. Measurements of the optical density and the thermoluminescent response of LiF:mg,Ti exposed to high doses of 60Co gamma rays. Radiat Prot Dosimetry. 2006;119(1–4):230–2. https://doi.org/10.1093/rpd/nci653.
Report No. 158 - AAPM TG 158: Measurement and calculation of doses outside the treated volume from external-beam radiation therapy. (2017).
Majdaeen M, Refahi S, Banaei A, Ghadimi M, Ardekani MA, Goushbolagh NA, Zamani H. A comparison of skin dose estimation between thermoluminescent dosimeter and treatment planning system in prostatic cancer: a brachytherapy technique. J Clin Transl Res. 2021;25(1):77–83. PMID: 34027203; PMCID: PMC8132188.
Marvi M, Gholami S, Barough M, Hosseini M, Nabavi M, Jaberi R, Mohammadkarim A. Evaluation of exit skin dose for intra-cavitary brachytherapy treatments by the BEBIG 60Co machine using thermoluminescent dosimeters. I Radiother Pract. 2021;20(1):49–54. https://doi.org/10.1017/S1460396919001018.
Lucas PA, Aubineau-Lanièce I, Lourenço V, Vermesse D, Cutarella D. Using LiF: Mg,Cu,P TLDs to estimate the absorbed dose to water in liquid water around an 192Ir brachytherapy source. Med Phys. 2014;41(1):011711. https://doi.org/10.1118/1.4851636. PMID: 24387503.
Chandola RM, Tiwari S, Kowar MK, Choudhary V. Monte Carlo and experimental dosimetric study of the mHDR-v2 brachytherapy source. J Cancer Res Ther. 2010 Oct-Dec;6(4):421–6. https://doi.org/10.4103/0973-1482.77068.
Hunt JG, da Silva FC, Mauricio CL, dos Santos DS. The validation of organ dose calculations using voxel phantoms and Monte Carlo methods applied to point and water immersion sources. Radiat Prot Dosimetry. 2004;108(1):85–9. https://doi.org/10.1093/rpd/nch002.
Reddy BR, Chamberland MJ, Ravikumar M, Varatharaj C. Measurements and Monte Carlo calculation of radial dose and anisotropy functions of BEBIG 60Co high-dose-rate brachytherapy source in a bounded water phantom. J Contemp Brachytherapy. 2019;11(6):563. https://doi.org/10.5114/jcb.2019.91224.
Bassi S, Berrigan L, Zuchora A, Fahy L, Moore M. End-to-end dosimetric audit: a novel procedure developed for Irish HDR brachytherapy centres. Phys Med. 2020;80:221–9. https://doi.org/10.1016/j.ejmp.2020.10.005.
Marcié S, Gerard JP, Dejean C, Feuillade J, Gautier M, Montagné L, Fuentes C, Hannoun-Levi JM. The inverse square law: a basic principle in brachytherapy. Cancer Radiother. 2022;26(8):1075–7. https://doi.org/10.1016/j.canrad.2022.04.002.
Tedgren CA, Hedman A, Grindborg J, Carlsson GA. Response of LiF:Mg,Ti thermoluminescent dosimeters at photon energies relevant to the dosimetry of brachythera py (< 1 MeV). Int J Med Phys Res Pract. 2011;38(10). https://doi.org/10.1118/1.3633892.
Lee JH, Chang LT, Shiau AC, Chen CW, Liao YJ, Li WJ, Lee MS, Hsu SM. A novel simple phantom for verifying the dose of radiation therapy. Biomedical Res Int. 2015;2015(934387). https://doi.org/10.1155/2015/934387.
Junell S, Dewerd L. SU-GG-T-241: determination of the Energy correction factor for TLD-100 in 6 and 10MV Photon beams relative to cobalt 60. Med Phys. 2008;35(6):2780. https://doi.org/10.1118/1.2961993.
Scarboro SB, Followill DS, Howell RM, Kry SF. Variations in photon energy spectra of a 6 MV Beam and their impact on TLD response. Med Phys. 2011;38(5):2619–28. https://doi.org/10.1118/1.3575419.
Abdel-Wahab M, Zubizarreta E, Polo A, Meghzifene A. (2017). Improving quality and access to radiation therapy - an IAEA perspective Semin Radiat Oncol, 27 (2) (2017), pp. 109–117, https://doi.org/10.1016/j.semradonc.2016.11.001
Acknowledgements
Special thanks goes to the Ghana Atomic Energy commission and all the Ghanaian health workers who provided help during this research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethical clearance (ECBAS 002/21–22) was sought from the College of Basic and Applied Sciences (CBAS) before the study commenced. Additionally, approval was also sought from the Cancer Center to use their HDR BT cobalt-60 machine (Flexitron, Elekta, Sweden).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doudoo, C.O., Gyekye, P.K., Emi-Reynolds, G. et al. A comparative study between MC simulation and TLD measurements of radiation doses to other parts of the body during gynaecological brachytherapy. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00905-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00905-z