Abstract
Copper (Cu) transporters have primary importance in maintenance of physiological limits of Cu homeostasis in plants. However, structural characterization of Cu transporters in many plant species is still limited. In this study, a total of 78 potential Cu transporter genes were identified from 18 different plant species. Study revealed that Cu transporters could be characterized with a CTR protein family (PF04145) domain, three putative transmembrane domains (TMDs), a single exon number, and a basic character. Met-rich motifs at N-terminal region, MXXXM motif in TMD-2, and GXXXG motif in TMD-3 could be essential for Cu transport since they were highly conserved in all analyzed species. In phylogeny, a clear distinction was observed between Cu transporter sequences of lower and higher plants. General topological features of Cu transporters in higher plants—monocots and dicots—were highly conserved compared to lower plants. Identification of Cu transporter homologous in various plant species and their comparative analysis at gene and protein levels will become valuable theoretical basis for future studies aiming to further characterization and molecular manipulation of Cu transporters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper (Cu) functions as a structural element in regulatory proteins as well as a cofactor in many enzymes such as Cu/Zn superoxide dismutase (SOD), cytochrome c oxidase, amino oxidase, laccase, plastocyanin, and polyphenol oxidases. It is also involved in variety of metabolic processes, including photosynthetic electron transport, mitochondrial respiration, superoxide scavenging, cell wall metabolism, and hormone signaling [1, 2]. Cu deficiency primarily affects the young leaves and reproductive organs, while its higher concentration or toxicity causes the chlorosis, necrosis, stunting, leaf discoloration, root growth inhibition, and formation of harmful reactive oxygen species (ROSs) [1, 3]. Thus, plants are required to have a sophisticated network to meditate the copper uptake, utilization, and detoxification [4]. In Arabidopsis, Cu+ is transported into cytosol via high-affinity six COPT1-6 transporters, while its efflux occurs through the P-type ATPases such as PAA1, PAA2, RAN1, HMA1, and HMA5. In addition, metallochaperones such as ATX1, CCH, and CCS1 distribute the intracellular Cu ions to their final destinations [5–8]. COPT transporters belong to CTR protein family [2], which contains three putative transmembrane domains (TMDs) with N- and C-terminal regions in extracellular space and cytosol, respectively (Fig. 1) [9, 10].
a Putative alignment of COPT family of Cu transporters in Arabidopsis. b Predicted topological structure of CTR/COPT proteins (modified from Peñarrubia [14]). Red, green, purple, and blue colors indicate Met-rich motifs, TMDs, Gly-rich, and Cys-rich motifs, respectively
N-terminal region of all Arabidopsis COPT members, except for AtCOPT4, contains a variable number of Met-rich motifs and His-rich residues. Studies of yeast CTR family have reported that Met-rich motifs selectively sequester Cu+ ions from oxidizing environment and stabilize them with thioester groups in Met residues to translocate in cytosol [9, 11, 12]. In green algae, CrCTR1 and CrCTR2 transporters included six putative Cu-binding motifs rich in Met and Cys residues [13]. A Met residue approximately at 20 amino acids before TMD-1 and an MxxxM motif within TMD-2 in Arabidopsis were reported to be essential in Cu transport [14]. Moreover, human and yeast CTR proteins assemble at membrane as trimers [15]. Yeast CTR1 mutants demonstrated that a conserved GxxxG motif in TMD-3 functions as a glycine zipper in helix packing and trimer assembly as well as for delivery of transporters to their targets [16]. In addition, MxxxMx12GxxxG motif signature is reported to be strictly conserved in CTR members [14]. Many CTR proteins, including AtCOPT1, 2, and 5 contain Cys residues (CxC motif) in their C-terminal regions (refer to Fig. 1). It has been also reported that Cys-rich motifs in C terminus of yeast CTR1 could sense the elevated levels of intracellular Cu concentration and regulate the Cu transport, thereby protecting the cells from Cu toxicity. Besides, C-terminal region was also reported to participate in Cu transfer to cytosolic metallochaperones [17]. Furthermore, transcriptional activation of Cu deficiency responsive genes such as FeSOD, COPT1, and COPT2 in Arabidopsis is controlled by transcription factor SPL7 via binding to reiterative cis-regulatory GTAC motifs in promoter [18]. Besides, a group of Cu-microRNAs such as miR397, miR398, miR408, and miR857 also include reiterative GTAC motifs in their promoters, which allow their transcriptional activation by SPL7 in response to Cu deficiency, leading to the degradation of mRNAs encoding non-essential cuproproteins [19]. Considering all these mechanisms, intracellular copper concentrations and its physiological demand are dynamically regulated.
In this study, we have identified potential Cu transporter genes from 18 different plant species; provided detailed information about their physicochemical properties; predicted putative TMDs and conserved motifs; constructed phylogenetic trees using protein sequences; and generated 3D models. Identification of Cu transporter homologs in various plant species and their comparative analysis at primary, secondary, and tertiary structural levels will become a valuable theoretical basis for future studies aiming to further characterize these sequences at structural as well as functional levels.
2 Materials and Methods
2.1 Retrieval of Cu Transporter Genes and Proteins
Six Arabidopsis COPT protein sequences such as COPT1 (Q39065.2), COPT2 (Q9STG2.1), COPT3 (Q9FGU8.1), COPT4 (Q8SAA5.2), COPT5 (Q93VM8.1), and COPT6 (Q8GWP3.1) were obtained from UniProtKB/Swiss-Prot database of NCBI (ncbi.nlm.nih.gov/). UniProtKB/Swiss-Prot database harbors the non-redundant, manually curated high-quality protein sequences [20]. These obtained sequences were used as queries in proteome datasets of 18 plant species including, Arabidopsis thaliana, Brachypodium distachyon, Brasica rapa, Chlamydomonas reinhardtii, Cucumis sativus, Eucalyptus grandis, Glycine max, Gossypium raimondii, Medicago truncatula, Oryza sativa, Phaseolus vulgaris, Physcomitrella patens, Populus trichocarpa, Prunus persica, Solanum lycopersicum, Sorghum bicolor, Vitis vinifera, and Zea mays in Phytozome database (phytozome.jgi.doe.gov/pz/portal.html) with a ≤ e-15 threshold value, except for C. reinhardtii (<e-2) and P. patens (<e-10). Later, redundant sequences were removed and remaining 78 putative Cu transporter sequences were used in this study. Phytozome is a plant-based genomic database containing the sequenced genomes of different plant species. It also provides wide search, comparison, and data visualization options with different specialized interfaces [21].
2.2 Sequence Analysis of Cu Transporters
Sequence length, molecular weight, and isoelectric point (pI) of Cu transporter proteins were determined by using ProtParam tool (web.expasy.org/protparam/), which allows the computation of physical and chemical parameters of protein sequences [22]. Subcellular localization of transporters was predicted by CELLO server (cello.life.nctu.edu.tw/). It is a multi-class support vector machine (SVM) classifier combining votes from four types of sequence compositions such as amino acid, di-peptide, partitioned amino acid, and sequence compositions, and makes a final decision [23]. Protein domain families were checked in Pfam (pfam.xfam.org/), which is a comprehensive protein family database employing the hidden Markov model (HMM) [24]. Transmembrane domains (TMDs) were predicted by SCAMPI server (scampi.cbr.su.se/), which uses a scale of experimentally obtained free-energy contributions from each amino acid to helix insertion efficiency to predict the putative TMDs [25]. Exon/intron numbers and organization of COPTs were analyzed by using GSDS server (gsds.cbi.pku.edu.cn/). Server requires CDS and genomic sequences in FASTA format for analysis [26].
2.3 Conserved Motif and Phylogenetic Analyses of Cu Transporters
Conserved motifs were analyzed by using MEME tool [meme-suite.org/tools/meme; 27] with following parameters; maximum number of motifs to find, 5; minimum width of motif, 6; and maximum width of motif, 50. Protein sequences were aligned by ClustalW (downloadable from clustal.org/clustal2/) using multiple alignment tool for 1000 bootstraps [28] and visualized by BioEdit (downloadable from mbio.ncsu.edu/bioedit/bioedit.html) Sequence Alignment Editor. Traditional or rectangular phylogenetic tree was constructed by MEGA 6 [downloadable from megasoftware.net/; 29] using maximum likelihood (ML) method for 1.000 replicates bootstrap.
2.4 3D Modeling and Interaction Partner Analyses of Cu Transporters
3D models of Cu transporters were predicted by I-TASSER server (zhanglab.ccmb.med.umich.edu/I-TASSER/), which is an integrated platform employing the composite approaches such as threading, ab initio modeling and atomic-level structure refinement in protein structure prediction [30]. The constructed 3D models were visualized by Pymol (downloadable from pymol.org/). It is an open-source tool allowing to produce the high-quality 3D images of biological molecules [31]. Model quality was checked by Ramachandran plot analysis (mordred.bioc.cam.ac.uk/~rapper/rampage.php), which shows the phi (φ)–psi (ψ) torsion angles of residues in a given protein structure and calculates the allowed and disallowed regions of amino acids which indicate the protein stability thereby model reliability [32]. Potential interaction partners of Cu transporters were predicted by using STRING server (string-db.org/), which harbors the putative interactions of physical and functional associations of known and predicted proteins [33].
3 Results and Discussion
3.1 Retrieval of Cu Transporter Sequences
Six high-affinity Arabidopsis COPT1-6 protein sequences were used as queries in Phytozome database to obtain the corresponding homologs of COPT members in 18 plant species. A total of 78 COPT gene sequences were retrieved from Phytozome database after having filtered for ≤ e-15 threshold value, with exception of C. reinhardtii (<e-2) and P. patens (<e-10), and having redundant sequences removed. Retrieved genes included 6 genes for A. thaliana; 2 genes for B. distachyon; 9 genes for B. rapa; 3 genes for C. reinhardtii; 3 genes for C. sativus; 6 genes for E. grandis; 6 genes for G. max; 8 genes for G. raimondii; 6 genes for M. truncatula; 3 genes for O. sativa; 5 genes for P. vulgaris; 2 genes for P. patens; 4 genes for P. trichocarpa; 5 genes for P. persica; 3 genes for S. lycopersicum; 3 genes for S. bicolor; 2 genes for V. vinifera, and 2 genes for Z. mays (Table 1).
3.2 Gene and Protein Features of Cu Transporters
A total of 78 potential Cu transporter sequences were identified from 18 different plant species. Later, we retrieved genomic, CDS, and protein sequences of these Cu transporters. Cu transporter proteins included a CTR Cu transporter family (PF04145) domain and had 14.0–18.9 kDa molecular weight (except for C. reinhardtii with 24.2–77.4 kDa) and 126–183 amino acid (aa) length (except for C. reinhardtii with 241–766 aa) with 5.17–10.21 pI value (Table 1). Protein sequences were mainly in basic character, and subcellular localizations were mainly predicted as plasma membrane. All copper transporters, except for C. reinhardtii (green alga), were predicted to have three putative TMDs with an extracellular N-terminal and cytosolic C-terminal regions. However, C. reinhardtii has four putative TMDs with cytoplasmic N- and C-terminal regions. Notably, all Cu transporter transcripts, except for C. reinhardtii, contained only a single exon, suggesting that COPT genes may have been well conserved during plant genome evolution. Previous studies have also made similar indications, in which COPT transporters were reported to belong CTR protein family and included three putative TMDs with N- and C-terminal regions in extracellular and cytosol, respectively (refer to Fig. 1) [2, 9, 10]. Therefore, we mention that physicochemical properties and topological features of Cu transporter sequences identified in this study comply with the general features of Cu transporters mentioned in literatures. Overall, we may report that in feature studies regarding the identification of Cu transporters in more plant species, CTR Cu transporter family (PF04145) domain, three putative TMDs, a single exon number, and basic (pI) nature of Cu transporters could be used in their characterization.
3.3 Conserved Motif and Primary Sequence Analysis
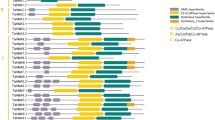
We have searched the most conserved five motifs in Cu transporter sequences using MEME tool (Table 2). Motif 1–3 were found to be related with CTR Cu transporter family (PF04145) and highly conserved in almost all species while motif 4 and 5 did not relate any protein family.
A total of 78 potential Cu transporter proteins from 18 plant species were aligned by ClustalW, and identical and similar residues were shaded as black and gray, respectively (Fig. 2). Approximate locations of three putative TMDs, and N- and C-terminal regions were determined based on the sequence alignment and general topological features of Cu transporter proteins mentioned in previous studies. Predicted locations of TMDs were labeled above the sequences as TMD 1–3. Variable number of Met-rich motifs in N-terminal region and an MXXXM motif in TMD-2 in Arabidopsis have been reported to be essential residues for Cu transport [14]. We have identified these Met-rich motifs at N-terminal region and MXXXM motif in TMD-2. MXXXM motif (framed with a red rectangular in Fig. 2) was observed to be strictly conserved in all aligned species in TMD-2. Variable number of Met-rich motifs at N-terminal region was also found but only most conserved ones were labeled due to sequence variations of aligned species. Studies of yeast CTR1 mutant demonstrated that a conserved GXXXG motif in TMD-3 functions as a glycine zipper, which is important for helix packing, trimer assembly and delivery of transporters to their targets [16]. This GXXXG motif (framed with green rectangular in Fig. 2) was also identified in TMD-3 of all aligned species. Highly conserved structure of this motif indicates that it may have a potential role as a glycine zipper in various plant species. Besides, MXXXMX12GXXXG signature motif, which is a combination of MXXXM motif in TMD-2 and GXXXG in TMD-3, is reported to be strictly conserved between CTR members [14]. This MXXXMX12GXXXG signature motif was also observed to be highly conserved in all 18 species. Moreover, any particular signature motifs were not observed in TMD-1, but only one column of Tyr, Leu, and Phe residues had some degree of conservation. Many CTR proteins, including AtCOPT1, AtCOPT2, and AtCOPT5, were also reported to include a CXC motif in their C-terminal regions as well as they involve in intracellular Cu regulation and Cu transfer to cytosolic metallochaperones [17]. In aligned sequences, this CXC motif, if present, was mainly in CAC form and located either at complete end or one residue before the end at C terminus. We have identified this motif in all sequences of AtCOPT1, AtCOPT2, AtCOPT5, and OsCOPT5.1 homologs (Fig. 2), therefore complying with the reports of previous studies [17]. In addition, this motif was identified in about 30 out of 78 aligned sequences. Therefore, presence or absence of this CXC motifs may be related with Cu tolerance in plant species since it is related with modulation of intracellular Cu concentration as well as Cu transfer to cytosolic metallochaperones, thereby protecting the cells from Cu toxicity [17]. However, to confirm this requires the further physiological and molecular Cu perturbation studies in different plant species.
Sequence alignment of Cu transporter proteins in 18 plant species. Protein IDs (Phytozome) were specified along with their corresponding homologs. Sequences were aligned by ClustalW, and identical and similar residues were shaded as black and gray, respectively. TMDs, and N terminus and C terminus regions were indicated above the sequences. Red rectangle shows the MXXXM motif, which is essential for Cu transport, in TMD-2, and green rectangle shows the GXXXG motif, which functions in helix packing and trimer assembly, in TMD-3. 1Met-rich motifs are located in N-terminal region of proteins and involved in Cu transport. Due to sequence variations of aligned sequences, only some were labeled. 2CXC motifs are located at end of C-terminal region and reported to function as an intracellular Cu sensor. Due to sequence variations of aligned sequences, its location was roughly indicated
Furthermore, we have also identified that most conserved three motifs, which also include CTR Cu transporter domain, are located within TMD 1–3 and N-terminal region of Cu transporters. This could indicate that TMD 1–3 and N-terminal region are the indispensable part of Cu transporters. Overall, Cu transporter sequences in analyzed species were identified to have three putative TMDs with an extracellular N-terminal and a cytoplasmic C-terminal regions, except for C. reinhardtii. Met-rich motifs at N-terminal region, MXXXM motif in TMD-2, and GXXXG motif in TMD-3 could be essential for Cu transport since they were highly conserved in all analyzed species. Thus, these residues could be also used in characterization of Cu transporter sequences in plant species.
3.4 Phylogenetic Analysis
Phylogeny was constructed using 78 potential Cu transporter proteins from 18 plant species. Protein sequences were labeled along with their corresponding homologs in A. thaliana and O. sativa since they showed the highest homology for these two species. Homology information was used as a benchmark to evaluate the clustering of protein sequences. For this, a separate phylogenetic tree was first constructed using experimentally characterized A. thaliana and O. sativa COPT members to find out their distribution among themselves (Fig. 3).
Phylogenetic analysis of COPT members involved in Cu transport in A. thaliana and O. sativa. The unrooted phylogenetic tree was constructed with Q39065.2, Q9STG2.1, Q9FGU8.1, Q8SAA5.2, Q93VM8.1, Q8GWP3.1, Q94EE4.1, Q60EN8.1, Q69P80.1, and Q7XTF8.1 NCBI accession numbers sequences by using maximum likelihood (ML) method in MEGA 6
Then, main phylogeny was constructed with identified Cu transporter sequences. Tree was divided into two main groups namely as A and B (Fig. 4). Later, group A was further subdivided into three subgroups such as A1, A2, and A3 based on tree topology. Subgroup A1 included AtCOPT1–4, and 6, and OsCOPT1, 2, and 6 homologs without any monocot/dicot separation with 88 % bootstraps. The clustering of these sequences in Arabidopsis and Oryza tree (Fig. 3) explained why these sequence homologs closely grouped in subgroup A1. Subgroup A2 contained AtCOPT5 and OsCOPT5.1 homologs without any monocot/dicot separation with 92 % bootstraps. Arabidopsis and Oryza tree (Fig. 3) also demonstrated that COPT5 sequences were distinctly separated from other COPT members. This also indicated that COPT5 homologs could have more conserved structure than other members. Subgroup A3 included P. patens (moss) sequences. Although they showed homology with AtCOPT5, they formed a separate group with 81 % bootstraps, indicating a separation between lower and higher plants in terms of Cu transporters. In group B, only C. reinhardtii (green alga) sequences clustered with 75 % bootstraps and demonstrated more homology to other species than plants. Overall, we may report that there could be a possible distinction between Cu transporter sequences of lower and higher plants.
Phylogenetic tree of Cu transporter proteins from 18 plant species. Protein sequences were specified along with their homologs. Homologs were used as benchmark to understand why certain sequences clustered together. Rectangular phylogenetic tree was constructed by MEGA 6 with maximum likelihood (ML) method for 1000 replicates bootstrap
3.5 3D Modeling
3D models were predicted by I-TASSER server using a representative sequence from each species to investigate the conservancy or divergency at structural level (Fig. 5). Crystallographic structures of investigated species have not been available yet therefore following templates of the highest significance in threading alignments were used in model construction. A total of 18 sequences from 18 different species were modelled, including A. thaliana (modelled sequence: AT2G26975.1; PDB template: 2LS3, 2LS4, 2LS2 and 4HW9), B. distachyon (Bradi1g24180.1; 2LS3, 3DTE_A, 2LS4 and 2LS2), B. rapa (Brara.B01109.1; 2LS3, 2LS4, 2LS2 and 3J5P_B), C. reinhardtii (Cre05.g247050.t1.2; 1LXL, 3W35_A, 4RKM_A, 4FGU_A, 2LS3 and 2LS4), C. sativus (Cucsa.146630.1; 2LS3, 2LS4, 2LS2 and 3J5P_B), E. grandis (Eucgr.J01992.1; 2LS3, 2LS4, 2LS2 and 4HW9), G. max (Glyma.07G141600.1; 2LS3, 2LS4, 2LS2 and 4HW9), G. raimondii (Gorai.003G127000.1; 2LS3, 2LS4, 2LS2 and 4HW9), M. truncatula (Medtr4g065123.1; 2LS3, 2LS4, 2LS2 and 4IAQ_A), O. sativa (LOC_Os01g56420.1; 2LS3, 2LS4, 2LS2 and 4HW9), P. vulgaris (Phvul.011G060400.1; 2LS3, 2LS4, 2LS2 and 4HW9), P. patens (Phpat.027G020800.1; 2LS3, 2WOE_A, 2LS4 and 2LS2), P. trichocarpa (Potri.009G038800.1; 2LS3, 2LS4, 2LS2, 4HZU_S and 2PNO), P. persica (ppa026783 m; 2LS3, 2LS4, 2LS2, 4HW9 and 4HZU_S), S. lycopersicum (Solyc08g006250.1.1; 2LS3, 2LS4, 2LS2 and 4K1C_A), S. bicolor (Sobic.005G059300.1; 2LS3, 4OOJ_A, 2LS4 and 2LS2), V. vinifera (GSVIVT01024838001; 2LS3, 2LS4, 2LS2 and 4K1C_A) and Z. mays (GRMZM2G042412_T01; 2LS3, 2LS4, 2LS2 and 4HW9). It was revealed that models were primarily developed on the human copper transport 1 templates such as 2LS2, 2LS3, and 2LS4.
3D models of Cu transporters in 18 plant species. TMDs were labeled in different colors such as TMD-1 in red; TMD-2 in green; TMD-3 in blue; TMD-4 in yellow and other structures colored in gray. Figures were arranged in an order from dicots to monocots and to lower plants. Last two figures, including lower plants were drawn slightly bigger to emphasize them
For each species, predicted top five 3D models were retrieved from I-TASSER server and evaluated with Ramachandran plot analysis and PyMOL viewer. Models with ≥ 90 % allowed residues in Ramachandran analysis (data not shown) and with general topological characteristics of CTR family were selected for visualization. Visualized models were arranged in order from higher (dicots and monocots) to lower plants to apparently observe the structural similarities or divergences between species (Fig. 5). Besides, putative TMDs were specified with different colors such as TMD-1 in red; TMD-2 in green; TMD-3 in blue, and, if present, TMD-4 in yellow, and other structures such as loops, non-TM helices, and beta strands were colored in gray. Topology of CTR family members were reported to have three putative TMDs with N- and C-terminal regions in extracellular and cytosol, respectively [9, 10]. We predicted all models, except for C. reinhardtii, with three potential TMDs with a long extracellular N-terminal and a relatively short cytoplasmic C-terminal regions. However, C. reinhardtii was predicted to have four putative TMDs with cytoplasmic N- and C-terminal regions. All these implicated that general topological features of Cu transporters could be highly conserved in monocots and dicots. However, there might be separation between higher and lower plants since lower plants had relatively different topology compared to higher plants.
Moreover, both N- and C-terminal regions also showed variations in the predicted 3D models. These conformational variations at coil and loop regions in proteins could be derived from the conformational flexibility of these regions.
3.6 Interaction Partner Analysis
Interactome map was constructed for O. sativa (LOC_Os01g56420.1; Fig. 6a) and A. thaliana (AT5G59030.1; Fig. 6b) as representatives of monocots and dicots, respectively. Ten potential interactors of both species were investigated. OsSPL9 (4338766), COX4 (4335339), cytochrome c oxidase copper chaperones (4347084 and 4330997), mitochondrial carrier protein (4332542), ferroportin1 domain-containing protein (4341302), and other uncharacterized expressed proteins were predicted among the interaction partners of O. sativa (Fig. 6a). OsSPL9 is a trans-acting element from SBP box gene family specifically binding to the consensus nucleotide sequence of 5′-TNCGTACAA-3′ [34]. It has been reported that transcriptional activation of some Arabidopsis Cu deficiency responsive genes and Cu-microRNAs such as miR397, miR398, miR408, and miR857 are controlled by TF SPL7 via interaction with reiterative cis-regulatory GTAC motifs in promoter [18, 19]. Both OsSPL9 and SPL7 belong to SBP box gene family and contain similar binding nucleotide sequences. Therefore, OsSPL9 might have a potential role in regulation of Cu homeostasis as a transcription factor. Cytochrome c oxidase copper chaperone is reported to bind two copper ions and deliver them to the Cu(A) site of COX [35]. Mitochondrial carrier protein transfers the molecules across mitochondrial membranes [36]. Considering the cytochrome c oxidases (COXs) localized in mitochondrial inner membrane, it is understandable that interaction of Cu transporters with this protein. Another interaction partner of Oryza is ferroportin1 domain-containing protein, which shows Fe ion transmembrane transporter activity [37].
For A. thaliana, SPL7, HMA5, RAN1, COX17, ATX1 (T6J19.6), MRS2, MRS2-2, MRS2-3, MRS2-4, and MRS2-7 proteins were predicted as putative interaction partners (Fig. 6b). Among these proteins, SPL7 is a TF binding to GTAC motifs to control the Cu-microRNAs such as miR397, miR398, miR408, and miR857, and modulate the transcriptional activation of some Arabidopsis genes under Cu deficiency [18, 19]. HMA5 is reported to involve in Cu compartmentalization and detoxification [38]. RNA1 involves in copper import to the ethylene receptors [39]. COX17, which is a cytochrome c oxidase copper chaperone, binds two copper ions and deliver them to the Cu(A) site of COX [35]. ATX1 is a Cu chaperone specifically delivering Cu to heavy metal P-type ATPases [40]. MRS2 gene family, including MRS2-2, MRS2-3, MRS2-4, and MRS2-7, is involved in magnesium transport [41]. Overall, it was revealed that putative interaction partners of O. sativa and A. thaliana Cu transporters, with exception of uncharacterized proteins, are directly or indirectly related with Cu homeostasis.
4 Conclusion
Cu functions as a structural element in regulatory proteins and as a cofactor in many enzymes as well as involving in various metabolic processes. Its deficiency or toxicity can lead to detrimental consequences in plants. Therefore, plants are required to possess a sophisticated network of Cu metabolism. Although Cu is an essential micronutrient for plant growth and development, functional and structural studies regarding the Cu transporter genes and proteins in many plant species are still limited. In this study, a total of 78 potential Cu transporter genes were identified from 18 different plant species and analyzed at primary, secondary, tertiary, and functional levels. Study revealed that Cu transporters could be characterized with a CTR protein family (PF04145) domain, three putative TMDs, a single exon number, and basic (pI) character. Met-rich motifs at N-terminal region, MXXXM motif in TMD-2, and GXXXG motif in TMD-3 could be essential for Cu transport since they were highly conserved in all analyzed species. In phylogeny, a clear distinction was observed between Cu transporter sequences of lower and higher plants. General topological features of Cu transporters in higher plants were highly conserved, whereas lower plants had relatively different topology compared to higher plants. Identification of Cu transporter homologs in various plant species and their comparative analysis at primary, secondary, and tertiary structural levels has led us to better understand the Cu transporters. Thus, results of this study will become a valuable theoretical basis for future studies of further characterization of Cu transporters.
Abbreviations
- TMD:
-
Transmembrane domain
- aa:
-
Amino acid
- Cu:
-
Copper
- SOD:
-
Superoxide dismutase
- ROS:
-
Reactive oxygen species
References
Marschner H (1995) Functions of mineral nutrients: macronutrients. Miner Nutr High Plants 2:379–396 (Academic Press, London)
Sancenón V, Puig S, Mateu-Andrés I, Dorcey E, Thiele DJ, Peñarrubia L (2004) The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J Biol Chem 279:15348–15355
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Huffman DL, O’Halloran TV (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70:677–701
Burkhead JL, Gogolin RKA, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Pilon M, Cohu CM, Ravet K, Abdel-Ghany SE, Gaymard F (2009) Essential transition metal homeostasis in plants. Curr Opin Plant Biol 12:347–357
Puig S, Peñarrubia L (2009) Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol 12:299–306
Puig S, Lee J, Lau M, Thiele DJ (2002) Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 277:26021–26030
Eisses JF, Kaplan JH (2002) Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem 277:29162–29171
Jiang J, Nadas IA, Kim MA, Franz KJ (2005) A Mets motif peptide found in copper transport proteins selectively binds Cu (I) with methionine-only coordination. Inorg Chem 44:9787–9794
Beaudoin J, Laliberté J, Labbé S (2006) Functional dissection of Ctr4 and Ctr5 amino-terminal regions reveals motifs with redundant roles in copper transport. Microbiology 152:209–222
Page MD, Kropat J, Hamel PP, Merchant SS (2009) Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell Online 21:928–943
Peñarrubia L, Andrés-Colás N, Moreno J, Puig S (2010) Regulation of copper transport in Arabidopsis thaliana: a biochemical oscillator? J Biol Inorg Chem 15:29–36
Aller SG, Unger VM (2006) Projection structure of the human copper transporter CTR1 at 6-Å resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci USA 103:3627–3632
Aller SG, Eng ET, De Feo CJ, Unger VM (2004) Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem 279:53435–53441
Wu X, Sinani D, Kim H, Lee J (2009) Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. J Biol Chem 284:4112–4122
Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T (2009) SQUAMOSA promoter binding protein—like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell Online 21:347–361
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945
Romiti M (2010) Entrez nucleotide and entrez protein FAQs. Gene 1:270
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD et al (2005) Protein identification and analysis tools on the ExPASy server. In: Walker John M (ed) The proteomics protocols handbook. Humana, Totowa, pp 571–607
Yu CS, Chen YC, Lu CH, Hwang JK (2006) Prediction of protein subcellular localization. Proteins 64:643–651
Sonnhammer EL, Eddy SR, Durbin R (1997) Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28:405–420
Bernsel A, Viklund H, Falk J, Lindahl E, von Heijne G, Elofsson A (2008) Prediction of membrane-protein topology from first principles. Proc Natl Acad Sci 105:7177–7181
Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Yi chuan = Hereditas/Zhongguo yi chuan xue hui bian ji 29:1023–1026
Timothy L, Mikael Bodén B, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:202–208
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (2015) The I-TASSER Suite: protein structure and function prediction. Nat Methods 12:7–8
DeLano WL (2002) The PyMOL molecular graphics system
Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, Richardson DC (2003) Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 50:437–450
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Jensen LJ (2013) STRING v9. 1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res 41:D808–D815
Yang Z, Wang X, Gu S, Hu Z, Xu H, Xu C (2008) Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 407:1–11
Amaravadi R, Glerum DM, Tzagoloff A (1997) Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet 99:329–333
Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin GJM, Brandolin GA, Pebay-Peyroula E (2006) Relations between structure and function of the mitochondrial ADP/ATP carrier. Annu Rev Biochem 75:713–741
Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N, Guerinot ML (2009) The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell Online 21:3326–3338
Andrés-Colás N, Sancenón V, Rodríguez-Navarro S, Mayo S, Thiele DJ, Ecker JR, Peñarrubia L (2006) The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J 45:225–236
Binder BM, Rodríguez FI, Bleecker AB (2010) The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J Biol Chem 285:37263–37270
Puig S, Mira H, Dorcey E, Sancenon V, Andrés-Colás N, Garcia-Molina A, Penarrubia L (2007) Higher plants possess two different types of ATX1-like copper chaperones. Biochem Biophys Res Commun 354:385–390
Gebert M, Meschenmoser K, Svidová S, Weghuber J, Schweyen R, Eifler K, Knoop V (2009) A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell Online 21:4018–4030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vatansever, R., Ozyigit, I.I. & Filiz, E. Genome-Wide Identification and Comparative Analysis of Copper Transporter Genes in Plants. Interdiscip Sci Comput Life Sci 9, 278–291 (2017). https://doi.org/10.1007/s12539-016-0150-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-016-0150-2