Abstract
Purpose
Endovascular aneurysm repair (EVAR) procedures are associated with high patient radiation doses. On the other hand, dosimetric data on EVAR procedures in literature is limited. The aims of this study were to estimate radiation dose and risk associated with typical EVAR procedures and provide normalized organ and effective dose data related to EVAR procedures.
Methods
Data from 28 consecutive patients who underwent EVAR procedures in a tertiary medical facility within an 18-month period were retrieved and analyzed for this study. Monte Carlo PCXMC 2.0.1.4 software was used for the patient dose estimation; analysis was done with Medcalc statistical package and then Microsoft Excel was used for the cancer risks.
Results
Mean Dose Area Product (DAP) for fluoroscopy was 132.96 Gy·cm2 and that for digital subtraction angiography was 100.46 Gy·cm2. The study showed strong correlation between total fluoroscopy time and total cumulative dose (r = 0.904, P < 0.0001). Estimated organ and effective dose conversion coefficients for EVAR procedure were 0.131 mGy/Gy·cm2 and 0.114 mSv/Gy·cm2 respectively. The highest cancer mortality risk was estimated as 342 per 100,000 cases for all cancers among age group < 60 years and least mortality risk was estimated as 5.7 per 100,000 cases for ages 70 years and above.
Conclusions
The study has estimated radiation dose and risk associated with typical EVAR procedures and determined normalized organ and effective doses. The dosimetric data was comparable with other studies reported. The dose conversion coefficient for organ and effective doses determined in this study may be used considering the procedure conditions from the study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Endovascular aneurysm repair (EVAR) procedures are indicated in patients with abnormal aorta in the abdominal or thoracic region, most commonly the abdominal region [1]. The procedures are frequently performed in dedicated interventional suits or surgical theatres equipped with mobile or fixed fluoroscopy c-arm machines [2,3,4]. There has been growing concern regarding radiation dose mainly due to prolonged fluoroscopy time [2,3,4,5]. Measurement of patient dose from EVAR procedure is possible using anthropomorphic phantoms and dosimeters such as thermoluminescent dosimeters (TLDs) [2,3,4,5,6]. Moreover, Monte Carlo (MC) codes with mathematical phantoms have been used to estimate energy deposition in patients undergoing EVAR procedures [4, 7,8,9]. Limited dosimetric information on EVAR procedures exists in literature. Few studies estimate entrance surface dose or peak skin dose using pre-calibrated radio-chromic films or published dose conversion formula for interventional procedures [3, 6, 10, 11]. However, a couple of these studies [2, 9, 12,13,14] report organ doses from EVAR procedures.
MC methods [2, 6] as well as methods based on physical anthropomorphic phantoms and dosimeters [2, 3, 6] are time consuming techniques. Furthermore, expertise is needed, and materials used that are not available in all hospitals. Normalized dose values comprise data independent of exposure parameters that can be used for dose estimation. To our knowledge, there are no normalized patient effective dose data in literature associated with EVAR procedures. The aims of this study were to (a) evaluate radiation dose and risk associated with typical EVAR procedures and (b) estimate normalized organ and effective dose data related to EVAR procedures.
2 Materials and methods
2.1 The angiography unit
EVAR procedures included in this study were performed with a Siemens Axiom Artis FA floor-mounted C-arm angiography system (Siemens, Erlangen, Germany). During these procedures, automatic exposure control (AEC) system was activated. The X-ray tube has an anode angle of 12°, an inherent filtration of 2.5 mm Al/80 kV, estimated total filtration of 5.5 mm Al and additional copper filters ranging from 0.1 to 0.9 mm.
2.2 Patients, projections performed and Ethics
Twenty-eight (28) consecutive patients who underwent EVAR procedures were included in this study. The patients’ dosimetric data (includes patient height and weight, age, field size and the projection angles) were extracted from the digital imaging and communications in medicine (DICOM) system for this retrospective study. Only patients with complete DICOM information were included in this study. The data collected were only for male patients because, until the time of this study, no female patients had reported with indication for the EVAR procedure. The fluoroscopic projections used were AP (anterior-posterior), LAO (left-anterior oblique) and RAO (right-anterior oblique) with respect to the image intensifier (II). As per the Helsinki declaration on ethical procedures for patient involved investigations, due process was followed to obtain ethical clearance. This approval was granted by the hospital ethical review committee.
2.3 Dose estimation with monte carlo PCXMC software
Monte Carlo PCXMC software version 2.0.1.4 [15] was used for the patient examination simulation to calculate the organ and effective doses. The software uses a hermaphrodite mathematical phantom model [16] whose height and weight can be adjusted to mimic that of a patient. Detailed dose calculations were performed for all the 28 EVAR procedures. The EVAR procedure was defined for each patient based on the DICOM images, considering the patients’ physical and exposure parameters. Simulation was performed for 2 million photons per energy level [17]. The specific kVp, tube filtration and DAP values for each projection and the tube anode angle were inputted for the dose calculation. The software calculated effective dose based on ICRP 60 [18] and ICRP 103 [19] recommendations for tissue weighting factors. ICRP 103 recommendations on tissue weighting factors supersedes that of ICRP 60, so only the former was reported for this study.
2.4 Dose measurements with physical anthropomorphic phantom
A physical RANDO anthropomorphic phantom (Alderson Research Laboratories, New York, USA) was used for the verification of results. The phantom represents an adult individual who weighs 74.6 kg and has a height of 1.74 m. This phantom is made up of 35 slices of transverse sections numbered 0 to 34. Each section is 25 mm thick with small holes aligned on a 30 mm × 30 mm grid to enable the insertion of the TLD-100 H and TLD 200 (Harshaw, USA) chips. To verify MC simulation results, organ doses (included the lungs, liver, thyroid, oesophagus, heart, kidneys, large and small intestines, bone marrow and stomach) were measured using 91 TLD-100 H and 77 TLD 200 chips. Annealing was done with Victoreen oven (Victoreen Inc.,Ohio, USA) according to manufacturer’s specifications. The chips were irradiated at 85 kV and the exposure was measured with RadCal ionization chamber (model 3035). A Harshaw 3500 TLD reader was used to read out the irradiated chips. The phantom’s slice-Sects. 21 to 32 were chosen to represent the possible location of the abdominal aorta and therefore, the most exposed location during the EVAR procedure. Consequently, 112 chips were inserted in the organ locations in the phantom and 55 chips were distributed on the surface to sample entrance and exit skin doses.
2.4.1 Normalized dose values
Organ doses were divided with the total DAP for each patient to provide organ-to-DAP normalized dose values. Similarly, measured organ doses D from the projections were divided with their corresponding DAP to produce normalized dose values i.e., the sum of the organ doses from each projection was divided by the sum of the DAP from the projections to obtain the normalized dose for each organ for each patient using Eqs. 1 and 2.
where, DCCorgan i is the normalized dose (dose conversion coefficient); Dorgan ij is the dose to the organ i from projection j; and DAP is the total dose area product associated with the procedure. E is the effective dose and Worgan i is the tissue weighting factor for the organ i.
2.4.2 Risk estimates
Lifetime attributable risk (LAR) of solid cancer mortality were calculated using the Biological Effects of Ionizing Radiation (BEIR VII phase 2) report [20]. Risk models for cancer mortality have been derived by the BEIR VII committee considering the age at exposure, the cancer site and gender. The risks models include leukemia, all solid cancers combined, solid cancers in some organs and the remainder organs. From the BEIR VII model, the risk of cancer mortality reduces with age. The potential risk of deterministic effects was also assessed for each patient. The organ doses from the PCXMC, varying patent age and the BEIR VII risk model equations for LAR were inputted into Microsoft excel for the estimation of the patient cancer risks.
2.5 Statistical analysis
Correlation between the organ and effective doses from Monte Carlo PCXMC simulations and TLD measurements was determined using linear regression analysis. Linear regression analysis was also used to examine the correlation between normalized organ and effective doses and patients BMI. Student’s t-test was used to compare means of simulated and measured doses. Bland-Altman statistical techniques was used to show the limits of agreement. Statistical significance was inferred when P < 0.05. Data analysis was performed using Medcalc statistical package [21] (Medcalc, Mariakerke, Belgium, version 18.11.6).
3 Results and discussion
The mean (range) age, weight and height of patients were 70.3 years (50–83 years), 83.4 kg (55–128 kg) and 1.7 m (1.6–1.9 m) respectively. Table 1 shows comparison of the fluoroscopy time (FT) and DAP in this study with other studies. The DAP values varied generally among patients in this study, but mean values were comparable to those presented in other studies [2, 9, 11]. The range of DAP values from this study, were comparable with those of Blaszak et al. [9] but 3 factors higher than those of Foerth et al. [2] and lower by a factor of 1.7 to that of Howells et al. [11]. Similarly, the mean FT of this study was comparable with that of Foerth et al. [2] but nearly twice that of Howells et al. [11]. Notwithstanding, the FT for this study compares well with that of Howells et al. [11] but three folds higher than that of Foerth et al. [2]. DAP and FT differences could be attributed to the variation in procedure techniques, the different equipment and AEC settings of the angiographic unit used for the procedure, typically the complexity of the aortic abnormality being treated and the experience of the interventional radiologist performing the procedure.
The details of patient physical and exposure parameters used for the MC simulations are presented in Table 2. A strong correlation was found between the total FT and the total DAP (r = 0.886, P < 0.0001) and with total cumulative dose CD (r = 0.904, P < 0.0001). The regression equation, relating the effective dose and CD was EmSv = 3.502 + 12.480 (CD Gy) with r = 0.98 and P < 0.001. Organ and effective doses depend on factors such as radiation field size, anatomical region, exposure parameters and exposure duration, number of angiographic acquisitions, and body mass index (BMI) of the patient [6]. For these reasons, considering the exposure factors alone in patient dose analysis for EVAR is misleading without taking into consideration the abnormality of the aorta which was treated. For an ideal procedure where, uniform angular distributions are simulated, the real effects of procedural parameters might be compromised mainly in the case of the organ dose estimates.
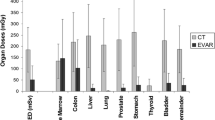
Table 3 shows the organ and effective doses obtained from simulations of patients’ EVAR procedures. Organ doses are strongly dependent on the anatomical region, examination projection, radiation beam quality and the radiation field size. A couple of other studies [2, 9, 12,13,14] have estimated the organ and effective doses in EVAR in the literature, have reported detailed information (presented in Table 3) on organ and effective doses to patients who underwent EVAR procedures of varying complexities. It could be seen that there are variations in the organ and effective doses reported in this study compared with those reported in the other studies in Table 3. Blaszak et al. [9] dose calculations were based on dose conversion coefficients obtained from voxel phantom models [22].
The phantom models used in Monte Carlo PCXMC software version 2, have some modifications in comparison with those of Monte Carlo PCXMC versions 1.2–1.5. Elemental composition and density of tissues have been changed and modified in PCXMC version 2. The differences, highlighted in the different versions of the software might account to some extent the differences in the dose results for some irradiation conditions in PCXMC 2 compared with PCXMC 1.5 [23]. Therefore, the differences in the results of the organ and effective doses from this study (Monte Carlo PCXMC 2.0.1.4) and those of Foerth et al. [2] (Monte Carlo PCXMC 1.5.1) as in Table 3 are to be expected.
The highest mean and median organ doses were recorded in the kidneys, 225.7 mGy and 164.9 mGy respectively. The median dose values in this study were mostly lower than those reported by Foerth et al. [2] by an average factor of 2.2, however, the range of dose values shows a wide variation. Mean doses to some organs in this study were mostly lower by an average factor of 2.2 (for men) and 2.0 (for women), but in some cases higher compared to the study of Blaszak et al. [9]. The differences in organ doses might be attributed to technique differences from one interventional radiology suit to the other, the complexity of the aortic abnormality being treated, the operators experience and the differences in the fluoroscopic equipment used. In addition, the differences in the phantom models used for the patient dose simulation could be contributory factors to these differences.
Some studies [2, 22, 24] have compared TLD and MC simulated dose results and the deviation between the two methods is observed to be within 10% for one of the studies [24] which was for pediatric dental procedures. The deviations in the case of EVAR procedures were observed to be about 20% for this study. The deviation in this study might be comparable with that of other studies [2, 22] in the literature for EVAR procedures although the calculation was not done.
Normalized dose values, also referred to as dose conversion coefficients (DCC) [25, 26] in some publications, have been determined in this study. DCC to convert from DAP to organ and effective doses were calculated with the Monte Carlo program and presented in Table 4. The average conversion coefficient for organ dose was determined to be 0.13 mGy/Gy·cm2 and that for effective dose was 0.11 mSv/Gy·cm2. Operators can use this data, which are technique- and instrumentation-independent values, to calculate organ and effective dose by multiplying total DAP of the procedure with a single conversion coefficient. A list of DCCs [25, 27] associated with interventional procedures can be found in literature, however, there has not been published DCC associated with EVAR procedures as presented in this study. Schultz et al. [25], demonstrated the importance of using procedure specific DCC, considering the conditions (i.e. tube voltage, filtration, beam projection, field size, FSD etc.) associated with its establishment. Therefore, this study has published the detailed conditions relating to the establishment of the DCC associated with EVAR procedure.
DCCs are used in medical physics to estimate the radiation dose received by various organs and tissues during radiological procedures. While DCCs can provide valuable information, they have limitations arising from standardization and variability, patient-specific factors, procedure variability, limited tissue differentiation, dynamic nature of the scan procedures, limited validation and incomplete representation. The dose conversion coefficients (DCCO and DCCE) play a crucial role in estimating the effective dose in the field of radiation protection. The DCCE provides a measure of the overall risk of harm from exposure to ionizing radiation, considering the different sensitivities of various tissues and organs in the human body. The significance of the dose conversion coefficient for the effective dose lies in key aspects such as Radiation Risk Assessment, comparison of radiation doses received in different situations or from various radiation sources, Optimization of Medical Procedures, guiding the development of radiation protection strategies, and as a valuable metric for communicating radiation risks to patients.
Estimated cancer mortality risks are shown for different age groupings in Table 5. The highest cancer mortality was 342 per 100,000 cases (about 1:330) for all cancers among age group < 60 years. The least mortality rate was associated with cancer of the liver, 5.6 per 100,000 cases (around 1:20,000) for 70 years and above. This is largely due to the age group of the patients included in this study. Usually, for that age group of patients, deterministic injuries are of more concern [9] because the cancer mortality risk coefficients decrease with increasing age.
Other studies [9, 28] have estimated the radiogenic effects due to EVAR. One study [9], reported that, cancer mortality risks were about 40% lower than the cancer incidence risks. The highest mean cancer incidence risk was 1:275 for the youngest group of patients (< 60 years) in that study. There were younger patients in their study and that could be a part of the reason for the percentage difference between the incidence and mortality risks. In another study [28], the estimated risk associated with EVAR patients was evaluated using the cumulative dose data of CT and interventional procedures. They reported that, the mean risk of exposure induced death was 1:130 persons.
4 Conclusion
This study has estimated the radiation dose and risk associated with typical EVAR procedures and determined normalized organ and effective dose data related to EVAR procedures. Though the patient cohort were males, the dosimetric data was comparable with other studies that reported both genders. The DCC for organ and effective doses determined in this study may be used considering the procedure conditions from the study. Due to the high radiation doses associated with EVAR procedures, necessary precautions are needed to ensure that the ALARA principle is adhered to always.
Data availability
This data was collected as part of a doctorial study and it is available upon request when necessary.
References
Sethi RKV, Henry AJ, Hevelone ND, Lipsitz SR, Belkin M, Nguyen LL. Impact of hospital market competition on endovascular aneurysm repair adoption and outcomes. J Vasc Surg. 2013;58(3):596–606. https://doi.org/10.1016/j.jvs.2013.02.014.
Foerth M, Seidenbusch MC, Sadeghi-Azandaryani M, Lechel U, Treitl KM, Treitl M. Typical exposure parameters, organ doses and effective doses for endovascular aortic aneurysm repair: comparison of Monte Carlo simulations and direct measurements with an anthropomorphic phantom. Eur Radiol. 2015;25(9):2617–26. https://doi.org/10.1007/s00330-015-3673-8.
Maurel B, Sobocinski J, Perini P, et al. Evaluation of radiation during EVAR performed on a mobile C-arm. Eur J Vasc Endovasc Surg. 2012;43(1):16–21. https://doi.org/10.1016/j.ejvs.2011.09.017.
Fossaceca R, Brambilla M, Guzzardi G, et al. The impact of radiological equipment on patient radiation exposure during endovascular aortic aneurysm repair. Eur Radiol. 2012;22(11):2424–31. https://doi.org/10.1007/s00330-012-2492-4.
Weerakkody RA, Walsh SR, Cousins C, Goldstone KE, Tang TY, Gaunt ME. Radiation exposure during endovascular aneurysm repair. Br J Surg. 2008;95(6):699–702. https://doi.org/10.1002/bjs.6229.
Panuccio G, Greenberg RK, Wunderle K, Mastracci TM, Eagleton MG, Davros W. Comparison of indirect radiation dose estimates with directly measured radiation dose for patients and operators during complex endovascular procedures. J Vasc Surg. 2011;53(4):885–894e1. https://doi.org/10.1016/J.JVS.2010.10.106.
Thakor AS, Winterbottom A, Mercuri M, Cousins C, Gaunt ME. The radiation burden from increasingly complex endovascular aortic aneurysm repair. Insights Imaging. 2011;2(6):699–704. https://doi.org/10.1007/s13244-011-0120-5.
Molyvda-Athanasopoulou E, Karlatira M, Gotzamani-Psarrakou a, Koulouris C. Siountas a. Radiation exposure to patients and radiologists during interventional procedures. Radiat Prot Dosimetry. 2011;147(1–2):86–9. https://doi.org/10.1093/rpd/ncr271.
Blaszak MA, Juszkat R. Monte Carlo Simulations for Assessment of Organ Radiation doses and Cancer risk in patients undergoing abdominal stent-graft implantation. Eur J Vasc Endovasc Surg. 2014;48(1):23–8. https://doi.org/10.1016/J.EJVS.2014.03.014.
Walsh C, O’Callaghan A, Moore D, et al. Measurement and optimization of patient radiation doses in endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2012;43(5):534–9. https://doi.org/10.1016/j.ejvs.2012.01.028.
Howells P, Eaton R, Patel AS, Taylor P, Modarai B. Risk of radiation exposure during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2012;43(4):393–7. https://doi.org/10.1016/j.ejvs.2011.12.031.
Harbron R, Abdelhalim M, Ainsbury L, Eakins J, Alam A, Lee C, Modarai B. Patient radiation dose from x-ray guided endovascular aneurysm repair: a Monte Carlo approach using voxel phantoms and detailed exposure information. J Radiol Prot. 2020;40. https://doi.org/10.1088/1361-6498/ab944e.
Efthymiou F, Metaxas V, Dimitroukas C, Kakkos S, Moulakakis K, Belavgenis A, Panayiotakis G. BMI-Based organ doses in endovascular aneurysm repair interventions utilising Monte Carlo simulation. Appl Radiat Isot. 2023;195:110740. https://doi.org/10.1016/j.apradiso.2023.110740.
Singh B, Andersson M, Edsfeldt A, Sonesson B, Gunnarsson M, Dias NV. Estimation of the added cancer risk derived from EVAR and CTA follow-up. J Endovasc Ther. 2023;0(0). https://doi.org/10.1177/15266028231219435.
Authority NS. A Monte Carlo program for calculating patient doses in medical x-ray examinations (2. 2008;(November).
Cristy M, Eckerman KF. Specific Adsorbed Fractions of Energy at Various Ages From Internal Photon Sources. Ornl/Tm-8381 V1-V7. 1987. 10.1.1.453.354.
Andreo P. Monte Carlo techniques in medical radiation physics. Phys Med Biol. 1991;36(7):861–920.
ICRP Publication 60. 1990 recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991;(21):1–3).
Agency HP Jr, Clarke JDB et al. RH,. ICRP Publication 103.; 2007.
BEIR. BEIR VII report, phase 2: Health risks from exposure to low levels of ionizing radiation. Natioanl Academies.
MedCalc. Software bvba, Ostend B. MedCalc Statistical Software.
Kramer R, Khoury HJ, Vieira JW. CALDose_X - A software tool for the assessment of organ and tissue absorbed doses, effective dose and cancer risks in diagnostic radiology. Phys Med Biol. 2008;53(22):6437–59. https://doi.org/10.1088/0031-9155/53/22/011.
STUK (Radiation and Nuclear Safety Authority). PCXMC - A Monte Carlo program for calculating patient doses in medical x-ray examinations - STUK. 2015.
Lee C, Park B, Lee SS, Kim JE, Han SS, Huh KH, Yi WJ, Heo MS, Choi SC. Efficacy of the Monte Carlo method and dose reduction strategies in paediatric panoramic radiography. Sci Rep. 2019;9:9691. https://doi.org/10.1038/s41598-019-46157-0.
Schultz FW, Zoetelief J. Dose conversion coefficients for interventional procedures. Radiat Prot Dosimetry. 2006;117(1–3):225–30. https://doi.org/10.1093/rpd/nci753.
Submitt To …. 2004;(16):1–10.
Hart D, Wall BF. Radiation Exposure of the UK Population from Medical and Dental X-ray Examinations. NRPB W-Series Reports. 2002;(March):41.
Brambilla M, Cerini P, Lizio D, Vigna L, Carriero A, Fossaceca R. Cumulative radiation dose and radiation risk from medical imaging in patients subjected to endovascular aortic aneurysm repair. Radiol Med. 2015;120(6):563–70. https://doi.org/10.1007/s11547-014-0485-x.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Institutional Review Board of the University of Cape Coast, Ghana.
Consent to participate
Informed consent was obtained through the ethics approval.
Consent to publication
The authors affirm that human research participants provided informed consent for the study, through the ethics approval.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Engmann, C.K., Boadu, M. & Owusu, A. Estimation of patient doses and cancer risk to patients during EVAR procedures. Health Technol. 14, 531–538 (2024). https://doi.org/10.1007/s12553-024-00828-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-024-00828-9