Abstract
Seventy composite samples of maize grains stored in five agro-ecological zones (AEZs) of Nigeria where maize is predominantly produced were evaluated for the presence of microbial metabolites with the LC-MS/MS technique. The possible relationships between the storage structures and levels of mycotoxin contamination were also evaluated. Sixty-two fungal and four bacterial metabolites were extracted from the grains, 54 of which have not been documented for maize in Nigeria. Aflatoxin B1 and fumonisin B1 were quantified in 67.1 and 92.9 % of the grains, while 64.1 and 57.1 % exceeded the European Union Commission maximum acceptable limit (MAL) for aflatoxin B1 and fumonisins, respectively. The concentration of deoxynivalenol was, however, below the MAL with occurrence levels of 100 and 10 % for its masked metabolite, deoxynivalenol glucoside. The bacterial metabolites had low concentrations and were not a source of concern. The storage structures significantly correlated positively or negatively (p < 0.01 and p < 0.05), respectively with the levels of grain contamination. Consumption of maize grains, a staple Nigerian diet, may therefore expose the population to mycotoxin contamination. There is need for an immediate action plan for mycotoxin mitigation in Nigeria, especially in the Derived Savannah zone, in view of the economic and public health importance of the toxins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays, L., Poaceae family) also known as corn, is the third most traded cereal after wheat and rice with a total production of 745 billion kg in over 1.60 million m2 in 2008 (FAOSTAT 2013). Nigeria is the world’s 8th largest producer of maize with production increasing from 5,570 kg in 2004 to 9,200 kg in 2012 (FAOSTAT 2013). The consumption pattern of maize-based diets by African adults is about 400 g/person/day, whereas, in the developed world, maize intakes are commonly less than 10 g/person/day (Shephard 2004). Maize can be cultivated in Nigeria for human consumption (78 %), feed and residual uses (17 %), and a small percentage set aside for re-planting (United States Department of Agriculture, 2012). A 4-week nationwide survey conducted in Nigeria between 2001 and 2003 showed that maize is the most widely consumed staple food in the country with an overall percentage frequency of consumption of 20.1 % (Maziya-Dixon et al. 2012) .
A number of toxic microbial metabolites abound in agricultural products due to the diversity of fungal and bacterial species that colonise products from field to store. There is ample evidence that the inhabitants of sub-Saharan Africa (SSA) are experiencing heavy dietary exposure to mycotoxins particularly aflatoxins and fumonisins (Gong et al. 2002, 2003; Turner et al. 2007). Mycotoxin contamination of foods begins with fungal infestation in the field which is carried into the store, and is further aggravated by poor storage conditions, leading to production of large amounts of toxic metabolites in many parts of SSA. A large number of mycotoxins can also co-occur in maize in SSA (Warth et al. 2012a; Abia et al. 2013). Literature is replete with information on the presence of aflatoxins, fumonisins, trichothecenes and some other Fusarium mycotoxins in Nigerian maize (Udoh et al. 2000; Afolabi et al. 2006; Adejumo et al. 2007a, b; Atehnkeng et al. 2008; El-Imam et al. 2012) including ochratoxin A that was only recently isolated from Niger State (Makun et al. 2013), but is lacking concerning other microbial metabolites. However, an array of microbial metabolites has been found in commercial poultry feeds in Nigeria, of which maize is a major constituent (Ezekiel et al. 2012).
In Nigeria, agricultural products are mostly produced by small-scale farmers and the products sold in local markets where “caveat emptor” remains the basic rule (Bandyopadhyay et al. 2007). Since these products rarely enter official channels of sales, surveillance of the level of toxin contamination rarely happens.
This study was conducted to screen maize matrices for fungal and bacterial metabolite pattern in order to to quantify the respective concentrations of the stored grains in the various agro-ecological zones of the country, and to establish possible relationships between storage structures and levels of mycotoxin contamination of the grains. Information on the occurrence, distribution and concentration of the contaminated grains will benefit food safety initiatives and enhance necessary interventions by relevant regulatory and health agencies in Nigeria.
Materials and methods
Study area
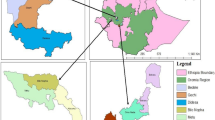
Surveys were conducted between August 2011 and February 2012 in five out of the seven AEZs of Nigeria where maize is predominantly produced (Adetunji, unpublished, 2013). The zones (Fig. 1) are: Sudan Savanna (Kano and Sokoto States), Northern Guinea Savanna (Kaduna State), Southern Guinea Savanna (Niger State), Derived Savanna (Ondo, Ekiti, Osun, Oyo and Nasarawa States) and Humid Forest (Lagos and Ogun States). The Sahel Savanna and Mid Altitude zones do not produce appreciable quantities of maize. The geographical location, temperature and rainfall pattern of the zones had been documented by previous workers (Udoh et al. 2000; Atehnkeng et al. 2008).
Sampling and sample preparation
Information about farmers who produce and store the grains in large quantities and their locations in the states was collected from the Agricultural Development Programme (ADP) offices of the various states. The modified EU (2002a) method was used for sampling and sample preparation in order to reduce variability. Briefly, the states were divided into ADP zones (based on climatic conditions and types of agricultural produce) which were further subdivided into agricultural blocks (local government) from which two local governments that produce maize in the highest amounts were selected (Table 1). A cell (village or town) that produce maize in the highest amount was selected from each of the two local governments, and maize grains randomly collected from three farmers in each cell.
Shape files of state boundaries, roads and major towns were loaded into Arcview GIS 3.2, where final map embellishments were carried out and sampling locations overlaid on them (Fig. 1). The grains that had been stored for a minimum of 1 month (except for grains in Lagos State which were stored for 2 weeks) were collected from the top, middle and bottom portions of the storage structures in the cells and the grains mixed together to form a composite sample per cell. The compositing of the maize grains was based on their storage structures. Maize grains with the same storage structure were pulled together as a composite sample, otherwise they were treated as a separate batch. The number of samples per state was not even, but depended on the number of ADP zones in the state and the type of storage structures found in each cell. The storage structures of the farmers were also correlated with mycotoxin contamination and the AEZs. A total of 70 composite samples were collected from 33 ADP zones and 55 cells (Table 1) made up of 11 samples each from the Sudan Savanna (SS), Northern Guinea Savanna (NGS) and Southern Guinea Savanna (SGS), 33 from Derived Savanna (DS) and 4 from Humid Forest (HF) zone. The samples were kept in well-labelled sterile polyethylene bags and transported to the laboratory for analysis.
The total weight of each composite sample was 3 kg. The samples were hand-mixed, coarse-ground and allowed to pass through a No.14-mesh screen. Subsamples of 500 g were taken from each lot, ground with a milling machine (Greiffenberger Antriebstenchnic, Germany) and further sieved with a 1-mm mesh. Subsamples of 50 g were further taken from the lots and placed in zip lock envelopes for fungal identification (unpublished data) and for multi-metabolite analysis by the LC-MS/MS technique. Samples used for the metabolite analysis were stored at −20 °C prior to analyses.
Chemicals
Methanol (LC gradient grade) and glacial acetic acid (p.a.) were purchased from Merck (Darmstadt, Germany), acetonitrile (LC gradient grade) from VWR (Leuven, Belgium), and ammonium acetate (MS grade) from Sigma-Aldrich (Vienna, Austria). Standards for fungal and bacterial metabolites were obtained from various research groups or from commercial sources. Water was purified successively by reverse osmosis with an Elga Purelab ultra analytic system from Veolia Water (Bucks, UK).
Extraction of maize grains and estimation of matrix effects
Five grams of each representative sample were weighed into a 50-ml polypropylene tube (Sarstedt, Nümbrecht, Germany) and 20 ml of the extraction solvent (acetonitrile/water/acetic acid 79:20:1, v/v/v) added. For spiking experiments, 0.25 g samples were used for extraction. Samples were extracted for 90 min on a GFL 3017 rotary shaker (GFL, Burgwedel, Germany) and diluted with the same volume of dilution solvent (acetonitrile/water/acetic acid 79:20:1, v/v/v), and the diluted extracts injected (Sulyok et al. 2007). Centrifugation was not necessary due to sufficient sedimentation by gravity.
Apparent recoveries of the analytes were cross-checked by spiking a sample that was not contaminated with mycotoxins with a multi-analyte standard on one concentration level, since previously generated maize data is available (Warth et al. 2012a; Abia et al. 2013). The spiked sample was stored overnight at ambient temperature to allow evaporation of the solvent and to establish equilibrium between the analytes and the sample. The extraction, dilution and analysis were as described earlier. The corresponding peak areas of the spiked samples were used for the estimation of apparent recoveries by comparison to a standard prepared and diluted in neat solvent. All concentrations of the naturally contaminated samples were corrected by a factor equivalent to the reciprocal of apparent recovery (1/R; where R is the apparent recovery value) of each analyte.
LC-MS/MS parameters
LC-MS/MS screening of target microbial metabolites was performed with a QTrap 5500 LC-MS/MS System (Applied Biosystems, Foster City, CA, USA) equipped with TurboIonSpray electrospray ionization (ESI) source and a 1290 Series HPLC System (Agilent, Waldbronn, Germany). Chromatographic separation was performed at 25 °C on a Gemini® C18-column, 150 × 4.6 mm i.d., 5 μm particle size, equipped with a C18 4 × 3 mm i.d. security guard cartridge (Phenomenex, Torrance, CA, USA). The chromatographic method, chromatographic and mass spectrometric parameters of 186 analytes under investigation are described elsewhere (Vishwanath et al. 2009). At present, this method has been transferred to another instrument and further expanded to 320 metabolites (unpublished data).
ESI-MS/MS was performed in the time-scheduled multiple reaction monitoring (MRM) mode both in positive and negative polarities in two separate chromatographic runs per sample by scanning two fragmentation reactions per analyte. The MRM detection window of each analyte was set to its expected retention time ±27 and ±48 s in the positive and the negative modes, respectively. Confirmation of positive analyte identification was obtained by the acquisition of two MRMs per analyte (with the exception of moniliformin and 3-nitropropionic acid, which exhibited only one fragment ion). This yielded 4.0 identification points according to European Union Commission decision 2002/657 (EU 2002b). In addition, the LC retention time and the intensity ratio of the two MRM transitions agreed with the related values of an authentic standard within 0.1 min and 30 % rel., respectively.
Relationship between storage structures and mycotoxin concentration of maize grains
The Pearson’s correlation method was used to establish relationship between identified storage structures and levels of mycotoxin contamination of the maize grains across the AEZs.
Statistical analysis
The levels of mycotoxin contamination of the maize grains were analysed with the SPSS for windows v.16.0 (SPSS, Chicago, IL, USA). The Duncan’s Multiple Range test (DMRT) was used to separate the means (p < 0.05). Simple descriptive analysis was used to evaluate the occurrence and concentration of fungal and microbial metabolites across the AEZs.
Results and discussions
LC-MS/MS quality assurance
Table 2 shows the performance characteristics data for the analytical method used as established from one spiked blank sample of maize. The limits of detection (LOD) ranged between 0.04 μg/kg (chloramphenicol) and 80 μg/kg (fusaproliferin). Quantitative standards for averufin, nidurufin, norsolorinic acid, versicolorins A and C were not available; these compounds were therefore semi-quantified using the response factor of averantin. Andrastin A was qualitatively determined in the maize samples from the peak area due to lack of internal standard. Apparent recoveries of other metabolites from the spiked samples were in the range of 60–160 % except for ophiobolin A, chanoclavine, skyrin and rugulosin which exhibited lower values.
Occurrence and distribution of microbial metabolites of stored maize grains in Nigeria
A total of 66 microbial (62 fungal and 4 bacterial) metabolites were detected in the maize grains (moisture content of between 12.41 and 13.29 %) across the five AEZs of Nigeria (Tables 3, 4). The large number of microbial metabolites detected in the grains point to the diversity of fungal and bacterial species that colonised the grains from field to the store. The metabolite concentrations ranged between 0.1 and 132,909 μg/kg, and the largest proportion of contaminated grains (aflatoxins including aflatoxin M1, fumonisins and deoxynivalenol) was found in the DS zone (Tables 3, 4) while the lowest was in the HF zone. The low amount of rainfall (650–1,300 mm), the prevailing high temperature (26–40 °C) (Atehnkeng et al. 2008) and long periods of dry season (6–9 months) in the SS and NGS zones (Udo et al. 2000; Atehnkeng et al. 2008) may be responsible for the low concentration of aflatoxins found in maize grains in these regions. Udo et al. (2000) did not detect aflatoxins in farmers’ stores in the NGS zone and attributed it to the fact that farmers usually store their grains in “rhumbu” (local granary) in this zone. The low concentration of aflatoxins in grains stored in the HF zone despite its high rainfall pattern (1,300–2,000 mm) and suitable temperature (26–28 °C) for growth of mycotoxigenic fungi (Udo et al. 2000) may be due to the fact that farmers in the zone do not usually store their maize for long periods, as they sell their maize in the fresh state because the zone is highly urbanised. The grains are usually consumed by the populace as snacks and used by local industries either for feed production or as raw material in other industrial purposes. On the other hand, the high concentration of aflatoxin observed in the DS zone, especially in Ondo and Nasarawa States, is probably due to the high amount (1,300–1,500 mm) of bimodal rainfall (Atehnkeng et al. 2008) usually recorded in this largest zone. Of the 66 microbial metabolites, 25 are regarded as mycotoxins addressed by regulations, and their derivatives and other notable mycotoxins were targeted by several analytical methods (Table 3), while the remaining 41 are rarely investigated (Table 4). Among the microbial metabolites detected in the stored maize, only the fungal metabolites, aflatoxins, diacetoxyscirpenol, deoxynivalenol, 3,mono-acetyldeoxynivalenol, fumonisins, ochratoxin A, zearalenone, and alpha-zearalenol, have been previously reported in Nigerian maize (Afolabi et al. 2006; Adejumo et al. 2007a, b; Atehnkeng et al. 2008; El-Imam et al. 2012), while the remaining 54 fungal and bacterial metabolites have not been documented.
Regulated and common mycotoxins of stored maize grains
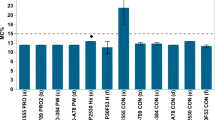
The mycotoxins detected in the maize grains are shown in Table 3. They are capable of being carcinogenic (aflatoxins, ochratoxin A and fumonisins), mutagenic (aflatoxins), teratogenic (ochratoxin A), estrogenic (zearalenone), haemorrhagic (trichothecenes), immunotoxic (aflatoxins and ochratoxin A), nephrotoxic (ochratoxin A), dermotoxic (trichothecenes) and neurotoxic (fumonisin B1) (IARC 1993, 2002; Bondy and Pestka 2000; CAST 2003). Aflatoxin B1, the most potent and prevalent form of the aflatoxins, was found in 67.2 % of the maize grains while aflatoxin G1 contaminated only 15.7 % of the grains. Aflatoxin B1 has been primarily linked to human primary liver cancer in which it acts synergistically with hepatitis B virus (HBV) infection (Li and Wu 2010). The aflatoxin levels of the stored maize were much higher than what was reported in a previous study in Nigeria: 0–1,506 μg/kg (Atehnkeng et al. 2008); and the DS AEZ had the highest aflatoxin level. This may be due to the fact that the region produces the highest amount of grains in the country. However, it differed from previous reports that implicated the SGS as the region with the highest aflatoxin levels (Atehnkeng et al. 2008); a pointer to regional variation and widespread contamination of maize grains by aflatoxins across the country. About 61.4 and 57.1 % of the maize grains exceeded the maximum acceptable limits (MAL) set by the European Union Commission (EU, 2006), for aflatoxin B1 (2 μg/kg) and total aflatoxins (4 μg/kg) in maize, respectively (Fig. 2). About 51.4 % of the maize grains had aflatoxin concentrations above the 10 μg/kg MTL recommended for unsorted food commodities by the National Agency for Food and Drug Administration and Control in Nigeria (Atanda et al. 2011).. Thus, chronic exposure to aflatoxins through maize consumption is evident in Nigeria, as with other parts of SSA (Bandyopadhyay et al. 2007; Atehnkeng et al. 2008; Diedhiou et al. 2011; Warth et al. 2012a; Abia et al. 2013).
Fumonisin B1, the most toxic of the fumonisins, has been be implicated as the causative agent of equine leukoencephalomalacia, a fatal neurological disease of horses, characterized by liquefactive necrosis of the white matter of the brain (Chilaka et al. 2012). Exposure to this mycotoxin has also been associated with the incidence of neural tube defects (Missmer et al. 2006). The mycotoxin was detected in 92.9 % of the maize grains and the range of contamination was between 1.8 and 10,447 μg/kg (Table 3), while fumonisin B2 and fumonsin B3 were found in the ranges of 12.8–3,455 and 6.4–720 μg/kg, respectively. The levels reported in this work are higher than the previous levels of fumonisin contamination in Nigerian maize (Afolabi et al. 2006; Adejumo et al. 2007b; El-Imam et al. 201) and maize from Cameroun, Democratic Republic of the Congo and Kwazulu Natal province of South Africa (Manjula et al. 2009; Chilaka et al. 2012; Abia et al. 2013). However, some stored maize grains intended for human consumption in Zambia (Kankolongo et al. 2009) and South Africa (Ncube et al. 2011) had fumonisin levels as high as 21,440 and 21,800 μg/kg, respectively, while in a recent study by Shephard et al. (2013), fumonisin B1 levels in good quality and moldy home-grown maize grains were also found to be as high as 17,120 and 190,100 μg/kg, respectively. The high levels of fumonisins in the stored maize point to lapses in implementing good on-field agricultural practices during planting and harvesting of maize grains, thus favouring the invasion of grains by fumonisin-producing Fusarium species and elaboration of toxins. About 55.7 % of the samples exceeded the MAL of 1,000 μg/kg for total fumonisins in maize (Fig. 2) set by EU (2006).
Deoxynivalenol, a trichothecene that causes nausea, vomiting and diarrhoea in agricultural animals when ingested in low and high doses, induces weight loss and food refusal in pigs and other farm animals (Pestka 2010a, b). This Fusarium toxin recorded a 100 % occurrence in the stored maize at a concentration range of 11–479 μg/kg. This is similar to the level of deoxynivalenol contamination (9.6–745.1 μg/kg) of maize previously reported in Nigeria (Adejumo et al. 2007a), Burkina Faso (31.4 μg/kg) and Mozambique (116–124 μg/kg) (Warth et al. 2012a), and for maize-based products such as cornflakes and popcorn (max = 63 μg/kg) from Belgium (De Boevre et al. 2012). In addition, the range of contamination of its masked form, deoxynivalenol glucoside, which is also reported for the first time in Nigeria maize was between 0.1 and 76 μg/kg, but similar to the levels (LOQ–82 μg/kg) found in Camerounian maize (Abia et al. 2013). Furthermore, deoxynivalenol and its glucoside were detected in human urine in Cameroun (Warth et al. 2012b). The mean level of contamination of nivalenol, another type B immunosuppressive, protein inhibitor trichothecene was 14 μg/kg, which was less than those recently reported for maize (34.1 μg/kg) from Mozambique (Warth et al. 2012a) and by Abia et al. (2013) for Cameroun (161 μg/kg). Zearalenone, an oestrogenic toxin which causes infertility in animals, has been mentioned in relation to human cervical cancer and outbreaks of precocious pubertal changes in children (CAST 2003). As reported by previous workers (Soleimany et al. 2012; Warth et al. 2012a; Abia et al. 2013). this Fusarium mycotoxin was also found in the stored maize grains (17.1 %) at concentration ranges of 0.4 to 2,044 μg/kg. In addition, a composite sample of maize grains had a high zearalenone concentration of 2,044 μg/kg. which was far above the MAL limit of 200 μg/kg.
Other emerging Fusarium mycotoxins found (Table 3) included enniatins, beauvericin, fusaproliferin and moniliformin (Jestoi 2008). These mycotoxins have not been documented for Nigerian maize except for enniatins which were found in maize from Southwestern Nigeria (Adejumo et al. 2007b). The co-occurrence of these emergent mycotoxins with other mycotoxins of known toxicology and many other metabolites of unknown toxicology is a source of concern. Beauvericin like enniatin is a cyclodepsipeptide with antibiotic, insecticidal, and cytotoxic effects most likely related to their ionophoric properties (Juan et al. 2013). Furthermore, beauvericin is genotoxic to human lymphocytes (Celik et al. 2010), while fusaproliferin is toxic to human non-neoplastic B-lymphocyte cell line IARC/LCL 171 (Logrieco et al. 1996) and moniliformin is cytotoxic to many mammalian systems (Jestoi 2008).
Ochratoxin A, a potent nephrotoxic metabolite from Penicillium species, Aspergillus ochraceus and A. carbonarius was detected in only 10.0 % of the maize grains and the range of contamination was between 4 and 580 μg/kg. The kidneys are the most susceptible organs to ochratoxin A where it can cause both acute and chronic kidney lesions (O’Brien and Dietrich 2005) in sufficiently high concentrations. However, only 8.6 % of the maize grains had ochratoxin A levels above the MAL of 5 μg/kg for maize grains (Fig. 2).
Non-regulated metabolites of stored maize grains
Thirty-seven fungal and four bacterial metabolites of the stored maize were non-regulated (Table 4). Metabolites of Aspergillus origin (averufin, averufanin, averantin, nidurufin, norsolorinic acid, versicolorins A and C) which are known aflatoxin precursors were found in about half of maize grains at low-to-moderate concentrations (Table 4), with the exception of physcion which was found in only 20.0 % of the stored grains, and kojic acid which had a maximum concentration of 132,909 μg/kg. The Fusarium metabolites found in the maize grains included aurofusarin, culmorin and its derivative (15-hydroxyculmorin), diacetoxyscirpenol, equisetin, fusaric acid, monocetoxyscirpenol and monocerin with equisetin having the highest percentage occurrence of 98.6 %. Penicillium metabolites occurred less frequently in the stored maize at moderate concentrations except for emodin, pestalotin and skyrin, which were found in more than two-thirds of the stored maize, and citreoviridin which had a mean concentration of 575 μg/kg. The presence of these non-regulated fungal metabolites corroborates previous studies where they were incriminated in the contamination of maize and maize beer (Abia et al. 2013). The bacterial metabolites, chloramphenicol, geldanamycin, monactin and nonactin (Table 4), had low concentrations in the maize grains and were therefore not a source of concern, although chloramphenicol (chlornitromycin) recorded a high incidence of 98.6 %.
Correlation between storage structures and levels of mycotoxin contamination of maize grains
Nine storage structures were identified across the AEZs and the storage structures depended on the local materials available for construction of the storage structures (Fig. 3). Storage of shelled maize grains kept in polyethylene bags on bare floors in store rooms was the most common method of storage as the farmers believe that it is easier, cheaper and more suitable for storing large quantities of grains, and this was followed by storage in “rhumbu” (local granary), while storage on roofs of residential buildings was the least common. The range of aflatoxin contamination of grains kept on bare floors was high and was between 13.25 and 656.24 μg/kg, with the least contamination in the HF and the highest in the SGS zones, respectively (Table 5). Furthermore, aflatoxin correlated positively (r = 0.79, p < 0.05) with storage structures in the DS zone and negatively with SGS and NGS zones (r = −0.734, r = −0.43; p < 0.01) respectively (Table 6). Fumonisin concentration was also high in all the storage structures across the AEZs. The highest contamination of fumonisin (4,175.72 μg/kg) was observed in maize stored in huts in the NGS zone and the least in maize stored in the HF zone (Table 5). Furthermore, a negative correlation (r = −0.79, p < 0.05) was observed between the storage structures and fumonisin contamination in the NGS zone, while a positive correlation was observed in the DS and HF zones (r = 0.41, p < 0.01; r = 0.873, p < 0.05). OTA was found only in storage structures in the DS (2.34–78.61 μg/kg), SS (3.51 μg/kg) and SGS (28.12–41.87 μg/kg) zones. The storage structures and OTA correlated positively with the NGS (r = 0.37, p < 0.01), SGS (r = 0.864, p < 0.05) and HF (r = 0.86, p < 0.05) zones respectively and negatively with the SS zone (r = −0.507, p < 0.01). DON was also present in all the storage structures across the AEZs and there was a positive correlation (r = 0.827, p < 0.005) between the storage structures and DON concentration in the HF zone (Table 6).
Co-occurrence of mycotoxins in stored maize grains
Occurrence of mycotoxin cocktails is an important aspect in the assessment of food safety because of the possible synergistic or additive effects produced in humans and animals by co-occurring toxins as a result of the interactions between the biological and toxicological properties of the toxins. In this study, several regulated mycotoxin combinations (2–17) were observed in the stored grains (Fig. 4). Aflatoxins and fumonisins co-occurred in about 65 % of the maize grains with repeated additions of ochratoxin A, deoxynivalenol, zearalenone and the emerging toxins. The co-occurrence of deoxynivalenol and/or its conjugate (deoxynivalenol glucoside) with fusaric acid are additional risks for consumers of the grains because fusaric acid is known to increase deoxynivalenol toxicity several folds, and the conjugate is capable of hydrolysing to its parent compound. The distribution of the mycotoxin mixtures were 2, 16 and 17 in the HF, DS and SGS zones, respectively.
Chronic exposure to mycotoxins, especially deoxynivalenol, aflatoxins and fumonisins through maize consumption is evident in Nigeria. This calls for concern due to chronic mycotoxicoses such as aflatoxicosis that may arise and possible additive and/or synergistic effects from constant daily exposure to the contaminated grains in addition to the possible large economic losses due to commodity rejection in international markets.
References
Abia WA, Warth B, Sulyok M, Krska R, Tchana AN, Njobeh PB, Dutton MF, Moundipa PF (2013) Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Cont 31:438–453
Adejumo TO, Hettwer U, Karlovsky P (2007a) Occurrence of Fusarium species and trichothecenes in Nigeria maize. Int J Food Microbiol 116:350–357
Adejumo TO, Hettwer U, Karlovsky P (2007b) Survey of maize from South Western Nigeria for zearalenone, α- and β-zearalenols, fumonisin B1 and enniatins produced by Fusarium species. Food Addit Contam 24:993–1000
Afolabi CG, Bandyopadhyay R, Leslie JF, Ekpo EJA (2006) Effect of sorting on incidence and occurrence of fumonisins and Fusarium verticillioides on maize from Nigeria. J Food Protect 91:279–286
Atanda OO, Ogunrinu MC, Olorunfemi MF (2011) A neutral red desiccated coconut agar for rapid detection of aflatoxigenic fungi and visual determination of aflatoxins. World Mycotox J 4(2):147–155
Atehnkeng J, Ojiambo PS, Donner M, Ikotun T, Sikora RA, Cotty PJ, Bandyopadhyay R (2008) Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int J Food Microbiol 122:74–84
Bandyopadhyay R, Kumar M, Leslie JF (2007) Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Addit Contam 24:1109–1114
Bondy GS, Pestka JJ (2000) Immunomodulation by fungal toxins. J Toxicol Env Health B 3:109–143
CAST (Council for Agricultural Science and Technology) (2003) Mycotoxins: risk in plants, animals, and human systems. CAST Task force report. No. 139. CAST, Ames, IA
Celik M, Aksoy H, Yilmaz S (2010) Evaluation of Beauvericin genotoxicity with the chromosomal aberrations, sister-chromatid exchanges and micronucleus assays. Ecotoxicol Environ Safety 73:1553–1557
Chilaka CA, De Kock S, Phoku JZ, Mwanza M, Egbuta MA, Dutton MF (2012) Fungal and mycotoxin contamination of South African comercial maize. J Food Agric Environ 10:296–303
DeBoevre M, Di Mavungu JD, Landschoot S, Audenaert K, Eeckhout M, Maene P, Haesaert G, De Saeger S (2012) Natural occurrence of mycotoxins and their masked forms in food and feed. World Mycotox J 5:207–219
Diedhiou PM, Bandyopadhyay R, Atehnkeng J, Ojiambo PS (2011) Aspergillus colonization and aflatoxin contamination of maize and sesame kernels in two agroecological zones in Senegal. J Phytopathol 159:268–275
El-Imam AMA, Ameh JB, Abdullahi IO (2012) Occurrence of fumonisins and deoxynivalenol in stored maize used in industrial productions in Zaria Nigeria. Afr J Food Sci 6:249–252
European Union Commission (2002a) Commission Directive (EC) No. 2002/27/EC of 13th March, 2002 amending Directive 98/53/EC laying down the sampling methods and the methods of analysis for the official control of the levels for certain contaminants in foodstuffs. Off J Eur Union L075:44–45
European Union Commission (2002b) Commision Decision (EC) No. 2002/657 of 12th August 2002. Implementing Council Directive EC No 96/23 concerning the performance of analytical methods and the interpretation of results. Off J Eur Union L221:8–36
European Union Commission (2006) Commission Regulation (EC) No. 1881/2006 of 19th December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364:15–24
Ezekiel CN, Bandyopadhyay R, Sulyok M, Warth B, Krska R (2012) Fungal and bacterial metabolites in commercial poultry feed in Nigeria. Food Addit Contam 29:1288–1299
Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP (2002) Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ 325:20–21
Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP (2003) Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int J Epidemiol 32:556–562
IARC (International Agency for Research on Cancer) (1993) IARC monographs on the evaluation of carcinogenic risk to humans. IARC Lyon, France 56: 445–466
IARC (International Agency for Research on Cancer) (2002) Traditional herbal medicines, some mycotoxins, napthalene, and styrene. IARC monographs on the evaluation carcinogenic risk to humans 82:1–556
Jestoi M (2008) Emerging Fusarium—mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—a review. Crit Rev Food Sci Nutr 48:21–49
Juan C, Riteni A, Marnes J (2013) Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem 141:1747–1755
Kankolongo MA, Hell K, Nawa IN (2009) Assessment for fungal, mycotoxin and insect spoilage in maize stored for human consumption in Zambia. J Sci Food Agric. doi:10.1002/jsfa.3596
Li Y, Wu F (2010) Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect 118:818–824
Logrieco A, Moretti A, Fornelli F, Fogliano F, Ritieni A, Caiaffa MF, Randazzo G, Bottalico A, Macchia L (1996) Fusaproliferin production by Fusarium subglutinans and its toxicity to Artemia salina, SF-9 insect cells, and IARC/LCL 171 human B lymphocytes. Appl Environ Microbiol 62:3378–3384
Makun HA, Adeniran AL, Mailafiya SC, Ayanda IS, Mudashiru AT, Ojukwu UJ, Jagaba AS, Usman Z, Salihu DA (2013) Natural occurrence of ochratoxin A in some marketed Nigerian foods. Food Cont 31:566–571
Manjula K, Hell K, Fandohan P, Abass A, Bandyopadhyay R (2009) Aflatoxin and fumonisin contamination of cassava products and maize grain from markets in Tanzania and Republic of Congo. Toxin Rev 28:63–69
Maziya-Dixon B, Akinyele IO, Oguntona EB, Nokoe S, Sanusi RA, Harris E (2012) Nigeria Food Consumption and Nutrition Survey. http://old.iita.org/cms/details/NFC.pdf. Accessed 5/4/2012
Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH Jr, Rothman KJ, Hendricks KA (2006) Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect 114:237–241
Ncube E, Flett BC, Waalwijk C, Viljoen A (2011) Fusarium spp. and levels of fumonisins in maize produced by subsistence farmers in South Africa. S Afr J Sci. doi:10.4102/sajs.v107i1/2.367
O’Brien E, Dietrich DR (2005) Ochratoxin A: the continuing enigma. Crit Rev Toxicol 35:33–60
Pestka JJ (2010a) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679
Pestka JJ (2010b) Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J 3:323–347
Shephard GS (2004) Mycotoxins worldwide: Current issues in Africa. In: Barug D, Van Egmond HP, López Garciá RW, Van Osenbruggen A, Visconti A (eds) Meeting the mycotoxin menace. Academic , Wageningen, pp 81–86
Shephard GS, Burger H-M, Gambacorta L, Krska R, Powers SP, Rheeder JP, Solfrizzo M, Sulyok M, Visconti A, Warth B, van der Westhuizen L (2013) mycological analysis and multimycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J Agric Food Chem 61:8232–8240
Soleimany F, Jinap S, Abas F (2012) Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem 130:1055–1060
FAO Statistics Division (2013) Food and Agricultural commodities production. http://faostat.fao.org/site/339/default.aspx. Accessed 18/3/2013
Sulyok M, Krska R, Schuhmacher RA (2007) Liquid Chromatography/tandem mass spectrometric multi-mycotoxin method for quantification of 87 analytes and its application to semi-quantitative screening of mouldy food samples. Anal Bioanal Chem 389:1505–1523
Turner PC, Collinson AC, Cheung YB, Gong YY, Hall AJ, Prentice AM, Wild CP (2007) Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol 36:1119–1125
Udoh JM, Ikotun T, Cardwell KF (2000) Storage structures and aflatoxin content of maize in five agroecological zones of Nigeria. J Stored Prod Res 36:187–201
United States Department of Agriculture (USDA) Foreign Agricultural Service (FAS) (2012) Nigerian grain and feed annual report, NII204. Global Agriculture Information Network (GAIN), Washington DC
Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R (2009) Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 395:1355–1372
Warth B, Parich A, Atehnkeng J, Bandyopadhyay R, Schuhmacher R, Sulyok M, Krska R (2012a) Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J Agric Food Chem 60:9352–9363
Warth B, Sulyok M, Fruhmann P, Mikula H, Berthiller F, Schuhmacher R, Hametner C, Abia WA, Adam G, Fröhlich J, Krska R (2012b) Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun Mass Spectrom 26:1533–1540
Acknowledgments
The authors give thanks to Mr Isaac Ogara of the Nasarawa State University, Lafia Campus for his assistance towards the collection of the maize grains. The co-authors from IFA-Tulln also acknowledge the Government of Lower Austria.
Source of funding
The authors are grateful to the Institute of Food Security, Environmental Resources and Agricultural Research, Federal University of Agriculture, Abeokuta, Nigeria for part funding (IFSERAR/UNAAB/IRG 70) the research work.
Conflict of interest
The Institute of Food Security, Environmental Resources and Agricultural Research (IFSERAR), Federal University of Agriculture, Abeokuta, Nigeria has no interest in the publication of the results and thus there is no conflict of interest. The authors are in full control of all the primary data reported in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adetunji, M., Atanda, O., Ezekiel, C.N. et al. Fungal and bacterial metabolites of stored maize (Zea mays, L.) from five agro-ecological zones of Nigeria. Mycotoxin Res 30, 89–102 (2014). https://doi.org/10.1007/s12550-014-0194-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-014-0194-2