Abstract
Thixoforming is one of the members of the family of semi-solid forming processes. It is possible to obtain complex shape of materials by this method and unconventional microstructures can be achieved due to its thixotropic properties. However, in this study thixoforming was used not only as forming method but also as a manufacturing method. Metallic composites were manufactured by a process of integration of powder metallurgy and semi-solid production technique. The mixture of Co72.5B15Si12.5 alloy and Fe powders become partially melted, compressed mechanically in semi-solid state and filled intricate cavities. Without changing alloy structure, it could be possible to reinforce it with Fe powders. Moreover, the final shape of the product could be achieved at the same time. Prior to the process, Co72.5B15Si12.5 alloy obtained in the form of stick was characterized by X-ray diffraction (XRD), scanning electron microscopy–energy dispersive spectroscopy (SEM–EDX), differential thermal analysis-thermogravimetry (DTA-TG), light microscope and micro-hardness machine. The stick was milled to get alloy powders and they were blended with iron. Characterization of the structures of the composite material was performed. It was concluded that the heating process of iron and alloy powders provides the formation of compact metal matrix composites.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Practical applications in metal forming have been made from the ancient centuries for 1000 years. In the prehistorical time, the ductile materials like gold and silver were used to produce thin sheets of valuable metals by cold working. They were hammered with stones and they were attained subtle sheets. Starting with the progression of the rolling mill, the sheet metal production breakthrough was transpired. The first known of a rolling mill was designed by Leonardo. His one of the drawings dated 1480 depicted in Fig. 1. With this drawing, it was described for the first time modifying the thickness by making a material pass between two cylindrical rollers with parallel axes [1,2,3].
In the modern life, forming can be simply defined as fabricating the plastic modification of a shape while keeping the mass and cohesion of the material. Additionally, the modification of a shape is performed with controlled geometry, thus forming differs from deformation with this property [1, 2].
In forming technology, desired geometry is generally obtained by multi-step processes however in the manufacturing technology, the state of the art must be reducing the steps in the production line. By present day processes, forming of metal alloys into complex configurations seems difficult. Some special requirements cannot be achieved at every application [4]. Besides, traditional methods have limitations for some materials with reputable problems related with producing, for instance residual stress [5].
At this point, thixoforming is appeared as a forming process which exploits metal rheological behaviour during solidus and liquidus range temperature. Superior mechanical properties and excellent formability components can be produced with this method, especially in automotive industries. Less casting defect components, such as macrosegregation, shrinkage and porosity, are obtained also. [6,7,8]. In this technique partially melted material retains a fraction of solid phase, behaves thixotropically when sheared, and it fills the intricate cavities in a single forming step. The material is brought to a phase in between liquid and solid state, and formed by using high frequency resistance heating. The thixotropic rheological behavior under shearing is a consequence of the (primary phase) spheroidal morphology within liquid. Current is carried to the sample by using an electrode (usually Cu). The electrode is cooled in water [9]. For successful process, solid spheroids in the liquid matrix of must be existed in the semi-solid state. Thus the material starts to behave thixotropically [10, 11]. On heating into the state of the semisolid, recrystallization can be substantiated and the liquid starts to penetrate the crystallized boundaries. Thereby, this leads to result in spheroids surrounded by the liquid. One of the advantages of this is that the alloys can be ensured in the extruded state in any circumstance. Another one; the spheroids are more fully rounded, and this can make the material become more thixotropic [12]. Below the temperature of recrystallization, the extrusion has taken place. This will threat the material to have long, thin grains in the extruded microstructure.
Although, thixoforming has been explained as a forming method in this article, it can be used to produce complex shapes of products. Fabrication of final product by using low forming forces, achieved time, energetic reduction and one step formation can be considered. It seems to be competitive when the intended part associates with intricate design, combining thin and thick section, to reduce and eliminate machining, and for high integrity parts [9].
Based on this, thixoforming was used as a fabrication method for a metal matrix composite material in this study. The powdered mixture of Co72.5B15Si12.5 and Fe powders was laid out in one technological chain by mechanical alloying. Then by a combination of powder metallurgy and thixoforming processes, net shape composite product was produced in a single step. Characterization of the produced structure was carried out by XRD, SEM–EDX, DTA-TG, light microscope and micro-hardness test machine.
2 Materials and Methods
Co72.5B15Si12.5 was used as composite metal matrix in crystalline phase. It was received in the form of sticks and they were milled in the high energy ball mill before the semi-solid process. In this step, cold welding repetition occurs, powder particles are fractured because of the high energy collision between balls and ceramic powder [13]. When the balls hit to the vial walls, a high-energy collision was emerged between the vial and the balls. This leads to an extreme deformation in the material mechanically. The reason for that can be the producing of the escalated stress concentration and lattice strains, and this created the generation of the fractures. Then owing to the impact of balls on the integrated powder particles, they were easily crushed, cold-welded, fractured, and re-welded.
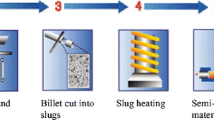
100 micron size pure Fe powder was used as an additive material to reinforce the composite. Schematic illustration of the process steps is demonstrated in Fig. 2 [14]. The powder mixture was compacted into the steel container which has 5 mm diameter and 30 mm height. In order to obtain better compaction, matrix and additive materials have been mechanically compressed during heating to semi-solid state. To fill the die cavity outright, it is required the heating of the material to the temperature of 1300 °C. Heating time was 58 s and cooling rate was 847 C/s. In the process, the most difficult steps are reflected as the heating of semi products speedily and conditioning the material in semi-solid state. The tread of heating of the material to the semi-solid state should be implemented with the high accuracy for supplying necessary temperature region in the heated semi product. Forming was carried out with a force of 7 kN. The main body of the die is manufactured from a titanium alloy. Copper electrodes provide the deformation and heating. The electrodes movement is consolidated by a hydraulic driver. The shape of the product is contingent upon the die cavity with shape insert which is changeable. Experimental equipment used in this study in University of West Bohemia is depicted in Fig. 3.
The hardness was determined using a Vickers hardness testing machine. Optical photographs were obtained by OLYMPUS OLS -3000 light microscope. The surface morphology and microstructure were characterized by a scanning electron microscope (TESCAN VEGA 3 SBH). The phase analysis of the materials was performed with the help of a Empyrean X-ray diffractometer with Cu Kα radiation (λ = 1.540 Å), in the 2θ range of 20–800. DTA-TG analysis was performed by using Simultaneous Thermal Analysis apparatus NETZSCH STA 409CD.
3 Results and Discussion
The material hardness is measured as 440 HV. SEM analysis depicted in Fig. 4 that the as-received alloy microstructural features. It can be seen that dendritic solidification is typical, and interdendritic eutectic silicon and cobalt exist.
Figure 5 shows optical microscopy analysis results. They demonstrate similar results with SEM images of Co72.5B15Si12.5 alloy. Figure 6 depicts its XRD analysis. The crystalline peaks of Co–Bi–Si alloy can be seen at 29°, 53.5°, 60.5° and 98.5°. Co2B peaks are appeared at 2θ values of 53, 51.5, 54.5 and 57.5. The major peaks of Co2Si are produced around 53.2°. It can be said that beside the Co–Bi–Si alloy, Co2B and Co2Si phases exists in the material.
As thixoforming is performed considering partial melting, DTA analysis for the experiment is that of the heating cycle. DTA-TG curves were implemented to view the thermal behavior of the alloy of Co72.5B15Si12.5 regarding the decomposition and phase formation at a heating rate of 10 °C/min in the temperature range of 25–1200 °C under argon atmosphere (Fig. 7a, b). The sample’s initial weight was 27.4 mg. Crystallization and liquidus temperatures of Co72.5B15Si12.5 are 913 °C and 1081 °C, respectively. As can be seen that the material has a moderate interval of the temperature in between the solid and liquid state in which the suitable working in a semi-solid state is performed. The results show the crystalline structure of the material and support the XRD results. Regarding the heating treatments (Fig. 7a), the melting peaks exists at 1047.7 °C. In the cooling stage, the crystallisation peak can be seen starting at 885.5 °C and ending at 962.5 °C. Moreover, an interesting feature observed is the expected delay in the transformations due to kinetic effects. Transformations are pushed to higher/lower temperatures during melting/solidification.
DTA heating/cooling rates are of 10 °C/min. The heating rate employed in the partial melting of the thixoforming slurry was 22 °C/s. The later is approx. 132 times larger. It can be compared and commented the consequences of this difference in terms of the kinetically affected phenomena and the reliability of the DTA technique used for this purpose. The fact that the applied heating rate is by far larger than the achieved via DTA can result in a considerable delay of the transformations (to higher temperatures) in the SSM operation when compared to the DTA prediction (heating cycle). On the other hand, this will explain why the slurry was still semisolid at the temperature of 1300 °C even if its predicted liquidus was below 1200 °C according to DTA results.
The liquid fraction (Flynn’s partial area integral of the DTA curve with relation to temperature) is an important parameter to choose the SSM working window and understand the metallurgical phenomena occurring in the semisolid state. The liquid fraction indicates at which temperature the eutectic is molten and at which liquid fraction the slurry is during forming. If the liquid fraction is too low, extremely refined spheroidal particles of primary phase are needed to achieve good thixoformability. If the liquid fraction is too high, solid particles can present a poorer (coarser and dendritic) microstructure. In the later case, the excessive liquid can compensate the morphology of solid resulting in acceptable apparent viscosity. On the other hand, high liquid fraction can result in a large amount of molten primary phase, which is undesirable in SSM operations.
The sensitivity (derivative of the liquid fraction curve with relation to temperature, i.e. dfl/dT) is another important parameter in the SSM process control and repeatability. The sensitivity is the ultimate information to define the SSM working window, since it predicts the thermodynamic stability of the slurry.
In Fig. 8, using the by thixoforming, cross section of the manufactured composite specimen can be seen. Rather than using liquid metal, the metal mixture was used partially solid and partially liquid and high frequency resistance heating. This procured the filling of the die by semi-solid metal in a non-turbulent manner. By this was, the contingent defects like harmful porosity could be substantially defeated [15,16,17]. As can be seen in the optical photographs (Fig. 8), the microstructure patterns are similar in the cavity part, Therefore, it can be evaluated that thixoforming has been done successfully in the cavity part and the compact structure has been obtained. Some eutectic phase division is observed towards edges of the cavity part with attendance of enriching of Co–Fe phases. This is typically seen in most thixoforming processes. Under forming pressures, it is engaged with the squeezing out of the liquid phase. A sound part with feasible uniform properties was produced by thixoforming. With respect to a die cast counterpart it was ascendant. Substantial dispersed porosity can be suffered in this manner [18].
Microstructural cross-sectional areas can be seen in Fig. 9. The structure is composed of polyedric grains of martensite. Partially Lamellar network clusters around these grains. The progress of this high fraction of martensite was eventuated due to the contribution of chemical composition. However, the reasons for that also can be accounted as the high cooling rate. The structure of obtained product was homogenous. On the cooling plate, a kind of fractional solidification occurred. This was in charge of the change in size and primary phase morphology by promoting further by the limited melt superheat [18]. The elements in the structure of the specimen can be seen by EDX-Line analysis (Fig. 10). It was observed that the martensite phase having higher content of Fe and Co. Nevertheless, in the network Si concentration is higher. It is said to be exhibiting typical microstructural patters of dendritic solidification [18]. The results demonstrated that the matrix was probably generated from Fe powder and the network formed from the metallic powder. Thus, Co diffused to the Fe-reach matrix thank to high temperature in the semi-solid state.
Figure 11 depicted XRD analysis of thixo-specimen. The crystalline phases can be easily seen. Besides, different from Fig. 6, Fe elements are appeared in the analysis. It is proved that without changing the alloy structure, Fe elements were doped to the composite. This is the difference in between alloys and composites as the main aim of this study.
Figure 12 demonstrates DTA analysis of the composite thixo-specimen. The initial weight was 27.7 mg. It could be possible to see crystallization peaks Fig. 12a. The first thermal phenomena is an exothermic reaction starts at 693.6 °C. Second thermal phenomenon started 891.3 °C and the peak seems at 902.9 °C. The second reaction was started at 1085.1 °C, and the accompanying peak is appeared at 1095.1 °C. Those endothermic reactions refer to the melting reactions of the materials. There were indicated different melting points from the graph, because the composite specimen has varied materials (matrix and additives). In Fig. 12b, three exothermic thermal reactions can be seen. These are corresponding to crystallization of the materials in the cooling process. The peaks are 809.6 °C, 836.0 °C, 1048.3 °C and respectively.
The expected delaying of endo/exothermic transformations to higher/lower temperatures due to kinetic effects is not seen for the peak at 730 °C (Fig. 12a) and the correspondent valley at 809.6 °C (Fig. 12b). Furthermore, in the cooling curve the lower temperature transformations are overlapped forming a single valley with two visible transformations apexes. This difference may be caused by inhomogeneity in the sample originated during forming. If the first DTA heating cycle was not discarded, this inhomogeneity probably caused the abnormal shape of the curve, which was not present in the second cycle since the material was homogeneized after melting.
To examine the mechanical properties of the product, hardness value was measured as 620 HV. It can be seen that by reinforcing the alloy with Fe powders the hardness is increased from 440 to 620 HV.
4 Conclusion
A metal matrix composite was manufactured by thixoforming process. As a process of integration of powder metallurgy and semi-solid production, the mixture of partially melted Co72.5B15Si12.5 alloy and Fe powders compressed mechanically in semi-solid state and filled intricate cavities. The fabrication of final product in one forming step, achieved time and energetic reduction was achieved. Having low number of defects, compact structure was achieved, distribution of Fe particles was admissible. The structure is composed of polyedric grains of martensite phase. Matrix was generated from Fe powder and the network formed from the metallic powder as in the network Si concentration is higher, it is exhibiting typical microstructural patters of dendritic solidification. By reinforcing the alloy with Fe powders the hardness of the alloy was increased.
References
K. Osakada, History of plasticity and metal forming analysis, ICTP 2008 The 9th International Conference on Technology of Plasticity. Osaka University, Japan (2008)
Schuler, Basic Principles of Metal Forming Metal Forming Handbook (Springer, Berlin, 1998)
Redazione, A short sheet metal history, Posted on 9 June 2014 in Sheet metal, Metal Work Magazine. http://www.metalworkingworldmagazine.com/a-short-sheet-metal-history/
C.H. Hamilton, F.B. Koeller, R.S. Raymond, M. Goldberg, Controlled environment superplastic forming of metals, US 3934441 A (1976)
T. Özel, D. Ulutan, Prediction of machining induced residual stresses in turning of titanium and nickel based alloys with experiments and finite element simulations. CIRP Ann. Manuf. Technol. 61, 547–550 (2012)
N.H. Husain et al., IOP Conf. Ser. Mater. Sci. Eng. 257, 012053 (2017)
Salleh, M.Z. Omar, J. Syarif, M.N. Mohammed, An overview of semisolid processing of aluminium alloys. ISRN Mater. Sci. (2013). https://doi.org/10.1155/2013/679820
T.Y. Liu, H.V. Atkinson, P. Kapranos, D.H. Kirkwood, S.C. Hogg, Metall. Mater. Trans. A 34, 1545–1554 (2003)
D. Aisman, H. Jirkova, L. Kucerova, B. Masek, Metastable structure of austenite base obtained by rapid solidification in a semi-solid state. J. Alloys Compd. 509S, S312–S315 (2011)
A. Rassili, H.V. Atkinson, A review on steel thixoforming. Trans. Nonferrous Met. Soc. China 20, 1048–1054 (2010)
A. Pola, M. Tocci, P. Kapranos, Microstructure and properties of semi-solid aluminum alloys: a literature review. Metals 181, 1–17 (2018). https://doi.org/10.3390/met8030181
G. Vaneetveld, A. Rassili, J.-C. Pierret, J. Lecomte-Beckers, Improvement in thixoforging of 7075 aluminium alloys at high solid fraction. Solid State Phenom. 141, 707–712 (2008)
P. Sharma, S. Sharma, D. Khanduja, On the use of ball milling for the production of ceramic powders. Mater. Manuf. Process. 30, 1370–1376 (2015)
J.C. Pierret, A. Rassili, G. Vaneetveld, J. Lecomte-Beckers, Stability of steel thixoforming process. Trans. Nonferrous Met. Soc. China 20, 937–942 (2010)
Q. Zhu, S.P. Midson, Semi-solid moulding: competition to cast and machine from forging in making automotive complex components. Trnas. Nonferrous Met. Soc. China 20, 1042–1047 (2010)
Midson S, Semi-solid processing. Retrieved on 01.02.2016 from http://www.themidsongroup.com/ssp.asp
B. Mašek, F. Vančura, D. Aišman, H. Jirková, M.F.-X. Wagner, Effect of input structure of blank on development of final structure when processing at temperatures between solidus and liquidus. Procedia Eng. 100, 722–729 (2015)
Y. Birol, Forming of AlSi8Cu3Fe alloy in the semi-solid state. J. Alloys Compd. 470, 183–187 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atay, H.Y., Aišman, D., Jirková, H. et al. Use of Thixoforming as a Manufacturing Method for Metallic Composites. Met. Mater. Int. 26, 1420–1429 (2020). https://doi.org/10.1007/s12540-019-00373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-019-00373-5