Abstract
Background
Cognitive behavioral therapy for insomnia (CBT-I) is a first-line therapy for insomnia disorders. We assessed changes in discrepancies between subjective and objective sleep measures and correlations between discrepancy changes and clinical insomnia severity for CBT-I in patients with primary insomnia
Methods
Fifty-two outpatients (mean age, 60.3 years; 26 women) with primary insomnia were treated by individual CBT-I (50 min, maximum six sessions, once every 1–2 weeks). One week before and after CBT-I, patients recorded a sleep log and wore an actigraphy device. Subjective and objective time in bed (TIB), total sleep time (TST), sleep-onset latency (SOL), wake time after sleep onset (WASO), and sleep efficiency (SE) were evaluated by averaging 1-week records. Relative values of sleep discrepancy in TIB, TST, SOL, WASO, and SE were calculated for estimating effects of CBT-I. The therapeutic effects were also evaluated using psychological scales before and after CBT-I.
Results
Subjective and objective discrepancies in sleep measures decreased by 36, 25, and 37 min in TST, SOL, and WASO, respectively, and 7% in SE (all P < 0.001) after CBT-I. Seven patients transitioned from underestimating SE before CBT-I to overestimating SE after CBT-I. Although CBT-I improved relative values of discrepancy in WASO and SE, alongside ISI, the improvement in insomnia severity only correlated with SOL discrepancy.
Conclusions
CBT-I may reduce the discrepancy between subjective and objective sleep measures in patients with primary insomnia. However, a greater therapeutic effect of CBT-I was observed in reducing the ISI, which was slightly influenced by improvements in sleep discrepancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia disorder is a common comorbidity with various medical conditions [1,2,3]. It causes distress at night, such as difficulty falling asleep, staying asleep, or waking up too early, and can also become a serious threat to daily activities and physical and mental health [4]. Therefore, it increases the risk of developing not only anxiety and mood disorders but also diverse mental and physical disorders [5]. Similar to comorbid mental disorders, the prevalence of insomnia disorder increases with age and is higher in women [1]. It has been speculated that there are significant pathological differences between patients with insomnia disorder with and without other medical conditions including mental disorders, which are reflected in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [6] and the International Classification of Sleep Disorders, 3rd edition (ICSD-3) [7].
Insomnia disorder is diagnosed based on subjective insomnia symptoms alongside insomnia-related daytime dysfunctions, according to the clinical criteria of the DSM-5, which shares the similar clinical criteria of the chronic insomnia disorder as the ICSD-3. Therefore, the severity of insomnia disorder has been generally assessed by self-completed questionnaires such as the Insomnia Severity Index (ISI) [8] or Pittsburgh Sleep Quality Index (PSQI) [9, 10], and insomnia symptoms have been evaluated by the total sleep time (TST), sleep-onset latency (SOL), wake time after sleep onset (WASO), and sleep efficiency (SE) [11] using a self-recorded sleep log in a clinical setting.
Primary insomnia, which is equivalent to insomnia disorder without any comorbid medical conditions, has been defined as the core pathological entity of insomnia disorder among the various insomnia subtypes in the ICSD, 2nd edition (ICSD-2) [12]. Sleep discrepancy, which connotes a significant difference between subjective and objective sleep parameters in TST, SOL, and WASO, is clearly defined as a core clinical feature of the paradoxical insomnia, which is also a subtype of insomnia disorder in the ICSD-2. Although sleep discrepancy is not considered a necessary component in the diagnostic criteria of chronic insomnia disorder in the ICSD-3, and its relationship with the pathology of insomnia disorder is under debate, it is frequently seen in patients with insomnia disorder, regardless of whether the patients have paradoxical insomnia. It has been suggested that patients with paradoxical insomnia obviously underestimate their TST, SOL, and WASO relative to the objective measures thereof by actigraphy or polysomnography (PSG) [13]. However, the ICSD-3 did not adopt all subtypes of chronic insomnia disorder owing to a lack of evidence to support separate groups including paradoxical insomnia [14]. Thus, sleep discrepancy to a greater or lesser extent is regarded as a common factor characterizing insomnia disorder and may occur owing to sleep state misperception, which has already been described in the 1st edition of the ICSD (ICSD-1) [15]. However, the cause of sleep misperceptions remains to be identified.
Previous studies examining the sleep structure of patients with insomnia using actigraphy and PSG suggested that subjective complaints, such as difficulty in falling asleep, increases in WASO, and reductions in TST, were rarely consistent with objective sleep measures obtained with these instruments [11, 16]. Patients with insomnia tended to report longer SOL and WASO values and shorter TST values than when these measures were obtained by PSG in a laboratory setting. Moreover, a meta-analysis suggested that patients with chronic insomnia showed similar sleep variables measured by PSG, including larger indices than those of normal controls [11]. A similar finding was also reported by using actigraphy. Patients with insomnia and post-traumatic stress disorder underestimated TST with SE and overestimated SOL compared to the corresponding sleep variables measured by actigraphy in home settings [17]. Although there may be a qualitative difference in objective sleep variables between PSG and actigraphy measurements, in addition to laboratory and home settings, this sleep discrepancy could be universally observed.
However, few studies have investigated how treatment changes sleep discrepancy. Cognitive behavioral therapy for insomnia (CBT-I) is recommended as a first-line therapy for insomnia disorder in international insomnia therapy guidelines [18]. CBT-I consists of components such as stimulus control [19], sleep hygiene [20], and sleep restriction [21]. It has been reported to have higher effectiveness [22,23,24], and incur more moderate side effects, than hypnotic medications [25] for insomnia disorder. Therapeutic targets of CBT-I include both subjective complaints of sleep difficulties and daytime dysfunctions. Thus, the effects of CBT-I have been chiefly assessed by subjective measures of insomnia disorder severity, in the form of ISI and PSQI scores, and sleep variables measured by a sleep log in previous studies [26, 27]. In contrast, the traditional therapeutic target of hypnotics is an improvement in the objective sleep variables, including SOL, WASO, and TST measured by PSG. Mitchell et al. reported that the therapeutic effects of CBT-I appeared to be greater in subjective sleep variables measured with a sleep log than in objective variables measured by actigraphy and PSG [18]. A meta-analysis revealed that CBT-I had significant effects on psychological scales, such as the ISI, PSQI, and Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS); it also showed significant effects on subjective sleep variables measured with a sleep log, while incurring a small effect on objective variables measured by actigraphy and PSG [23].
Harvey et al. focused on cognitive distortions in patients with insomnia [28, 29]. They speculated that the sleep discrepancy might be caused by distorted cognitive processes, such as cognitive arousal, information processing bias, and attention bias. Therefore, they also pointed out that a distorted perception of sleep, including the sleep discrepancy, could serve as a therapeutic target of CBT-I. Actually, CBT-I includes the sleep restriction process, in which therapists control the time in bed (TIB) based on the patients’ sleep efficiency, as estimated by their sleep log, to restore the homeostatic drive and improve SE [27]. To assess the effectiveness of CBT-I on these cognitive distortions, especially on the information processing and attention biases, sleep discrepancy between the objective and subjective measures of sleep continuity could be useful. Harada et al. evaluated the effect of two-session group CBT-I with self-written sleep logs and actigraphy in 33 patients with insomnia, after which they reported that the discrepancies between those sleep measures decreased by 33.3 min for TST and 18.4 min for SOL following the treatment [30]. However, no study has elucidated the effect of individual CBT-I on sleep discrepancy in patients with insomnia.
This study was undertaken to clarify the effects of CBT-I on the discrepancy between subjective and objective sleep evaluations in patients with primary insomnia. The ISI and DBAS, which reflect the severity and some cognitive biases of insomnia, respectively, were also used to assess CBT-I efficacy. Subjective and objective sleep measures were evaluated with a sleep log and actigraphy, respectively. This study also focused on the relationship between sleep discrepancy and insomnia severity as assessed by the ISI and DBAS. In addition, a reduction in and withdrawal from sleep medication, which are important outcomes reflecting an improved insomnia disorder, were quantitatively evaluated in light of the recent findings on the efficacy of CBT-I for discontinuing hypnotics in patients with primary insomnia. Because hypnotics could also facilitate sleep discrepancies by enhancing cognitive distortions [31], this relationship was assessed collaterally.
Methods
Patients
Sixty-two native Japanese outpatients with primary insomnia, who had been treated at our sleep clinic from April 2011 to March 2015, underwent individual CBT-I. The patients had been diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) [32] by psychiatrists with more than 10 years of clinical experience. Since the DSM IV-TR differentiates primary insomnia from comorbid insomnia with other medical conditions or mental disorders, four patients with a coexisting disease (anxiety disorder, major depressive disorder, or moderate and severe obstructive sleep apnea) and six patients who did not consent to individual CBT-I, due to the time required, were excluded. As such, 52 patients (mean age, 60.3 ± 15.5 years; 26 women) were included in this study. The most frequent reason cited by patients declining to participate in the study was refusal to wear an actigraphy device. All 52 patients were willing to reduce or discontinue their doses of hypnotics, as confirmed by the psychiatrists participating in the study.
Exclusion Criteria
Patients diagnosed with complications, or with mental, neurological, or sleep disorders, except for primary insomnia, during diagnostic psychiatric interviews were excluded from the research study. Patients with acute and chronic physical illnesses were also excluded by referring the clinical records. Those who showed 15 or more apnea or hypopnea events per hour (apnea hypopnea index, respiratory event index), as assessed by a home sleep apnea testing apparatus, and those with scores of 11 or higher on the International Restless Legs Syndrome Rating Scale [31] were excluded. Patients with scores of 53 or higher on the Self-rating Depression Scale (SDS) Japanese version [33] and those who had any social difficulties linked to their participation in the study (e.g., requiring a leave of absence from work) were also excluded. In addition, patients with irregular sleep–wake rhythms, including shift-workers and international travelers, were excluded.
Ethics
This study was approved by the ethics committee of Shiga University of Medical Science on October 26, 2010 (the approval number: 22 -91). In accordance with the tenets of the Declaration of Helsinki, each patient received an explanation from the therapist regarding the study purpose, potential risks associated with participation in this study, no detriment due to non-participation, and ability to withdraw consent. All participating patients provided written informed consent.
Treatment Protocol of Individual CBT-I and Therapeutic Strategies
Fifty-two patients entered the individual CBT-I course. In total, a maximum of six sessions (50 min for each session) were conducted once every 1–2 weeks. All CBT-I sessions were performed by a single psychologist who had more than 10 years of clinical experience and who had been certified by the Japanese Association of Behavioral and Cognitive Therapies. The CBT-I course consists of the sleep hygiene guidance, stimulus control therapy [18], sleep restriction therapy [20, 21], and other cognitive behavioral techniques [34, 35].
As sleep hygiene guidance, psychoeducation was provided to patients whose sleep habits had aggravated their sleep quality. Sleep hygiene guidance was carried out at the initial session for all patients. Initially, based on the chronological table of sleep duration prepared by Ohayon et al. [36], patients were educated on a consistent sleep–wake schedule in daily life, sleep duration, and fixed wake-up time, according to the actual daily life of the patient and his/her age-group. In addition, patients were taught about appropriate routine pre-sleep behaviors, daily habits associated with sleep health, and bedroom environments for achieving good sleep health.
Stimulus control therapy is a technique that facilitates an association between environmental cues in a bedroom (e.g., bed) with psychophysiological sleepiness. To utilize the Pavlovian conditioning process, patients were encouraged to do nothing but sleep in the bed and avoid stimuli that may promote their arousal. At every session, patients received feedback on their subjective sleep evaluation data obtained with a sleep log and objective sleep measurement data obtained by actigraphy devices, after which their sleep-arousal patterns and lifestyle habits were discussed based on these data.
Sleep restriction therapy was tailored to the patient’s sleep–wake patterns, as determined by referencing the data derived from sleep logs and actigraphy devices. Patients were instructed not to go to bed too early, stay in bed beyond the prescribed bedtime, or take a nap. The initial TIB was set at the average total sleep time at night over the course of the previous week, as recorded with a sleep log and actigraphy device. To delay the TIB, a behavioral activation approach was applied to increase environmental reinforcements and reduce punishments in line with sufficient sleep hygiene [37]. Sleep restriction could increase homeostatic sleep drive and improve circadian-dependent sleep–wake patterns.
When the severity of the patient’s insomnia disorder was reduced and reached the subthreshold (cutoff) level of the ISI (less than seven points) before the final CBT-I session, the following protocol of sleep medication reduction was additionally introduced. In patients with single drug administration, the dose was first decreased by half every week (i.e., the taper method); thereafter, the dose was decreased to one-fourth the original dosage, and discontinuation was attempted in combination with the alternate day administration method. For polymedicated patients, dose reduction was attempted sequentially for each medication, with the aim of single administration; thereafter, dose reduction or withdrawal was attempted by a protocol similar to that of single drug administration.
The CBT-I session was generally conducted six times. However, therapy was terminated even if the session had been conducted fewer than six times, if the patient’s insomnia was in remission and the patient could completely discontinue their sleep medication. The observation was performed from 7 days prior to the initiation of the CBT-I session to 7 days after the termination of all CBT-I sessions. The observation periods for all patients ranged from 7 to 14 weeks.
Measures
Psychological Measures
All paper-based psychological measurement scales were filled out twice by the patients themselves at the beginning of the first CBT-I session and the end of the last CBT-I session. The Insomnia Severity Index Japanese version (ISI-J) [8, 38], which is a five-point Likert scale with seven items regarding insomnia symptoms and daytime dysfunction that were present during the 2 weeks prior to the test, was used to assess insomnia severity. The total score ranges from 0 to 28. The ranges of the total score from 0 to 7, from 8 to 14, from 15 to 21, and from 22 to 28 correspond to non-insomnia, subthreshold insomnia, moderate insomnia, and severe insomnia, respectively.
The Japanese version of the Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-J) [39, 40], an 11-point Likert scale with 16 items regarding beliefs and attitudes about sleep, was used to evaluate cognitive biases in sleep-related beliefs and attitudes regarding insomnia. The total score ranges from 0 to 160.
The Japanese version of the Epworth Sleepiness Scale (ESS) [41, 42], a four-point Likert scale with eight items regarding daytime sleepiness, was adopted to evaluate the aspects of daytime dysfunction due to insomnia. The total score ranges from 0 to 24; the cutoff score is 11 or higher.
The Self-rating Depression Scale (SDS) [33, 43], a four-point Likert scale with 20 items regarding depressive symptoms, was adopted to evaluate the psychological consequences of insomnia based on other aspects of daytime dysfunction. Two, eight, and 10 out of 20 items evaluate main emotions, physiological accessory symptoms, and psychological accessory symptoms, respectively. The total score ranges from 20 to 80.
Sleep Evaluation Measures
A sleep log was used for subjective sleep evaluations. In a sleep log, a patient describes his or her own everyday sleep-arousal patterns on a paper-based timetable. In the description, into-bed time, estimated sleep-onset time, estimated arousal time, bed-leaving time, TST, medication, and SE were included. Patients were instructed to fill in the into-bed time and the bed-leaving time precisely by watching a clock and the estimated sleep-onset time and the estimated arousal time approximately without watching it. Subjective TIB (sTIB), TST (sTST), SOL (sSOL), SE (sSE), and WASO (sWASO) were calculated from the obtained data and used as subjective sleep evaluation measures.
Objective sleep evaluations were performed based on the amount of body motion measured by actigraphy (Actiwatch2, Philips Respironics, Amsterdam, the Netherlands) [44]. Recorded time-series motion data with a sampling rate of 32 Hz were analyzed with Actiware CT software (Philips Respironics, Version 6.01). Actigraphy generates signals corresponding to the level and frequency of body motions using a built-in accelerometer and records body motions as activity counts. If the participant is moving, the activity counts for the four adjacent sampling epochs are taken into account and scored for sleeping and waking. Each epoch is graded as mobility or immobility in 15 s increments. Patients were required to press the event button on a side surface of the instrument when going to bed and when leaving bed to record objective into-bed and bed-leaving times. Analyzing parameters were set to a 5-min epoch length and medium sensitivity, and both activity and marker selections were set at the default settings. Objective sleep parameters were derived using the subject-marked event markers (MARK mode). To ensure objectivity, the extraction and analysis of actigraphy data were blindly performed by a clinical technologist who was separated from the CBT-I therapist. Following the algorithm developed by Cole et al. [45], objective TIB (oTIB), TST (oTST), SOL (oSOL), SE (oSE), and WASO (oWASO) were calculated and used as objective sleep evaluation measures. In addition, we calculate the relative values of sleep discrepancy between subjective and objective sleep measures (i.e., sTST/oTST) to evaluate the effects of CBT-I on objective-subjective sleep discrepancy while controlling for the absolute values of each objective sleep measure. We interpolated a value of 1 as a dummy variable when the subjective sleep measure was 0 in calculating the relative values of sleep discrepancy.
The data from the sleep log and actigraphy were recorded every day from 7 days before the start of CBT-I to 7 days after the end of therapy, and data averaged for 7 days before therapy and for 7 days after therapy were used in this analysis.
Dose of Sleep Medication
The dose of sleep medication was analyzed based on diazepam conversion according to a previous study by Inada and Inagaki [46]. Two patients who had taken ramelteon or suvorexant were excluded from the analysis target for the dose variation analysis.
Statistical Analyses
All statistical analyses were conducted by the psychologist who conducted all CBT-I sessions. Data were analyzed using SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA). Unpaired t-tests were used to calculate the mean differences in demographic data, psychological measures, and subjective and objective sleep measures between the therapy completion group and the dropout group, alongside the mean difference between the doses of sleep medication before and after the treatment. Paired t-tests were used to calculate the mean difference in relative values of each sleep discrepancy measure before and after the treatment. The Holm’s method was used to adjust the level of significance of multiple measurements [47]. A variance analysis of a single factor was used to analyze the factors associated with the successful withdrawal from sleep medication. The Spearman’s product-moment coefficients were calculated for analyzing the correlations between changes in relative values of sleep discrepancy and psychological measures. The McNemar test was used to assess the conversion ratio between underestimated sleep and overestimated sleep. To explore the relationship between hypnotics reduction/discontinuation and reduction in relative values of sleep discrepancy, a one-way factorial ANOVA was utilized for each relative value of sleep discrepancy. P < 0.05 was considered statistically significant. Effect sizes were calculated from the mean and standard deviation using Cohen’s d.
Results
Patient Characteristics
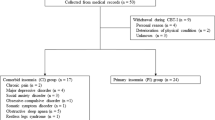
Fifty-two patients agreed to participate in this study (Fig. 1). Forty-three patients (mean age, 61.4 years) completed the CBT-I course, and nine patients (17.3%) dropped out. The breakdown of the dropout group was as follows: six patients did not seek further medical attention, two patients were admitted to the hospital because they developed other physical problems, and the family of one patient wanted to terminate the therapy. Forty patients (77%) were taking hypnotics at the beginning of the treatment, compared to the 16 patients (31%) on hypnotics at the end of the treatment. A flow diagram of this study is shown in Fig. 1. There was no statistically significant difference between the completion and dropout groups, except for the frequency of sessions (Table 1).
Effects of CBT-I
Changes in Psychological Measures, Subjective, and Objective Sleep Evaluation Measures, and Medication Dose Through CBT-I
The ISI, DBAS, and SDS values became significantly lower (P < 0.001 for all) after CBT-I compared to that before CBT-I (Table 2). Although these pre- and post-treatment scores were significantly different, the effect sizes ranged from small to large.
Subjective sleep evaluation measures showed significant changes after CBT-I: sTIB decreased by 18.6 min, sTST increased by 30 min, sSOL decreased by 28.2 min, sWASO decreased by 48.6 min, and sSE improved (8.8%) with small to medium effect sizes.
As for objective sleep measures, only oTIB showed a significant decrement (22.9 min, P = 0.03). The diazepam equivalent doses of medication significantly decreased (P < 0.001) with an intermediate effect size after CBT-I.
Changes in Sleep Discrepancies after CBT-I
We evaluated effects of CBT-I on the subjective–objective discrepancy by using relative values of sleep discrepancy in TIB, TST, SOL, WASO, and SE at the pre- and post-treatment points. There was no change in the relative values of sleep discrepancy in TIB (t(42) = −0.77, P = 0.44), TST (t(42) = 0.80, P = 0.43), and SOL (t(42) = 0.44, P = 0.66). For WASO and SE, the relative values of sleep discrepancy significantly improved (WASO: t(42) = 3.80, P < 0.001, SE: t(42) = −2.85, P = 0.007) with small effect sizes (d[0.38; 0.32]) after CBT-I (Fig. 2).
Relative values of sleep discrepancy before and after cognitive behavioral therapy for insomnia (CBT-I). Relative values of sleep discrepancy (subjective sleep measures divided by objective sleep measures) in wake time after sleep onset (WASO) and sleep efficiency (SE) significantly improved after CBT-I. N.S. not significant, **significant. The white and black bars with gray lines indicate mean values with standard deviations in each relative value of sleep discrepancy in time in bed (TIB), total sleep time (TST), sleep onset latency (SOL), WASO, and SE at pre- and post-CBT-I, respectively. The level of significance was adjusted using the Holm method
Correlations Between Changes in Sleep Discrepancy and Psychological Measures
The correlation between the pre- to post-treatment changes in relative values of sleep discrepancy and each psychological measure were analyzed. There was a positive correlation between changes in the relative value of sleep discrepancy in SOL and changes in the ISI score (r = 0.352, p = 0.02). However, changes in the other psychological measures did not show any correlation with changes in the relative values of sleep discrepancy (−0.15 < all r < 0.03; all P > 0.10).
Under and Overestimation of Sleep
When objective sleep measures showed better scores than subjective measures, the patient was considered to have underestimated sleep. Conversely, when subjective sleep measures showed better scores than objective measures, the patient had overestimated sleep. The TIB, TST, SOL, SE, and WASO scores listed in Table 3 indicate that there were a certain number of patients who had overestimated sleep. In SE, there were seven patients who had underestimated sleep before treatment, which was then overestimated after treatment, which reflected a significant change when the McNemar test was performed (χ2 = 4.5, P = 0.03). As for TIB, TST, SOL, and WASO, the conversion of sleep evaluations was not significant (all P > 0.08).
Reduction of Hypnotics and Changes in Sleep Discrepancies
To assess the relationship between hypnotics reduction/discontinuation and reduction in sleep discrepancies, one-way factorial ANOVAs were conducted. There was no significant difference in changes in relative values of sleep discrepancy in TIB (F(1,41) = 0.16, P = 0.69), TST (F(1,41) = 0.08, P = 0.78), SOL (F(1,41) = 0.30, P = 0.59), WASO (F(1,41) = 0.39, P = 0.55, and SE (F(1,41) = 0.01, P = 0.92) between the group achieving a reduction by more than half of their doses of hypnotics (n = 10) and the group that did not achieve a reduction by more than half of their doses of hypnotics (n = 33).
Discussion
Reduced Discrepancy in Subjective–Objective Sleep Evaluation After CBT-I
The sleep discrepancies in WASO and SE significantly improved after CBT-I. TIB is under the control of each patient and is recognized with an alert state; thus, sTIB and oTIB may be similarly decreased after CBT-I. Conversely, the results in TIB indicate that the stimulus control and sleep restriction therapies were appropriately conducted. In addition, relative values of sleep discrepancy in TST and SOL also did not significantly change after CBT-I even though average values of them were seemed to be improved. Although CBT-I did not clearly improve objective sleep measures, it sufficiently improved subjective measures, especially of WASO, SE, and SOL. Because CBT-I essentially aims to improve SE and reduce invalid TIB, both reduced WASO and SOL, and improved SE may be achieved. A previous meta-analysis also indicated that CBT-I improves the same subjective sleep measures as the above, and even better so than TST [27]. In line with those previous findings, it would not be surprising to observe non-significant changes in sleep discrepancy in TST after CBT-I. However, sleep discrepancy in SOL also showed non-significant changes after CBT-I. One reason for this might be the relatively greater standard deviation in the relative value of sleep discrepancy in SOL compared to those in the other measures.
In contrast to subjective sleep measures, the effects of CBT-I on objective sleep measures are scarcely examined. Lund et al. examined CBT-I effects on sleep discrepancy by using a sleep log and PSG measurements [16]. In their study, the objective sleep variables measured by PSG did not show any change after the entire course of CBT-I; however, the discrepancy between sleep log and PSG measurements decreased after CBT-I. However, objective sleep measurements assessed by overnight PSG may not appropriately reflect habitual sleep states and can fail to match the averaged sleep log data. Although their study protocol was methodologically limited, their results, along with the current study findings, strongly suggest that objective sleep measures are more stable than subjective sleep measures, and that CBT-I could independently affect these subjective sleep measures.
It has been suggested that even healthy participants could overestimate and underestimate their own sleep durations [48]. Recent large cohort studies indicated that middle-aged and older people strongly tend to overestimate their sleep states [49, 50]. Considered alongside these previous findings, CBT-I could improve the underestimation of sleep states and enhance the healthy overestimation of sleep states as a positive bias, which presumably contributes to alleviating the severity of insomnia disorder. Variations in sleep discrepancy after CBT-I were also reported by Kay et al. [51]. They demonstrated reductions in the discrepancy and decentration in subjective–objective sleep evaluations, as well as a conversion from over- to underestimation in terms of the WASO after CBT-I.
In this study, all patients received feedback on their sleep log and actigraphy data during the sleep restriction process in every CBT-I session. It remains unclear whether simple self-monitoring/biofeedback [52], which may have a strong therapeutic effect in CBT, directed adequate attention to the physiological status based on the current CBT-I procedure [37]. Tang and Harvey mentioned that sleep misperception can be improved via patients’ own voluntary feedback by actigraphy [53]. Taken together, it is speculated that the feedback of objective sleep information possibly contributes to improving insomnia pathology by improving patients’ sleep misperceptions.
Effects of CBT-I on Psychological Measures Associated with Insomnia Pathology
Contrary to expectations, changes in the ISI score more accurately reflected the effects of CBT-I than decreases in sleep discrepancies, as reflected in effect size calculations. Moreover, changes in the ISI score only correlated with changes in SOL discrepancy. Interestingly, the improvement in SOL discrepancy was found to correlate with improvement in insomnia severity, which, however, did not show an overall significant change after CBT-I, suggesting that there might be individual differences in the effects of CBT-I. These results also suggest that reductions in sleep discrepancies are collaterally achieved by CBT-I, and that sleep discrepancy per se is scarcely associated with insomnia pathology. The DBAS and SDS scores may reflect some aspects of cognitive bias and daytime dysfunction caused by insomnia disorder. The lack of a correlation between changes in these scores and sleep discrepancies could also imply an indirect influence on insomnia treatment by CBT-I.
However, the ESS score did not show any significant reduction after CBT-I. Previous studies have reported a reduction in the ESS score as a measure of the efficacy of CBT-I [54, 55]. A possible explanation for this inconsistency is as follows: patients in this study had low ESS scores even before treatments of CBT-I, such that a ceiling effect may have had an impact here. In addition, patients with coexisting sleep disorders, such as obstructive sleep apnea and restless legs syndrome, which may cause significant sleep degeneration, were excluded; this may have resulted in the low average ESS score before the treatments. Moreover, the reduction of and withdrawal from sleep medications presumably prevented drug-induced excessive daytime sleepiness after CBT-I.
The current results also suggest that CBT-I not only improves ISI, DBAS, and SDS scores but also leads to the reduction of and withdrawal from sleep medications. Twenty-seven patients (51.9%) withdrew from taking sleep medication after CBT-I. The ISI score after CBT-I was the only significant factor related to the withdrawal. This implies that an improvement in insomnia was a major factor that enabled the reduction of or withdrawal from sleep medications. Thus, other factors, such as age, dosing duration, and initial insomnia severity, which have been reported to cause difficulty with regard to withdrawal from sleep medication [56, 57], might be secondarily influenced. In this study, the timing of the start of the drug reduction program was adjusted by monitoring the ISI scores, which could have influenced the results.
CBT-I seemed to facilitate the reduction of hypnotics, and 10 patients reduced their dose of hypnotics by more than half. However, no relationship between the reduction in hypnotics and reduction in sleep discrepancies was found. It has been indicated that benzodiazepine hypnotics are beneficial for insomnia treatment but facilitate cognitive distortion and could enhance sleep discrepancy [58]. In contrast, the current results suggested that the reduction in hypnotics with CBT-I did not influence sleep discrepancy. It should be considered that CBT-I may chiefly alleviate the severity of insomnia disorder, and the impact of hypnotics on sleep discrepancy seemed minor based on the current results. The long-term use of benzodiazepine hypnotics could lead to residual cognitive distortions [59], which could have also influenced the results.
Limitations
In this study, a randomized comparison with a control group was not conducted, and no follow-up was conducted after therapy. Thus, long-term prognoses were not observed. In addition, all patients were outpatients who sought medical attention in a specialty outpatient clinic of a university hospital. Although insomnia disorder is highly comorbid with various medical conditions, especially with mental disorders, patients with comorbid insomnia were excluded to prevent any contamination by psychotropic medications except for hypnotics. Thus, the characteristics of the group of research participants may have differed from those of patients seeking local primary medical care, and caution is warranted when generalizing the results of this study to all patients with an insomnia disorder. Furthermore, this study did not include any patients that met the criteria of paradoxical insomnia in ICSD-2, according to the subjective TST. Patients with paradoxical insomnia typically state that they did not sleep at all in the morning while the PSG shows adequate and relatively “normal” sleep time. Although sleep misperception is also included as a characteristic of paradoxical insomnia, typical paradoxical insomnia could share some pathologies associated with mental disorders [13]. Our exclusion criteria of the study participants could have caused this limitation. Moreover, the averaged ISI score of the study participants points to the moderate severity of insomnia disorder. Patients with irregular sleep–wake habits such as shift work and frequent international travel were also excluded. However, particular attention was not given to minor changes in the sleep–wake schedule of daily life; this could also be a limitation.
The assessment of objective sleep by actigraphy is also a major limitation, since PSG is the standard technique for the measurement of objective sleep variables. It has been reported that although correlations are relatively strong between PSG and actigraphy, actigraphy tends to overestimate sleep; compared with PSG, actigraphy tends to report shorter SOL, longer TST, shorter WASO, and higher SE in patients with insomnia. Withrow et al. reported that actigraphy (Philips Healthcare, Bend, OR) is fairly accurate in measuring TIB, TST, SOL, and SE but significantly underestimates WASO as compared to PSG [55]. Thus, the subjective–objective discrepancy in WASO could be underestimated and was used for patient feedback during CBT-I in the current study. This could have facilitated the conversion of sleep evaluation after CBT-I. PSG is intrinsically sensitive to unconsciously emerged micro-arousal states during sleep. However, such micro-arousal states could influence the pathophysiology of sleep misperception per se [60]. Thus, further technologies, such as high-density electroencephalography, could be appropriate for measuring sleep continuity and subjective sleep quality, which may also be associated with insomnia pathology [61]. In contrast, actigraphy is highly suitable to obtaining objective sleep data for assessing patients’ habitual sleep states compared to PSG administered at a lab or hospital. Recently developed high-precision wearable and portable electroencephalography methods could be available for assessing sleep discrepancy at home [44, 62]. Future studies should use more reliable methods to ensure transferability across measures.
This study was a pilot exploratory study, and the sample size was not defined based on a priori power analysis. In addition, although objective data were processed by a blinded technologist, all statistical analyses were conducted by the same psychologist who conducted all CBT-I sessions. Thus, this could also be construed as a limitation.
Conclusion
This study assessed changes in sleep discrepancy during the CBT-I for primary insomnia patients. Cognitive biases, including sleep discrepancy, could be reduced by CBT-I. Sleep discrepancy seemed to convert negative to positive bias directions in accordance with insomnia severity reduction by CBT-I. Although sleep discrepancy decreased with CBT-I, this appeared to partially contribute to improvements in primary insomnia. Further longitudinal randomized control trials are warranted to clarify the possible specificity of CBT-I in treating sleep discrepancy, in addition to improving the long-term resilience of patients with regard to the potential development of primary insomnia.
Change history
20 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12529-021-10015-z
References
Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111.
Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10.
Uchiyama M, editor. J, Group research group on guidelines for diagnosis and treatment of sleep disorders. Suimin shogai no taio to chiryo gaidorain. [Management and Treatment Guidelines for Sleep Disorders (Author’s transl)] 2nd ed. 2002.
Kadotani T, Kadotani H, Arai H, et al. Comparison of self-reported scales and structured interviews for the assessment of depression in an urban male working population in Japan : a cross-sectional survey. Sleep Sci Pract. 2017;1:9.
Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9:S3–9.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: VA. American Psychiatric Pub; 2013.
American Academy of Sleep Medicine. Sleep Med. 3rd ed. Am. Acad. 2014. Elsevier:ICSD-3 international classification of sleep disorders.
Morin CM. Insomnia: psychological assessment and management. New York, NY: Guilford Press; 1993.
Buysse D, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97:165–172.
Baglioni C, Regen W, Teghen A, et al. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18:195–213.
American Academy of Sleep Medicine. ICSD-2 international classification of sleep disorders. 2nd ed. American Academy of Sleep Medicine: Diagnostic coding Man; 2005.
Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective–objective sleep discrepancy: a review. Sleep Med Rev. 2018;40:196–202.
American Sleep Disorders Association, Diagnostic Classification Steering Committee, Thorpy MJ. The international classification of sleep disorders: diagnostic and coding manual. American sleep disorders association. 1990.
Lund HG, Rybarczyk BD, Perrin PB, Leszczyszyn D, Stepanski E. The discrepancy between subjective and objective measures of sleep in older adults receiving CBT for comorbid insomnia. J Clin Psychol. 2013;69:1108–20.
Ghadami MR, Khaledi-Paveh B, Nasouri M, Khazaie H. PTSD-related paradoxical insomnia: an actigraphic study among veterans with chronic PTSD. J Inj Violence Res. 2015;7:54.
Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40.
Bootzin RR. Stimulus control treatment for insomnia. Proc Am Psychol Assoc. 1972;7:395–6.
Hauri P. Current concepts: the sleep disorders. The Upjohn Company, Kalamazoo, MI; 1977. pp. 21–35.
Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56.
Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. J Am Med Assoc. 2009;301:2005–15.
Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9:24–34.
Jan Y-W, Yang C-M, Huang S-H, Lee H-C. Treatment effect of cognitive-behavior therapy for insomnia combined with usual medication. Sleep Biol Rhythms. 2019;17:311–21.
Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16.
Merrigan JM, Buysse DJ, Bird JC, Livingston EH. Insomnia JAMA. 2013;309:733.
Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191–204.
Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93.
Harvey AG, Tang NKY, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25:593–611.
Harada D, Yamadera W, Sato M, et al. Effects of two-session group cognitive behavioral therapy for psychophysiological insomnia: a preliminary study. Sleep Biol Rhythms. 2015;13:348–56.
Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88.
Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text revision). Washington, DC: American Psychiatric Pub; 2000.
Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70.
Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behav Res Ther. 2002;40:741–52.
Perlis ML, Smith MT, Jungquist C, Nowakowski S, Orff H, Soeffing J. Cognitive-behavioral therapy for insomnia. In: Attarian H, editor. Clinical Handbook of Insomnia. Humana Press; 2010. pp. 281–296.
Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73.
Kay-Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ. 2016;354:2123.
Munezawa T, Morin CM, Inoue Y, Nedate K. Development of the Japanese version of the Insomnia Severity Index (ISI-J). Japanese J Psychiatr Treat. 2009a;24:219–25.
Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30:1547–54.
Munezawa T, Morin CM, Inoue Y, Nedate K. Development of the Japanese version of Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-J). Japanese J Sleep Med. 2009b;3:396–403.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5.
Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10:556–65.
Fukuda K, Kobayashi S. A study on a self-rating depression scale (author’s transl). Seishin shinkeigaku zasshi Psychiatr Neurol Jpn. 1973;75:673–9.
Matsuo M, Masuda F, Sumi Y, et al. Comparisons of portable sleep monitors of different modalities: potential as naturalistic sleep recorders. Front Neurol. 2016;7:1–11.
Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9.
Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69:440–7.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Aritake S, Uchiyama M, Tagaya H, et al. Time estimation during nocturnal sleep in human subjects. Neurosci Res. 2004;49:387–93.
Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41:1–9.
Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54.
Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res. 2015;24:32–9.
Saha S, Dey D, Bhattacharyya IM, Das A. An investigation on biofeedback analysis and psychosomatic applications. 2015 International Conference on Recent Developments in Control, Automation and Power Engineering. 2015. pp. 38–43.
Tang NKY, Harvey AG. Altering misperception of sleep in insomnia: Behavioral experiment versus verbal feedback. J Consult Clin Psychol. 2006;74:767–76.
Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31:1527–33.
Lichstein KL, Nau SD, Wilson NM, et al. Psychological treatment of hypnotic-dependent insomnia in a primarily older adult sample. Behav Res Ther. 2013;51:787–96.
Murakoshi A, Takaesu Y, Komada Y, Ishikawa J, Inoue Y. Prevalence and associated factors of hypnotics dependence among Japanese outpatients with psychiatric disorders. Psychiatry Res. 2015;230:958–63.
Takaesu Y, Utsumi T, Okajima I, et al. Psychosocial intervention for discontinuing benzodiazepine hypnotics in patients with chronic insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2019;48:101214.
Mendelson WB. Long-term follow-up of chronic insomnia. Sleep. 1995;18:698701.
Barker MJ, Greenwood KM, Jackson M, Crowe SF. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: A meta-analysis. Arch Clin Neuropsychol. 2004;19:437–54.
Halász P, Terzano M, Parrino L, Bódizs R. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23.
Sterpenich V, Perogamvros L, Tononi G, Schwartz S. Fear in dreams and in wakefulness: Evidence for day/night affective homeostasis. Hum Brain Mapp. 2020;41:840–50.
Withrow D, Roth T, Koshorek G, Roehrs T. Relation between ambulatory actigraphy and laboratory polysomnography in insomnia practice and research. J Sleep Res. 2019;28:e12854.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Shiga University of Medical Science on October 26, 2010 (approval no. 22-91).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was corrected to unblind information in the Ethics section.
Rights and permissions
About this article
Cite this article
Nishikawa, K., Kuriyama, K., Yoshiike, T. et al. Effects of Cognitive Behavioral Therapy for Insomnia on Subjective–Objective Sleep Discrepancy in Patients with Primary Insomnia: a Small-Scale Cohort Pilot Study. Int.J. Behav. Med. 28, 715–726 (2021). https://doi.org/10.1007/s12529-021-09969-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12529-021-09969-x