Abstract
The large gorgonian genus Leptogorgia Milne Edwards & Haime, 1857 is abundant and widely distributed along coastlines and around oceanic islands in the Eastern Tropical Pacific, where it comprises 31 species with a wide bathymetric distribution. Material of Leptogorgia dictynna sp. nov. was collected at mesophotic reefs bordering marine protected areas off Pacific Costa Rica and Panamá. The species is characterised by a conspicuous, irregularly pinnate branching pattern; almost flat polyp-mounds with a lateral, biserial distribution on the branches; coenenchymal sclerites mostly long and thin spindles, up to 0.17 mm in length; and white colony and sclerites. The finding of this new species confirmed the biodiversity hot spot status of the protected areas where it was sampled and the need to increase their protection. Herein, the new species is described, and it is compared with other members of the genus in the Eastern Tropical Pacific from both a morphological and a molecular point of view.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gorgonian genus Leptogorgia Milne Edwards & Haime, 1857 is widely distributed in the Eastern Tropical Pacific (ETP) where it is abundant along continental shorelines and around oceanic islands. The genus comprises 31 valid species in the ETP, with a wide bathymetric range. Normally, it occurs shallower than 30 m in depth; however, several species have been found in deeper waters (Bayer 2000; Breedy and Guzman 2007; Breedy and Cortés 2011). Leptogorgia styx Bayer, 2000 has the deepest record for the genus at 1900–1950 m depth on a seamount in the East Pacific Rise, 500 km SSW of Acapulco, México (Bayer 2000). Some other species have been recorded from shallower, mesophotic habitats (35–150 m deep), such as Leptogorgia regis Hickson, 1928, down to 50 m and Leptogorgia filicrispa Horvath, 2011, down to 87 m (Breedy and Guzman 2007; Horvath 2011).
The species boundaries of Leptogorgia (as in many other octocorals) are difficult to draw (Breedy and Guzman 2007); however, morphological characters, such as colony and sclerite shapes, sizes, and colours, together with field observation (e.g. habitat, bathymetry), represent valuable information to determine and delimit species. In Leptogorgia, the colouration of the colonies and sclerites has been traditionally used to separate groups of species (Guzman and Breedy 2008; Breedy and Cortés 2011), which has been corroborated by molecular phylogenetic studies of eastern Pacific octocorals (Vargas et al. 2014; Ament-Velasquez et al. 2016).
A previously undescribed mesophotic species of Leptogorgia collected in Las Perlas Archipelago, Panamá, was recently also discovered during an exploratory immersion by the submersible DeepSee at mesophotic reefs off the Osa Conservation Area, Costa Rica. The exploration was part of the Osa Peninsula-Pristine Seas Expedition to document the unique and iconic biodiversity of the region and to support efforts to create a large Marine Protected Area off the Osa Peninsula (Friedlander et al. 2019). Herein, we describe the new mesophotic species based on material collected in Costa Rica and Panamá, compare it with related species in the genus, and provide a molecular phylogenetic analysis supporting our conclusions.
Material and methods
Sites and collections
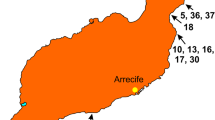
The holotype was collected at Coquito point, Bajo del Diablo pinnacle, off Caño Island by the submersible DeepSee of the M/V Hundersea Hunter at 50 m depth. The paratypes were incidentally collected from mesophotic reefs in southeast Las Perlas Archipelago in the Gulf of Panamá, Panamá, by a small trawling net at 40–50 m from the Smithsonian Institution’s R/V Urraca.
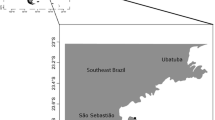
Caño Island Biological Reserve is a protected area located off the south Pacific coast of Costa Rica, approximately 15 km off mainland Osa Peninsula (8° 43′ N, 85° 52′ W) (Fig. 1). It is part of the Osa Conservation Area, which comprises 55.4 km2 of marine area with coral reefs, sandy bottoms, rhodolith beds, rocky outcrops, and beaches (Guzman and Cortés 2001; Cortés et al. 2009; Friedlander et al. 2019). The south section of the Island is deeper, more exposed to wave action and currents, and less explored. Several rocky reefs and submarine pinnacles are found in the south (Guzman and Cortés 1989), one of them is Bajo del Diablo, located about 2 km south-west Caño Island (Fonseca et al. 2010). Coquito point, south Bajo del Diablo, is the deepest point down to 55 m in depth. There are twenty octocoral species reported from shallow waters (down to 30 m depth) in Caño Island, being one of the most diverse sites in terms of Octocorallia in Costa Rica (Cortés et al. 2009; Friedlander et al. 2019). The collected specimen was photographed fresh on deck, air dried, and a fragment fixed in 95% ethanol.
Las Perlas Archipelago is a marine protected area located within the 50–m isobath 70 km off the Pacific coast of Panamá City (8° 39.274′ N, 79° 3.708′ W), composed of ca. 250 basaltic rock islands and islets, encompassing 1,688 km2 (Guzman et al. 2008). The protected area is the fourth largest in Panamá, surrounded by reef and coral communities with reports of over 19 species of scleractinian corals and 38 octocoral species (Guzman et al. 2008). The southern part of the archipelago (San Telmo and Galera islands and Trollope Bank) contains the highest diversity of corals. The collected specimens were air-dried.
Morphological analysis
For microscopy, fragments of the tips of the colonies were treated with 5% sodium hypochlorite to dissociate sclerites from the tissues. The structures were washed several times in distilled water and dehydrated with 100% ethanol and posteriorly dried at 40 °C in an oven. For optic microscopy, sclerites were mounted in water or glycerine and photographed with an Olympus LX 51 inverted microscope. For scanning electron microscopy (SEM), sclerites were mounted on SEM stubs by double stick carbon tape and silver paint bridges between the tape and the stubs were made to increase the electronic conduction. The samples were then sputter-coated with gold, 30–60 nm layer, in an Eiko IB-5 Ion Coater, and the images were obtained using a Hitachi SEM S-3700 N. Measurements of the sclerites were obtained from both the optical microscope and the SEM images. The length of the sclerites was measured from one tip to the other, and the width was taken from the most distant points across the sclerites, reporting the largest sizes found in the samples. The diameter of the branches, branchlets, and stems is given taking into account the height of the polyp-mound. Morphological characters of colonies and sclerites and comparisons with related species in the genus (Breedy and Guzman 2005, 2007) are presented in Table 1.
The holotype is deposited in the Museo de Zoología, Universidad de Costa Rica (MZUCR), and paratypes are deposited in the Smithsonian Tropical Research Institute (STRI) octocoral collection in Panamá.
Molecular phylogenetic analyses
DNA was extracted from ethanol-preserved tissues with the DNeasy PowerSoil Pro Kit (Qiagen, USA) according to the manufacturer’s instructions and kept at − 20 °C until further processing. A partial region of the mitochondrial mismatch repair gene (mtMutS) and the 28S nuclear ribosomal gene was amplified with primers ND42599F (5′-GCCATTATGGTTAACTATTAC-3′; France and Hoover 2002) and MUT3458R (5′-TSGAGCAAAAGCCACTCC-3′; Sánchez et al. 2003) and 28S-Far (5′-CACGAGACCGATAGCGAACAAGTA-3′) and 28S-Rar (5′-TCATTTCGACCCTAAGACCTC-3′) (McFadden and Ofwegen 2012), respectively. All the reactions were carried out in 50 μl volume with 10–50 ng DNA, 2.5 units Taq DNA polymerase (DreamTaq, Thermo Scientific, Waltham, MA), 1X DreamTaq Buffer, 0.2 mM of each dNTP, 0.3 μM of each primer, and 50 μg of BSA. The amplification protocol for mtMutS consisted of 2 min of initial denaturation at 94 °C followed by 35 cycles of 30 s at 94 °C, annealing at 50 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min and for 28S was 5 min of initial denaturation at 94 °C followed by 35 cycles of 60 s at 94 °C, annealing at 46 °C for 30 s, extension at 72 °C for 60 s, and a final extension at 72 °C for 10 min. The resulting PCR products were purified and sequenced by Macrogen Inc. (Seoul, Korea), using the same forward and reverse PCR primers. The PCR purification was done by Macrogen, and they used the ExoSAP-IT purification method. The resulting PCR products were purified (ExoSAP-IT purification method) and sequenced by Macrogen Inc. (Seoul, Korea). Sequences from MZUCR3304 have been deposited in GenBank under accession numbers MZ322102 (28S rDNA) and MZ320325 (mtMutS).
Phylogenetic inference was done using the programs RAxML 8.2.11 and MrBayes 3.2.6 and the GTR + G model of sequence evolution. For the maximum likelihood analysis, we assessed support using 1000 bootstrap replicates. For the Bayesian analysis, we ran the MCMCMC for 10,000,000 generations sampling every 1,000 generations. We checked for convergence using Tracer and removed 25% of the sampled trees as a burn-in before calculating a Bayesian consensus tree. For the phylogenetic analyses, we focused the analyses on octocoral sequences from the genera Pacifigorgia, Eugorgia, and Leptogorgia (family Gorgoniidae) deposited in public databases (SI-Table 1). Finally, for mtMutS and 28S, we calculated the genetic (K2P) distance between L. dictynna sp. nov. and related Leptogorgia species.
Results
Taxonomy
Class Anthozoa Ehrenberg, 1834
Subclass Octocorallia Haeckel, 1866
Order Alcyonacea Lamouroux, 1816
Family Gorgoniidae Lamouroux, 1812
Genus Leptogorgia Milne Edwards & Haime, 1857
Synonymy in Breedy and Guzman 2007
Type species: Gorgonia viminalis Pallas, 1766 (from the Mediterranean Sea), by subsequent designation, Verrill 1868: 420.
Diagnosis: The genus is characterised by having variable branching patterns: pinnate-like, dichotomous, irregular, or filiform. Branch anastomosis is rare, normally absent, or limited to few branches or branchlets. Colonies have a horny axis that has a narrow cross-chambered central core with a network of organic filaments frequently mineralised with deposits of carbonate hydroxylapatite. Polyps are fully retractile into the coenenchyme, forming mound-shaped protuberances which may be slightly raised, or prominent around the polyp apertures. Coenenchymal sclerites are basically capstans and/or spindles and derivatives of them. Anthocodial sclerites usually are flat rods and platelets. The colour of the colonies and sclerites is variable: white, yellow, orange, red, violet, brownish, or mixtures thereof, and also bicoloured.
Genus distribution: Eastern Pacific (from southern California to Chile), Atlantic Ocean, western and southern Africa, Caribbean Sea, Mediterranean Sea, and one record for the Subantarctic (Williams and Lindo 1997).
Leptogorgia dictynna sp. nov.
http://zoobank.org/75E4A2F2-D8B8-4D59-84DD-5869006920DB
Holotype: MZUCR 3304, dry/fragment in ethanol, Coquitos Point, Bajo del Diablo, Caño Island, Osa Peninsula, Costa Rica, 50 m depth, DeepSee submersible, Dive 2979, pilot S. Blum, 17 March 2019. GenBank accession numbers MZ322102 (28S rDNA) and MZ320325 (mtMutS).
Paratypes: STRI 678, 691, 705, 706, dry, southern Las Perlas Archipelago, Pacific coast of Panamá, 40–50 m depth, small trawling net from the Smithsonian’s R/V Urraca, R. Robertson, June 2003.
Diagnosis: Colonies flabellate, irregularly pinnate, branching in several planes and with long terminal twigs. Branches and twigs mostly bilaterally and closely arranged. Polyps fully retractile into mostly flat mounds biserially arranged on the branches and branchlets. Coenenchymal sclerites mostly straight, long spindles with marked waists and up to 10 whorls of separate tubercles and up to 0.17 mm in length. Capstans straight with marked bare waists and warty ends. Anthocodiae with weak points of colourless, long, and narrow rods, up to 0.10 mm in length. Colour of colony white, sclerites whitish to transparent.
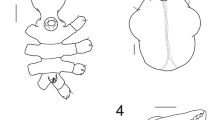
Description of the holotype: The holotype is an erect richly branched, flabelliform colony 42 cm long and 30 cm wide (Fig. 2a). Branching is irregularly pinnate and in several planes. The main stem is 3.5–4.0 cm long; it is round in cross section and has a diameter of 6 mm just above the holdfast. The colony has up to about seven orders of branches. The stem and the branches contain subramifications arranged bilaterally. These subramifications consist mostly of short and simple twigs and a small and variable number of longer branches which originally were also twigs. Although the branches and twigs are arranged bilaterally, those on one side of the stem or branch are not uniformly spaced relative to those on the other side; some are subopposite; others are offset to varying degrees such that in a few cases they appear to be alternating; and in a few places, they are uniserial over short distances. Branches are slightly flattened, 4.0–5.0 mm in diameter; twigs are around 1.0–1.5 mm in diameter. There are usually 5 to 7 twigs per 3 cm along each side of a branch. The twigs are closely spaced, mostly 10–20 mm apart. One characteristic feature of the twigs is that they are not very uniform in length along a branch; very short ones often occur between much longer ones, from 2–54 mm, mostly 20–30 mm in length (Fig. 2a). They can be straight or curved to varying degrees (at 30°–45° angles) and are usually directed distally relative to the branch on which they occur (Fig. 2a). The highest order branches found at the outer perimeter of the colony also possess twigs arranged bilaterally. They are variable in length with the longest ones not necessarily those at the proximal end of the branch from which they arise (Fig. 2a). The holdfast is oval, 2 cm in diameter; both the stem and the holdfast are devoid of polyps. Longitudinal grooves extend along the main branches. They are very distinct at the base and can be traced, in a sinuous manner along the stem and main branches. The axis is horny, with a chambered central core filled with organic filaments mineralised with microspheres of carbonate hydroxylapatite, typical for the genus. A few lower branches are pseudo-anastomosed. The polyp mounds are mostly flat. Polyps are usually biserial, arranged in two rows on each side of the branches, 6–9 polyp apertures/cm, and further apart in thicker branches. Polyps are fully retractile into the coenenchyme (Fig. 2b). The polyp apertures are oval, 0.24–0.63 mm in diameter in the dry holotype (Fig. 2b). Coenenchymal sclerites are mostly straight, long spindles 0.07–0.17 mm in length and 0.03–0.05 mm in width, with marked waists, 5 to 10 whorls of tubercles that are mostly separated and with small and scattered warts along the ends (Fig. 3a). The spindles may have both ends acute or one blunt and the other acute; a few of them are slightly curved at the ends (Fig. 3a). Capstans are straight with marked bare waists, two warty whorls of tubercles that can be incomplete, and warty ends. The capstans are 0.05–0.06 mm in length and 0.02–0.03 mm in width (Fig. 3b). Some immature forms appear in the samples (Fig. 3c). The anthocodiae have a weak, point-like arrangement of long and narrow rods. These sclerites are colourless, flat, 0.07–0.10 mm in length, and 0.008–0.02 mm in width, with scalloped margins or with few short, lateral projections (Fig. 3d). The colour of the colony is white, and sclerites are whitish to transparent.
Variability: The large colonies match the holotype, and the smaller colonies observed from the submarine grow mostly in one plane but keep the characteristic irregularly pinnate growth pattern of the branches. We observed colonies larger than the holotype, perhaps reaching up to 50–60 cm tall and branching in more than three planes (Fig. 4a–b). The paratypes from Panamá are from 8 to 30 cm long, matching the holotype morphology in all aspects.
Etymology: The species name is derived from the Greek myth of the nymph Britomartis (= sweet or blessing maiden), worshipped in Crete where she also received the name Dictymna or Dictynna (= lady of the net; net = dictya). The nymph was associated with fishers and sailors and considered the protectress of harbours and navigation. She was beloved by Artemis, but Minos was attracted to her and chased her throughout Crete. In order to escape from Minos, she threw herself into the sea, where she got entangled in nets deployed by fishers. Instead of dying, Artemis saved her unharmed and turned her into a goddess (Smith 1813–1893, Hard 2019). The name L. dictynna refers to the first specimens collected that were entangled in nets that alludes to the Greek myth of the goddess Dictynna, lady of the nets. Dictynna is used as the epithet of the species.
Habitat and distribution: Coquitos Point is characterised by large massive rocks covered by red crustose algae and sandy patches between the rocks (Fig. 4a–b). The shoal is known for the number of large fishes living there. The colonies of L. dictynna were found in clusters on large rocks, facing the dominant strong current (Fig. 4b) down to 50–54 m in depth. The paratypes from Panamá were incidentally obtained from rocky reefs down to 50 m while collecting fish for another research expedition.
Phylogenetic analysis: Our molecular phylogenetic analyses positioned L. dictynna sp. nov. together with other eastern Pacific Leptogorgia species. Leptogorgia dictynna sp. nov. did not form part of the clade of Leptogorgia species with exclusively white sclerites in either the 28S or the mtMutS phylogenies. In the mtMutS phylogeny, L. dictynna sp. nov. was closely related to clades including Leptogorgia flexilis (Verrill, 1868) and Leptogorgia ramulus (Milne Edwards & Haime, 1857), Leptogorgia cuspidata (Verrill, 1865) and Leptogorgia rigida Verrill, 1868 and the white species of Leptogorgia, but was not included in any of these groups (Fig. 5). In the 28S phylogeny, this species was sister to a clade including L. flexilis, L. cuspidata, L. rigida and a dubious specimen attributed to L. alba in need of verification (Fig. 6). The genetic distances between eastern Pacific Leptogorgia species are generally low and difficult to use in isolation for species discovery and delimitation. In the case of L. dictynna sp. nov. K2P distances (Table 2) ranged between 0.0014 (L. dictynna sp. nov. vs. L. ramulus) and 0.0073 (L. dictynna sp. nov. vs. L. flexilis) for mtMutS and between 0.0070 (L. dictynna sp. nov. vs. L. cuspidata) and 0.0214 (L. dictynna sp. nov. vs. L. alba) for 28S.
Growth forms of eastern tropical Pacific colonies of Leptogorgia spp. a Leptogorgia alba, in situ colonies, 16 m depth, Roca Matapalito, Osa Peninsula; b L. alba, in situ colony, 20 m depth, Shark Cave, Isla del Caño; c L. cortesi, MZUCR 2126, preserved specimen, 15 m depth, Islotes, Osa Peninsula. a–b Photographs by Kike Ballesteros, Pristine Seas, National Geographic Society
Phylogenetic placement of L. dictynna sp. nov. mtMutS Bayesian phylogeny. The values above and below the branches correspond to the posterior probability and bootstrap support of the branch, respectively. The maximum likelihood and Bayesian phylogenetic trees for these markers can be found in the Supplementary materials
Discussion
Bajo del Diablo is one of the richest places in Caño Island with regard to octocoral diversity. Between 10 to 30 m, about 12 octocoral species coexist (Cortés et al. 2009). Deeper than 30 m, the number of species decreases. Down to 50 m, there is a shift in octocoral species diversity, and at Coquitos Point, the only species found was L. dictynna sp. nov..
As the colour is considered an important trait to separate groups of Leptogorgia in the eastern Pacific, in the present case, the white colour of the colonies of L. dictynna is the first characteristic that could separate it from the other coloured species in the genus. There are seven valid species in the eastern Pacific that exclusively have white colonies (Table 1); among them, the closest comparable species to the new one are Leptogorgia cortesi Breedy & Guzman, 2012, L. alba, and L. styx. This is mainly due to the irregularly pinnate growth of these colonies that could be compared to the new species. Leptogorgia cortesi (Fig. 7c) has bushy colonies of longer sparse branchlets than L. dictynna sp. nov.. Although L. alba present variable pinnate morphologies (Fig. 7a–b), the pinnate state is more irregular than in L. dictynna sp. nov. and has thicker branchlets; therefore, neither this species nor L. cortesi (Fig. 7c) have the conspicuous branching pattern of L. dictynna sp. nov. which has a large number of relatively short, simple, bilaterally arranged branchlets (twigs) occurring throughout the colony from the stem to the highest order branches (Fig. 4). Although there is not much information about L. styx, the colony growth is in one plane, different from L. dictynna. The spindles (the most abundant kind of sclerites in the Leptogorgia group) of L. cortesi, L. alba, and L. styx are very similar, with noticeable variation in size and ornamentation (Table 1). The spindles in L. dictynna sp. nov. are clearly less complex, with larger median spaces and generally thinner than the ones in these species supporting the description of a new species for the new specimens. Leptogorgia cortesi and L. alba are also found around the Peninsula de Osa, the first one is found in shallow waters down to 20 m depth, and the latter is down to 40 m in the mesophotic zone.
Phylogenetic placement of L. dictynna sp. nov. 28S rRNA Bayesian consensus phylogeny. The values above and below the branches correspond to the posterior probability and bootstrap support of the branch. The maximum likelihood and Bayesian phylogenetic trees for this marker can be found in the Supplementary materials
Molecular phylogeny reconstructions of eastern Pacific gorgoniids offered support to all morphological groups defined for Leptogorgia (Ament-Velasquez et al. 2016; Vargas et al. 2014), corroborating the monophyly of all Leptogorgia species with exclusively white sclerites described for the eastern Pacific (Vargas et al 2014). In the case of L. dictynna sp. nov. a Leptogorgia species with exclusively white sclerites and a white colony, our molecular analyses did not include this species in the clade of previously described white Leptogorgia species, supporting its description as a new Leptogorgia species different from the widespread and morphologically diverse L. alba. The analysis of the genetic distance between L. dictynna sp. nov. and related Leptogorgia species revealed that the closest species to L. dictynna sp. nov. is represented by a GenBank specimen classified as L. ramulus, a species with white and pink morphs. That species clearly differs from the new species in the composition of its sclerome, which is dominated by capstans instead of spindles as in the new species, and consistently shows light orange anthocodial sclerites. Leptogorgia ramulus is only known from museum specimens and has neither been found along the coasts of Costa Rica or Panama. The L. ramulus sequence deposited in GenBank should be considered dubious, and the taxonomic status of the associated voucher, if any exists, must be evaluated. Finally, considering that L. dictynna sp. nov. exclusively occurs in the mesophotic zone, its discovery suggests that the exploration of this zones’ ecosystems will reveal new gorgonian lineages that are possibly not closely related to the shallow-water species already described and characterised for the region. Thus, the systematic study of the eastern Pacific mesophotic zone will result in a better understanding of the octocoral diversity of this region.
Conclusions
The mesophotic reefs around Peninsula de Osa and Las Perlas Archipelago have been scarcely explored, and the discovery of L. dictynna sp. nov. exposes the need for continuous exploration of deep habitats within this zone to reveal undiscovered species diversity. Both protected areas support two important centres of biodiversity in the region, requiring special attention to destructive fishing practices (e.g. trawling) potentially affecting mesophotic habitats. This work is a contribution to the knowledge of the octocorals from mesophotic habitats.
References
Ament-Velasquez SL, Breedy O, Cortés J, Guzman H, Wörheide G, Vargas S (2016) Homoplasious colony morphology and mito-nuclear phylogenetic discordance among Eastern Pacific octocorals. Mol Phylogenet Evol 98:373–381. https://doi.org/10.1016/j.ympev.2016.02.023
Bayer FM (2000) A new species of Leptogorgia from the eastern Pacific (Coelenterata: Octocorallia: Holaxonia). Proc Biol Soc Wash 113(3):609–616
Breedy O, Guzman HM (2002) A revision of the genus Pacifigorgia (Coelenterata: Octocorallia: Gorgoniidae). Proceedings of the Biol Soc Wash 115(4):782–839
Breedy O, Guzman HM (2005) A new species of Leptogorgia (Coelenterata: Octocorallia: Gorgoniidae) from the shallow waters of the eastern Pacific. Zootaxa 899:1–11. https://doi.org/10.11646/zootaxa.899.1.1
Breedy O, Guzman HM (2007) A revision of the genus Leptogorgia Milne Edwards & Haime, 1857 (Coelenterata: Octocorallia: Gorgoniidae) in the eastern Pacific. Zootaxa 1419:1–90. https://doi.org/10.11646/zootaxa.1419.1.1
Breedy O, Cortés J (2011) Morphology and taxonomy of a new species of Leptogorgia (Cnidaria: Octocorallia: Gorgoniidae) in Cocos Island National Park, Pacific Costa Rica. Proc Biol Soc Wash 124(2):62–69
Breedy O, Guzman HM (2012) A new species of Leptogorgia (Cnidaria: Anthozoa: Octocorallia) from Golfo Dulce, Pacific, Costa Rica. Zootaxa 3182:65–68. https://doi.org/10.11646/zootaxa.3182.1.7
Cortés J, Guzmán HM, Fonseca AC, Alvarado JJ, Breedy O, Fernández C, Segura A, Ruiz E (2009) Ambientes y organismos marinos de la Reserva Biológica Isla del Caño, Área de Conservación Osa, Costa Rica. Serie Técnica: Apoyando los esfuerzos en el manejo y protección de la biodiversidad tropical, Número 13. The Nature Conservancy (TNC), San José, Costa Rica. 48 p
Duchassaing P, Michelotti G (1864) Supplement au mémoire sur les Coralliaires des Antilles. Memorie Accad Sci Torino (ser. 2) 23:97–206
Ehrenberg CG (1834) Beiträge zur physiologischen Kenntniss der Corallenthiere im Allgemeinen, und besonders des rothen Meeres, nebst einem Versuch zur physiologischen Systematik derselben. Abhandlungen der Königlichen preussischen Akademie der Wissenschaften zu Berlin. Aus dem Jahre 1832. Erster Theil 1–380
Fonseca AC, Guzmán HM, Cortés J, Soto C (2010) Marine habitat map of “Isla del Caño”, Costa Rica, comparing Quickbird and Hymap images classification results. Rev Biol Trop 58:373–381
France SC, Hoover LL (2002) DNA sequences of the mitochondrial COI gene have low levels of divergence among deep-sea octocorals (Cnidaria: Anthozoa). Hydrobiologia 471:149–155. https://doi.org/10.1023/A:1016517724749
Friedlander A, Salinas de León P, Ballesteros E, Breedy O, Cortés J, Hernández N, Mayorga J, Mirallés M, Muñoz Wilson A, Naranjo-Elizondo B, Thompson C, Sala E (2019) Península de Osa: Biodiversidad y recomendaciones de conservación. Informe de la expedición National Geographic Pristine Seas, Asociación Conservación Osa y la Universidad de Costa Rica. Marzo del 2019. 74 p
Gray JE (1859) On the arrangement of zoophytes with pinnated tentacles. Ann Mag Nat Hist Ser 4(3):439–444
Guzman HM, Breedy O (2008) Leptogorgia christiae (Octocorallia: Gorgoniidae) a new shallow gorgonian from Pacific Panama. J Mar Biol Assoc UK 88(4):719–722. https://doi.org/10.1017/S0025315408001240
Guzman HM, Cortés J (1989) Coral reef community structure at Caño Island. Pacific Costa Rica Mar Ecol 10(1):23–41
Guzman HM, Cortés J (2001) Changes in reef community structure after fifteen years of natural disturbances in the Eastern Pacific (Costa Rica). Bull Mar Sci 69(1):133–149
Guzman HM, Benfield S, Breedy O, Mair JM (2008) Broadening reef conservation across the Tropical Eastern Pacific Seascape: distribution and diversity of reefs in Las Perlas Archipelago, Panama. Environm Conserv 35:1–9. https://doi.org/10.1017/S0376892908004542
Haeckel E (1866) Generelle Morphologie der Organismen. Berlin, 1036 pp
Hard R (2019) El gran libro de la mitología griega. Décima Edición. Traducción de Jorge Cano Cuenca
Hickson SJ (1928) The Gorgonacea of Panama Bay together with a description of one species from the Galapagos Islands and one of Trinidad. Videnskavelige Meddelelser Fra Den Naturhistoriske Forening i Kovenhavn for Aarene 85:325–422
Horvath EA (2011) An unusual new “sea fan” from the northeastern Pacific Ocean (Cnidaria: Octocorallia: Gorgoniidae). Proc Biol Soc Wash 124(1):45–52. https://doi.org/10.2988/10-27.1
Lamouroux JVF (1812) Extrait d’un mémoire sur la classification des Polypiers coralligènes non entièrement pierreux. Nouv Bull Sci Soc Philom 3:181–188
Lamouroux JVF (1816) Histoire des Polypiers coralligènes flexibles, vulgairement nommés Zoophytes. Caen lxxxiv +560pp.
McFadden CS, van Ofwegen LP (2012) Stoloniferous octocorals (Anthozoa, Octocorallia) from South Africa, with descriptions of a new family of Alcyonacea, a new genus of Clavulariidae, and a new species of Cornularia (Cornulariidae). Invertebr Syst 26:331–356. https://doi.org/10.10071/is12035
Milne Edwards H, Haime J (1857) Histoire naturelle des coralliaires ou polypes proprement dits, Vol. I. Libraire Encyclopédique de Roret, Paris, pp. I-xxxiv + 1–326, 8 plates, numbered A1–6, B1–2
Pallas PS (1766) Elenchus zoophytorum systems generum adumbrations generaliores et specierum cognitarum succinactas descriptions cum selectis auctorum synonymis, Hagae Comitum, 451 pp
Sánchez JA, McFadden CS, France SC, Lasker HR (2003) Molecular phylogenetic analyses of shallow-water Caribbean octocorals. Mar Biol 142:975–987. https://doi.org/10.1007/s00227-003-1018-7
Smith W (Ed) 1813–1893 A dictionary of greek and roman biography and mythology. John Murray, London p 257–258
Vargas S, Guzman HM, Breedy O, Wörheide G (2014) Molecular phylogeny and DNA barcoding of tropical eastern Pacific shallow-water gorgonian octocorals. Mar Biol 161:1027–1038. https://doi.org/10.1007/s00227-014-2396-8
Verrill AE (1865) Synopsis of the polyps and corals of the North Pacific Exploring Expedition, under Commodore C. Ringgold and Captain John Rodgers, U.S.N., from 1853 to 1856. Collected by Dr. Wm. Stimpson, naturalist to the Expedition. With description of some additional species from the West coast of North America. Proc Essex Inst 4:181–196
Verrill AE (1868) Notes on Radiata in the Museum of Yale College. No. 6. Review of the corals and polyps of the West Coast of America. Trans Conn Acad (Second Edition) 1(2):377–422
Williams GC, Lindo KG (1997) A review of the octocorallian genus Leptogorgia (Anthozoa: Gorgoniidae) in the Indian Ocean and Subantarctic, with description of a new species and comparisons with related taxa. Proc Cal Acad Sci 49:499–521
Acknowledgements
Our gratitude to Bert Hoeksema and anonymous reviewers for their suggestions and comments that improved our publication. Our appreciation to Dennis Opresko for his review and remarks on morphological characters and Stephen Cairns for revising a previous version of the manuscript. We thank Alex Rodríguez, Luis Solís, and Andrea Moya (UCR) for their contribution in this study. We thank the Servicio Nacional de Áreas Protegidas de Costa Rica for allowing our research at the Área de Conservación Osa and Instituto Costarricense de Pesca y Acuicultura. This research was conducted under the permits ACOSA-INV-010-19-SINAC, ACOSA-PI-PC-026-19, and AJDIP/342-2017. We thank Ross Robertson, Smithsonian Tropical Research Institute, for collecting the samples from Panamá. Our appreciation to Pristine Seas, National Geographic Society, Washington DC, USA, for making this project possible, to the crew of the submersible DeepSee and M/V Argo (UnderSea Hunter Group), and to Osa Conservation, Golfito, Costa Rica.
Funding
The expedition was funded by Pristine Seas, National Geographic. Vicerrectoría de Investigación, Universidad de Costa Rica (projects B5738, B6773 and B5159), and Smithsonian Tropical Research Institute partially funded the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
Genetic sequence data generated in this study were deposited in GenBank with Accession Numbers MZ322102 and MZ320325. All other data analysed during this study are included in this published article and its supplementary information files. The specimens analysed in this study are deposited in public repositories.
Author contribution
OB conceived and designed the research, collected specimens, made morphological analyses, taxonomic interpretation, and wrote the article. HMG facilitated samples from Panamá and wrote the article. CMC made the molecular analyses and the interpretation and wrote the article. SV made the phylogenetic analyses and interpretation, analysed taxonomic data, and wrote the article. All authors have revised and agreed to the submitted version of the manuscript.
Additional information
Communicated by B.W. Hoeksema
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/9DF61046-7C58-4ECB-B746-D735ED39A301
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Breedy, O., Guzman, H.M., Murillo-Cruz, C. et al. Discovery of a new species of Leptogorgia Milne Edwards & Haime, 1857 (Anthozoa: Octocorallia: Gorgoniidae) from eastern tropical Pacific mesophotic reefs. Mar. Biodivers. 51, 95 (2021). https://doi.org/10.1007/s12526-021-01232-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01232-6