Abstract
Phylogeographic studies contribute to the description of geographic patterns and evolutionary histories of animal populations. Such studies also shed light on the taxonomic status of widely distributed species complexes. This study assessed the phylogeographic pattern, taxonomic status, and demographic history of two polychaete species, Perinereis anderssoni Kinberg, 1866 and Perinereis ponteni Kinberg, 1866 along the Brazilian coast. The molecular markers COI and 16S and external morphological features were used to characterize samples from 15 sites. Our analysis indicates that P. ponteni represents a single widely distributed taxon along the Brazilian coast, whereas P. anderssoni encompasses two allopatrically distributed cryptic species (North and South Clades). The North Clade is well structured and demographically stable, with numerous apparently ancient haplotypes. The South Clade does not display population structure. High gene flow probably occurs among sites and haplotype network suggests an expansion scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike terrestrial animals, marine organisms seldom use visual clues for partner choice during their reproductive processes (Klautau et al. 1999). As a result, morphological features often used for taxonomical classification do not suffice to distinguish cryptic marine invertebrates (Mayr and Ashlock 1991). Non-recognition of such species may have consequences, such as the underestimation of local diversity and the failure to identify species at risk of extinction (Galetti-Jr et al. 2008). Thus, a combination of morphology with other classification approaches should allow for a more accurate taxonomic assessment.
Marine invertebrates encompass a number of truly cosmopolitan species that inhabit a wide geographic range (Knowlton 2000). Dispersion potential varies depending on environmental drivers and species-specific characteristics (Knowlton 1993; Palumbi 1994; Russo et al. 1994). Among marine invertebrates, polychaetes are one of the groups with truly cosmopolitan species (Ahrens et al. 2013). However, the former cosmopolitan status of several widely distributed species of polychaetes has been challenged (Grassle and Grassle 1976; Barroso et al. 2010; Nygren et al. 2010; Silva et al. 2017). Many of them are currently considered as complexes of cryptic species in the grounds of low morphological disparity and high molecular divergence (a proxy for evolutionary time), according to a recently proposed conceptual framework (Struck et al. 2018). In such a context, phylogeographic studies provide the evidence for extant ties between geographic patterns and evolutionary histories. Moreover, such studies may also shed light on the true taxonomic status of entire species complexes.
Over the past two decades, a growing number of studies addressed the phylogeography of polychaetes, often indicating the occurrence of complexes of cryptic species (e.g., Schulze et al. 2000; Bleidorn et al. 2006; Barroso et al. 2010; Borda et al. 2013; Cossu et al. 2014; Einfeldt 2014; Park and Kim 2007; Park and Kim, 2017; Sampértegui et al. 2013; Seixas et al. 2017). Despite the wide geographical scale of many of the above species complexes, Nygren (2014) stated that cryptic polychaetes are not restricted to widely distributed nominal species but are also common even in sympatry.
The Nereididae Blainville, 1818 currently includes 45 genera and approximately 750 species (Blainville 1818; Rouse and Pleijel 2001; Santos et al. 2005; Bakken and Wilson 2005; Read and Glasby 2017). Nereidids are one of the most widely distributed groups among the polychaetes and thus may provide good candidates for phylogeographic surveys (Audzijonyte et al. 2008; Virgilio et al. 2009; Liu et al. 2012). The polyphyletic genus Perinereis Kinberg, 1865 encompasses more than 60 species (Bakken and Wilson 2005). Nine species are known for the Brazilian coast, two of them originally described from Rio de Janeiro (type-locality): P. ponteni Kinberg, 1866 and P. anderssoni Kinberg, 1866.
Specimens of P. anderssoni usually live in sand-muddy beaches, or associated with macroalgae in rocky shores. Previous studies have reported P. anderssoni along the Brazilian coast in the Atlantic Ocean (Santos and Steiner 2006; Amaral et al. 2014), as well as in the Pacific Ocean (Salazar-Vallejo and Londono-Mesa 2004). P. ponteni occurs along the Brazilian coast in sympatry with P. anderssoni but reaches as far north as the Mexican eastern coast (Santos and Steiner 2006). Some authors have considered P. ponteni and P. anderssoni a single species (Hartman 1948; Rioja 1960; Salazar-Vallejo and Jiménez-Cueto 1996; Espinosa et al. 2007). Others, however, have distinguished the two species on the basis of the number of proboscidial paragnaths in areas I and V, the shape of notopodial ligules, the position of dorsal cirri, and the color pattern of living animals (Lana 1984; Santos and Steiner 2006; De León-González and Goethel 2013; Coutinho et al. 2015). In addition, a cytogenetic analysis has revealed significant chromosomal differences between the two species and showed that chromosomal variation did not necessarily result in morphological differences in nereidids (Ipucha et al. 2007).
In such context, our study assesses the phylogeographic pattern, taxonomic status, and demographic history of P. anderssoni and P. ponteni populations along the Brazilian coast.
Material and methods
Sampling
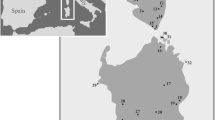
Samples were collected from 15 sites along the Brazilian coast, spanning more than 3000 km and 20° of latitude (Fig. 1). Site coordinates, number of specimens for each species, and the fixation method used for morphological and molecular assessments are provided in Fig. 1 and Table 1.
Specimens were collected in intertidal rocky shores during lower spring tides and sorted after removal of algal and Brachydontes sp. beds, where both P. anderssoni and P. ponteni are usually found. Specimens used for morphological analysis were fixed in formalin and those for molecular analyses were fixed and stored in ethanol.
Morphological analysis
All individuals collected for morphological analysis, 269 specimens of P. anderssoni and 236 of P. ponteni, were observed with the aid of compound and stereoscopic microscopes (Olympus CX31 and SZ51, respectively), coupled to a digital camera (Sony 13MP). Most specimens used for morphology were deposited in the Collection (UFFNER), at the Laboratory of Systematics and Ecology of Polychaetes (LASEPOL), Federal Fluminense University.
A previous morphometric analysis was performed by Coutinho et al. (2015) based on the same specimens. Nevertheless, we will provide additional data on the number of paragnaths, a character commonly used for the delimitation of species of nereidids.
DNA extraction and amplification
The posteriormost part of the body of each worm was used for DNA extraction using a commercial kit (Purege® Gentra Systems) or a hot lysis protocol developed for nematodes (Floyd et al. 2002). Vouchers of all specimens used for molecular analysis were deposited in the “Edmundo Ferraz Nonato” Polychaete Collection of the Rio de Janeiro Federal University (IBUFRJ).
Two mitochondrial fragments (COI and 16S ribosomal) were amplified through PCR reactions. The P. anderssoni COI amplifications were performed using the universal primers LCO1490 and HCO2198 (Folmer et al. 1994). PCR reactions took place in 1× Taq buffer, 2.0 mM of magnesium chloride, 0.2 mM of dNTPs, 0.6 mM of each primer, 1 unit of Taq DNA polymerase, and 1 μL of DNA in a final volume of 25 μL with the following thermal cycling conditions: 95 °C: 2′ + 35 cycles (94 °C: 40s, 51 °C: 60s, 72 °C: 60s) + 72 °C: 5′. The P. anderssoni 16S amplifications were performed using 16Sar-L and 16Sbr-H primers (Palumbi et al. 1991), or alternatively the reverse 16SAN-R (Zanol et al. 2010). PCR reactions took place in 1× Taq buffer, 2.0 mM of magnesium chloride, 0.2 mM of dNTPs, 0.6 mM of each primer, 0.2 mg/mL of BSA and 1 unit of Taq DNA polymerase, and 1 μL of DNA in a final volume of 25 μL with cycling conditions: 94 °C: 4′ + 35 cycles (94 °C: 50s, 51 °C: 50s, 72 °C: 50s) + 72 °C: 5′. The P. ponteni COI amplifications were performed using COI-EUR and ACOI-AF primers (Zanol et al. 2010). The PCR conditions were the same used for P. anderssoni COI except for cycling conditions: 95 °C: 2′ + 35 cycles (94 °C: 60s, 47 °C: 60s, 72 °C: 60s) + 72 °C: 7′. The P. ponteni 16S amplifications were performed using the primers 16Sar-L (Palumbi et al. 1991) and 16SAN-R (Zanol et al. 2010). The PCR conditions were the same used for P. anderssoni 16S except for cycling conditions: 94 °C: 3′ + 35 cycles (94 °C: 30s, 50 °C: 30s, 72 °C: 30s) + 72 °C: 7′.
Purification and sequencing were performed both at the Macrogen Inc. laboratory and at the Molecular Biodiversity Laboratory of the Federal University of Rio de Janeiro using an ABI 3500 (Applied Biosystems®).
Molecular divergence
Sequences were edited with the Mega 5.0 software (Tamura et al. 2011) and aligned using Clustal W (Thompson et al. 1994). Pairwise genetic distances among populations were calculated using Kimura’s two parameters (K2P) with Mega 5.0 (Tamura et al. 2011). Molecular diversity parameters were estimated through DNAsp 5.0 (Librado and Rozas 2009).
Phylogenetic analysis and haplotype network
Because some authors consider P. ponteni and P. anderssoni a single species, phylogenetic analysis was performed for both species together with their sequences deposited on GenBank (Accession numbers MH128330-MH128354 for 16S and MH143495-MH142526 for COI). As outgroups were used sequences from Neanthes acuminata (KJ538980.1 for 16S and KJ539102.1 for COI), Pseudonereis variegata (KC833489.1 for 16S and JX503029.1 for COI) and Perinereis nuntia (JX644015.1 for 16S and JX420257.1 for COI). Models of nucleotide substitution were performed using the ModelTest tool integrated to the Mega 5.0 software (Tamura et al. 2011). A maximum likelihood (ML) tree was built with PhyML 3.0 (Guindon et al. 2010), and branch support was estimated by 1000 replicate bootstraps. Bayesian inference (BI) trees were constructed using MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) with 1,000,000 generations of the Markov-Monte Carlo chains (MCMC) with two runs and four chains each, being sampled each 1000 generations with a burn-in of 25%. Both analyses were performed using GTR+G+I as the evolutionary model selected by ModeltTest.
A haplotype network was built through statistical parsimony (Templeton et al. 1987), with a confidence level of 95%, using the Pegas package (Paradis 2010) of the R environment (R Core Team 2012).
Demographic history and population structure
The demographic history and neutral evolution hypothesis were assessed through Tajima’s D neutrality (Tajima 1989) and Fu’s Fs (Fu 1997) tests in DNAsp 5.0 (Librado and Rozas 2009), with the species clustered within each collection site or within the entire sampling space. Mismatch distribution analysis was performed for those populations that deviated from the null hypothesis of neutral evolution. Parameter estimation was performed using Arlequin 3.5 (Excoffier and Lischer 2010) under scenarios of both population and spatial expansion plotted with DNAsp 5.0 (Librado and Rozas 2009) under a population expansion scenario. Time from the last population and/or spatial expansion were calculated through τ parameter (Rogers and Harpending 1992) using the equation t = τ/2u, where t is the time from the last expansion event and u is the product of mutation rate (μ) and number of base 8 pairs. A mutation rate (μ) of 3.5 × 10 was assumed for the COI fragment, as previously calculated for Eurythoe complanata (Pallas, 1766) polychaete populations separated by the Isthmus of Panama (Barroso et al. 2010). A μ of 1.6 × 10−8, previously defined for invertebrates, was assumed for the 16S fragment (Collado and Mendez 2012). The degree of structuration among populations was assessed through analysis of molecular variation (AMOVA) using Arlequin 3.5 (Excoffier and Lischer 2010) with 10,000 permutations. Groups used in AMOVA were defined a priori based on the results of the phylogenetic analysis.
Results
Morphological and molecular delimitation of Perinereis anderssoni and Perinereis ponteni
Morphological delimitation
Specimens of P. anderssoni have a fully pigmented dark prostomium. Differences between species on numbers of paragnaths in each area had already been presented by Coutinho et al. (2015), but a synthesis of the number per area is given in Table 2. Pharynx area I has five conical paragnaths, while area V has only three. Dorsal cirri are long and surpass the tip of notopodial ligules along the body. Median and posterior notopodial ligules are expanded and display no glandular structures (Fig. 2a–c). In contrast, specimens of P. ponteni have two oval non-pigmented areas in the prostomium and shorter dorsal cirri, which rarely surpasses the tip of the notopodial ligules. Proboscis area I of P. ponteni has 13 to18 conical paragnaths, while area V has only one (Table 2). Notopodial ligules of the median and posterior segments are expanded and display glandular agglomerates (Fig. 2d–f).
Perinereis anderssoni—a anterior end, dorsal view; b pharynx, frontal view; c view of the 45th parapodium (posterior region). Perinereis ponteni—d anterior end, dorsal view; e pharynx, lateral view; f view of the 45th parapodium (posterior region). av area V, aI area I, dc dorsal cirri, nol notopodial ligule. Scales: a 1.63 mm, b 4.02 mm, c 0.22 mm, d 2.00 mm, e 2.34 mm, f 0.19 mm
Within-species variations in the number of paragnaths for each pharynx area were significant different only for P. anderssoni populations (see also Coutinho et al. 2015). One northern population (Pacheco—CE) presented higher number of paragnaths in areas II, IV, and V than two southern (Martin de Sá—SP, and Itaipú—RJ) and two northern (Tambaba—PB, and Baía da Traição—PB) populations (Table 2).
Molecular delimitation
Phylogenetic analyses of molecular markers clearly distinguished P. anderssoni from P. ponteni, recovering two clades with high support for both methods of analysis (BI and ML) and both markers (COI and 16S, Figs. 3 and 4). Mean genetic distance (K2P) between clades was 24.2% for COI and 13.0% for 16S. Both BI and ML analyses indicated a complex relationship of P. anderssoni and P. ponteni with other species. While for 16S, P. anderssoni (represented by two sister clades) was more related to Perinereis cultrifera (Grube, 1840) and both are the sister group of P. ponteni, for COI, P. anderssoni was more related to Pseudonereis variegata (Grube, 1857) while P. ponteni was related to Perinereis nuntia (Lamarck, 1818).
Phylogenetic tree of Perinereis anderssoni and Perinereis ponteni. COI Bayesian inference tree clades, statistical support refers to posterior probability (PP) of Bayesian inference/bootstrap (BS), support lower than 50% not shown and represented by “..”. CE, PE, BA: Ceará, Pernambuco and Bahia, states in the northeastern region of Brazil; ES, RJ, SP: Espírito Santo, Rio de Janeiro and São Paulo, states in the southeastern region of Brazil; PR: Paraná, a state in southern Brazil
Phylogenetic tree of Perinereis anderssoni and Perinereis ponteni. 16S Bayesian inference tree clades, statistical support refers to posterior probability (PP) of Bayesian inference/bootstrap (BS), support lower than 50% not shown and represented by “..”. CE, PE, BA: Ceará, Pernambuco and Bahia, states in the northeastern region of Brazil; ES, RJ, SP: Espírito Santo, Rio de Janeiro and São Paulo, states in the southeastern region of Brazil; PR: Paraná, a state in southern Brazil
Perinereis anderssoni
Phylogenetic analysis and population structure
The phylogenetic analysis of COI fragments allowed for the discrimination of two clades, one encompassing the populations found in the northeastern states of Ceará and Pernambuco (North Clade) and one with all southern populations (South Clade) (Fig. 3). Bayesian inference analysis of the 16S fragment also supported both clades while maximum likelihood analysis recovered only the South Clade within a paraphyletic group of northern population specimens (see Fig. 4 for clade support, topology not shown).
The average distance between COI-defined clades was 8.7% (K2P), while the South Clade 16S fragment differed from northern samples by only 2.2% on average. A similar pattern emerges from the COI haplotype network (Fig. 5) and from AMOVA analysis (Table 2). The North and South Clades defined by the COI fragment were connected through their central haplotypes by 47 mutational steps. The South Clade displayed a star-like appearance with a central haplotype composed of 26 copies separated from surrounding haplotypes by only one or two mutational steps. Conversely, the North Clade network had a single haplotype in the center, separated from surrounding haplotypes by 1 to 11 mutational steps.
When considering the 16S fragment, northern and southern populations were separated by seven mutational steps between the central northern haplotype from PE and the RJ main haplotype (Fig. 6). Southern populations again displayed a star-like appearance with a central haplotype represented by 23 samples, and eight rare or unique haplotypes separated from the center by only one or two mutational steps (Fig. 6).
Two different scenarios were defined for the COI AMOVA. A first scenario separated the North and South Clades (PE+CE|ESP+RJ+SP+PR), and, in a second scenario, the North Clade was split into two groups (PE|CE|ESP+RJ+SP+PR) (Tables 3 and 4). A high variation was observed between the North and South Clades in the first scenario (94.1%) and among the three groups in the second scenario (94.8%), while variation among populations within a single group or clade was lower (< 2%) than within populations (> 4%). Only one scenario was defined for the AMOVA of 16S (PE|ES+RJ+SP+PR). The variation between the northern and southern groups was significant (86.7%, p < 0.05). Variation within populations was higher than observed with COI AMOVA (7.39%), possibly because of the separation of RJ samples from other populations within the southern group as presented in the haplotype network (Table 5).
Demographic history
Both neutrality tests Tajima’s D and Fu’s Fs were applied to the groups obtained as a result of the phylogenetic analysis. Considering each population separately, neutrality was rejected only for COI in three populations: PR (D = − 1.5999, p < 0.005), PE (Fs = − 5.3254, p < 0.005) and RJ (Fs = − 3.10496, p < 0.005) for only one test each. When all populations were gathered as a single clade, population changes were observed only for the South Clade with significant negative values (COI: D = − 1.93695 and Fs = − 9.13840, p < 0.05, 16S: D = − 1.551 and Fs = − 3.639, p < 0.05). Because evolution neutrality was rejected, this population was submitted to mismatch distribution. Regarding COI, it was not possible to reject either the hypothesis of both spatial expansion (SSD = 0.0147, p = 0.601, raggedness index (RI) = 0.1454, p = 0.643) or the hypothesis of demographic expansion (SSD = 0.0017, p = 0.704, and RI = 0.0379, p = 0.833). A similar pattern was obtained with 16S for both spatial (SSD = 0.0147, p = 0.601; RI = 0.1454, p = 0.643) and demographic expansion (SSD = 0.0309, p = 0.318; RI = 0.1454, p = 0.421).
The τ parameter was used for estimating the time since a last expansion event. According to the analysis, the North Clade, PE, defined with the COI fragment, underwent the last expansion 79,000 years ago. The South Clade underwent the same process between 34,000 (entire clade) and 26,000 years ago (PR and RJ).
Perinereis ponteni
Phylogenetic analysis and population structure
Analyses of both markers similarly indicated that all specimens belonged to the same clade (Figs. 3 and 4). Therefore, site-specific clades could not be identified. The average distance (K2P) among samples from different sites was 0.3% with a maximum of 1.4% for the COI and 1% for the 16S fragments, respectively. Because of the short genetic distance and the few specimens collected per site, the estimates of molecular diversity and demographic history were performed with all specimens, as if they belonged to one panmitic population.
The COI haplotype network was composed of two central haplotypes, separated by two mutational steps, which corresponded to the haplotypes shared by most individuals, and the unique descendant haplotype (Fig. 7). The 16S haplotype network was composed by a central haplotype that corresponded to the haplotype shared by most individuals and the remaining descendant haplotypes (Fig. 8). Population structure analysis could not be performed because of the small sample sizes.
Perinereis ponteni—16S haplotype network. Localities according to Fig. 7
Demographic history
Neutrality tests rejected the null hypothesis of neutral evolution only with regard to 16S, with both tests yielding significant negative values (D = − 2.10224, p < 0.05; Fs = − 6.80980, p < 0.005). Mismatch distribution analysis indicated the existence of population expansion, with non-significant SSD values and raggedness indexes (p > 0.05). Regarding last expansion estimates, results diverged. Demographic expansion was estimated to have occurred 128,000 years ago, while spatial expansion may have occurred as late as 12,000 years ago. Neutrality tests performed with the COI fragment were not significant (p > 0.05), with values of − 1.5538 and 2.217 for Tajima’s D and Fu’s Fs, respectively.
Discussion
Our results indicate that P. anderssoni and P. ponteni are distinct species, as previously suggested by morphological analyses (Lana 1984; Santos and Steiner 2006; De León-González and Goethel 2013; Coutinho et al. 2015), cytogenetics (Ipucha et al. 2007), and morphometry (Coutinho et al. 2015). The two have different evolutionary histories and incongruent phylogeographic patterns along the Brazilian coast.
Despite their common taxonomy history, P. anderssoni and P. ponteni do not even seem to be sister species, since both analyses indicated a closer relationship to other species (Perinereis nuntia, Perinereis cultrifera, and Pseudonereis variegata) depending on the marker. The grouping of P. anderssoni with a species from another genus, Pseudonereis variegata, observed on COI analysis is not unexpected since both genera, Perinereis and Pseudonereis, are closely related (Santos et al. 2005) and until now can not be regarded as monophyletic groups owing to the lack of phylogenetic support for these nominal genera (Bakken and Wilson 2005). Differences in the number of paragnaths, shape and size of dorsal and ventral cirri, posterior notopodial ligule length, number of chaetigers, and total body length were recorded by Coutinho et al. (2015), separating both species. The number of paragnaths, in all proboscidial areas, revealed significant interspecific differences, with, as expected, the number of paragnaths of area V, one of the criteria used in prior species classification, being the most significant to discriminate the species (Figure 4, p. 4). Additionally, a PERMANOVA analysis on paragnath numbers also indicated significant differences between the two species. Likewise, P. anderssoni and P. ponteni also differed in shape significantly according to Coutinho et al. (2015) (Fig. 2, p. 3). In general, out of all measured variables measured by the authors, notopodial cirri length from chaetigers 30 and the notopodial ligule length, in the 45th chaetiger, explained 45% of variation found between species.
The analyses also indicate that P. anderssoni consists of two probably cryptic species with non-overlapping distribution. In contrast, P. ponteni represents a widely distributed species with genetic flow along the Brazilian coast, a result corroborated by a morphometric analysis (Coutinho et al. 2015).
The genetic distance between North and South Clades of P. anderssoni (COI = K2P 8.7%/16S = K2P 2.2%) suggests that these populations evolved separately. Similar results were previously used to discriminate cryptic species within larger complexes, including the Eumida sanguinea (Örsted, 1843) complex (Phyllodocidae) (COI: 6.5 to 18.5%) (Nygren and Pleijel 2011); the Eurythoe complanata (Pallas, 1766) complex of the Amphinomidae family found on the Brazilian Atlantic coast (COI 10%) (Barroso et al. 2010); Diopatra Audouin and Milne Edwards, 1833 (Onuphidae) (16S: 1%) (Rodrigues et al. 2009); Archinome rosacea (Blake, 1985) (Amphinomidae) (Borda et al. 2013); and the deep sea species Ophryotrocha labronica Bacci and La Greca, 1961 (Dorvilleidae) (COI: 17.2%) (Cossu et al. 2014), among others.
Sampértegui et al. (2013) evaluated the real status of the Chilean Perinereis gualpensis Jeldes, 1963 and Perinereis vallata (Grube, 1857), previously included in the Perinereis nuntia species group, and considered them as two clearly defined species based on molecular and morphological data. Genetic distances were not provided, but differences in paragnath number were recorded: Perinereis gualpensis with 6–10 in area VI and P. vallata with 11–14. Another species group, Perinereis nuntia from Korea, was analyzed by Park and Kim (2007) based on molecular (COI) analysis and morphology. Divergence values found for Perinereis mictodonta (Marenzeller, 1879) and Perinereis wilsoni Glasby and Hsieh, 2006 varied from 18.88 to 19.39% being also associated with differences in paragnath numbers and length of dorsal cirri and notopodial dorsal ligule. More recently, Park and Kim (2017) surveyed the Korean species Perinereis cultrifera (Nereididae), a group with putative wide distribution. They described a new species with a genetic distance ranging from 24.7 to 25.6% when compared to P. cultrifera specimens from Europe. Similar divergences were found along the Brazilian coast among four species of the annelid genus Capitella Blainville, 1828 (Capitellidae) (COI: 17.5 to 26.5%, 16S: 24.4 to 46.4%), previously recorded as Capitella capitata (Fabricius, 1780), a well-known species complex. Seixas et al. (2017) investigated the distribution of Timarete punctata (Grube, 1858) (Cirratulidae), including a number of sites on the Brazilian coast, and found two cryptic species (COI: 21.0%, 16S: 14%). Both studies also described morphological differences among congeners.
Haplotype networks of the north and south groups of P. anderssoni identified here with both markers suggest the prevalence of different demographic histories. The northern lineage encompasses a greater number of haplotypes and appears to be older. On the other hand, the southern lineage displays a star-like appearance, where haplotypes that are more recent have separated from a central frequent haplotype by few mutational steps (Slatkin and Hudson 1991). This lineage likely emerged later in time and displays no indication of a recent population expansion event. The population expansions of both lineages occurred approximately 80,000 (north) and 30,000 years (south) ago. Thus, climate changes caused by Pleistocene glaciation and interglaciation events may have affected the phylogeographic distribution pattern of P. anderssoni. Two scenarios could potentially account for such pattern: (1) a northern species spread south after glaciation and gave rise to a new lineage and (2) a widely distributed species became separated by glaciation events, giving rise to two independent lineages. Favoring the first scenario, Pleistocene glaciation drastically limited species distribution to lower latitudes, with expansion taking place after this period. Glaciation more drastically affected species in temperate and/or estuarine regions than tropical and/or oceanic regions (Hewitt 2000). This phenomenon was well documented in polychaetes of the North Hemisphere (Jolly et al. 2006; Virgilio et al. 2009). The effects of glaciation have not been well established in the South Hemisphere and are still the subject of debate (Lessa et al. 2003). Sea level decline provoked by glaciation in tropical marine regions may have promoted the latitudinal migration of species. Such migration was reported in Australia and New Zealand for the sea star Coscinasterias muricata (Waters and Roy, 2003) and the gastropod Potamopyrgus antipodarum (Neiman and Lively, 2004), both of which display a North/South differentiation pattern. Other evidence lends support to the second scenario. Along the Brazilian coast, the sea level decline transformed part of the continental shelf into a terrestrial environment, possibly exposing the Vitoria-Trindade Chain (Ferrari and Riccomini 1999). The sea level dropped 100 to 150 m below current levels (Jouzel et al. 2007). The event probably caused changes in the currents and water masses of the South Atlantic West, restricting the gene flow between populations on the north and south of the Vitoria-Trindade Chain. The newly formed landmass could have separated a widely distributed population into two subpopulations. After a long period, such subpopulations would have diverged enough to be considered different species.Water masses and biogeographic divisions may also have restricted gene flow. The northeastern coastal waters are warmer and nutrient poor, while southern and southeastern coast waters are cold and nutrient-rich (Floeter et al. 2001). The two P. anderssoni lineages lie in distinct biogeographic provinces as determined by Palacio (1982) and Spalding et al. (2007) based on species, water temperature, and geological history.
A comparison of previous studies of P. anderssoni unveils morphological differences, which, in hindsight, could be related to the existence of two lineages (Lana 1984; Santos and Steiner 2006; De León-González and Solís-Weiss 1998; Coutinho et al. 2015). In the study area, intraspecific differences were observed for notopodial cirrus length and numbers of paragnaths, the latter being statistically different among populations for some of the pharyngeal areas. Nevertheless, the population pattern of differentiation was not the same northern/southern geographic pattern observed in the molecular analysis.
Nygren (2014) argued that morphological assessments alone result in the underestimation of polychaete species number. According to him, unless cryptic species are taken into account with the use of combined approaches, our understanding of biogeographical patterns will be severely limited. As stated above, topologies from phylogenetic analysis and the level of molecular divergence observed between clades of P. anderssoni are high enough to consider them as different evolutionary entities. The lack of any noticeable morphological disparity, mainly when compared to other non-cryptic species with similar levels of molecular divergence, allows us to consider both P. anderssoni clades as cryptic species conforming to Struck et al. (2018) conceptual framework.
In contrast, P. ponteni displays low population structuration with a high degree of genetic homogeneity across the geographic range evaluated. Phylogenetic analysis, for both markers, indicated the existence of a single clade with short inner genetic distances. Low population structuration may result from high gene flow among the sites. Genetic homogeneity also became apparent in the haplotype network structure. The pattern composed of few central haplotypes and many descendant haplotypes with few mutational steps suggests expansion began from a small gene pool. Altogether, the molecular diversity values, neutrality test results, and the haplotypic diversity of the southern population of P. ponteni suggest the occurrence of a bottleneck or founder effect for this group (Grant and Bowen, 1998).
Pleistocene glaciation and interglaciation do not seem to have affected the evolutionary history of populations of P. ponteni, indicating that colonization occurred after this period. The estimation of time since the last expansion was not conclusive, because demographic expansion and spatial expansion analyses yielded τ values of 12,775 and 128,826 years, respectively.
Our study indicates that P. ponteni is a single, widely distributed taxon along the Brazilian coast, whereas P. anderssoni encompasses two allopatrically distributed cryptic species (North and South Clades). The northern populations are well structured and demographically stable. On the other hand, the South Clade does not display population structuration and high gene flow probably occurs among sites. Haplotypes suggest an expansion scenario.
References
Ahrens JB, Borda E, Barroso R, Paiva PC, Campbell AM, Wolf A, Nugues MM, Rouse GW, Schulze A (2013) The curious case of Hermodice caranculata (Annelida: Amphinomidae): evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basins. Mol Ecol 22:2280–2291
Amaral ACZ, Nallin SAH, Steiner TM, Forroni TO, Gomes-Filho D (2014) Catálogo das espécies deAnnelida Polychaeta do Brasil. http://wwwibunicampbr/museu_zoologia/files/lab_museu_zoologia/Catalogo_Polychaeta_Am aral_et_al_2012_0pdf Acessed 27 December 2017
Audouin JV, Milne Edwards H (1833) Classification des Annélides et description de celles qui habitent les côtes de la France. Ann Sciences Nat 29:195–269
Audzijonyte A, Ovcarenko I, Bastrop R, Väinölä R (2008) Two cryptic species of the Hediste diversicolor group (Polychaeta, Nereididae) in the Baltic Sea, with mitochondrial signatures of different population histories. Mar Biol 155:599–612
Bacci G, La Greca M (1961) Una nuova specie di Ophryotrocha delle costa tirreniche. Boll Zool 16:413–416
Bakken T, Wilson RS (2005) Phylogeny of nereidids (Polychaeta, Nereididae) with paragnaths. Zool Scr 34:507–547
Barroso R, Klautau M, Solé-Cava AM, Paiva PC (2010) Eurythoe complanata (Polychaeta: Amphinomidae), the ‘cosmopolitan’ fireworm, consists of at least three cryptic species. Mar Biol 157:69–80
Blainville HD (1818) Memoire sur la classe des Sétipodes partie des Vers à sang rouge de M. Cuvier, et des Annélides de M. de Lamarck. Bull Soc Philom 1818:78–85
Blainville H (1828) Mollusques, Vers et Zoophytes. In: Levrault FG (ed) Dictionnaire des Sciences Naturelles, Strasbourg & Paris. 57:1–443
Blake JA (1985) Polychaeta from the vicinity of deep-sea geothermal vents in the eastern Pacific. I: Euphrosinidae, Phyllodocidae, Hesionidae, Nereididae, Glyceridae, Dorvilleidae, Orbiniidae and Maldanidae. Bull Biol Soc Wash 6:67–101
Borda E, Kudenov JD, Chevaldonné P, Blake JA, Desbruyères D, Fabri M-C, Hourdez S, Pleijel F, Shank TM, Wilson NG, Schulze A, Rouse GW (2013) Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps. Proc R Soc B 280:20131876. https://doi.org/10.1098/rspb.2013.1876
Bleidorn C, Kruse I, Albrecht S, Bartolomaeus T (2006) Mitochondrial sequence data expose the putative cosmopolitan polychaete Scoloplos armiger (Annelida, Orbiniidae) as a species complex. BMC Evol Biol 6:47–59
Collado GA, Mendez MA (2012) Phylogenetic relationships and taxonomy of Altiplano populations of Biomphalaria (Gastropoda: Planorbidae): inference from a multilocus approach. Zool J Linn Soc-Lond 165:795–808
Cossu P, Maltagliati F, Pannacciull FG, Simonini R, Massamba-N'Siala G, Casu M, Lardicci C, Prevedelli D, Castelli A (2014) Phylogeography of Ophryotrocha labronica (Polychaeta, Dorvilleidae) along the Italian coasts. Mar Ecol 35:1088–1097
Coutinho MCL, Paiva PC, Santos CSG (2015) Morphometric analysis of two sympatric species of Perinereis (Annelida: Nereididae) from the Brazilian coast. J Mar Biol Assoc UK 95:953–959
De León-González JA, Solis-Weiss V (1998) The genus Perinereis (Polychaeta:Nereididae) from Mexican littoral waters, including the description of three new species and the redescriptions of P. anderssoni and P. elenacasoae. P Biol Soc Wash 111:674–693
De León-González JA, Goethel C (2013) A new species of Perinereis (Polychaeta, Nereididae) from Florida, USA, with a key to all Perinereis from the American continent. ZooKeys 312:1–11
Einfeldt AL, Doucet JR, Addisona JA (2014) Phylogeography and cryptic introduction of the ragworm Hediste diversicolor (Annelida, Nereididae) in the Northwest Atlantic. Invert Biol 133:232–241
Espinosa VV, Dıáz OD, Liñero-Arana I (2007) Nereididae Lamarck, 1818 (Annelida: Polychaeta) de la costa occidental de Venezuela. Bol Inst OceanogrVenezuela 46:119–127
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Fabricius O, Berry SS, Roper CFE (1780) Fauna Groenlandica: systematice sistens animalia Groenlandiae occidentalis hactenus indagata, quoad nomen specificum, triviale, vernaculumque: synonyma auctorum plurium, descriptionem, locum, victum, generationem, mores Othonis Fabricii, pp 1:450
Ferrari AL, Riccomini C (1999) Campo de esforços Plio-Pleistocênico na Ilha de Trindade (Oceano Atlântico Sul, Brasil) e sua relação com a tectônica regional. Rev Bras Geoc 29:195–202
Floeter SR, Guimarães RZP, Rocha LA, Ferreira CEL, Rangel CA, Gasparini JL (2001) Geographic variation in reef-fish assemblages along the Brazilian coast. Glob Ecol Biogeogr 10:423–431
Floyd R, Abebe E, Papert A, Blaxter M (2002) Molecular barcodes for soil nematode identification. Mol Ecol 11:839–850
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fu YX (1997) Statistical tests of neutrality of mutation against population growth, hitchhiking and background selection. Genetics 147:915–925
Galetti PM Jr, Rodrigues FP, Solé-Cava AM, Miyaki CY, Carvalho D, Eizirik E, Veasey EA, Santos FR, Farias IP, Vianna JA, Oliveira LR, Weber LI, Almeida-Toledo LF, Francisco MR, Redondo RAF, Siciliano S, Del Lama SN, Freitas TRO, Hrbek T, Molina WF (2008) Genética da conservação brasileira. In: Frankham R, Ballou JD, Briscoe DA (eds) Fundamentos de Genética da Conservação, 1st edn. Editora SBG, Ribeirão Preto, pp 244–274
Glasby CJ, Hsieh H-L (2006) New species and new records of the Perinereis nuntia species group (Nereididae: Polychaeta) from Taiwan and other Indo-West Pacific shores. Zool Studies 45:553–577
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 30. Syst Biol 59:307–321
Grant WS, Bowen BW (1998) Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J Hered 89:415–426
Grassle JP, Grassle JF (1976) Sibling species in the marine pollution indicator Capitella (Polychaeta). Science 192:567–569
Grube E (1840) Actinien, Echinodermen und Würmer des Adriatischen- und Mittelmeers, nach eigenen Sammlungen beschrieben von Adolph Eduard Grube. Koenigsberg pp 92
Grube AE (1857) Annulata Örstediana. Enumeratio Annulatorum, quae in itinere per Indiam occidentalem et Americam centralem annis 1845-1848 suscepto legit cl. A. S. Örsted, adjectis speciebus nonnullis a cl. H. Kröyero in itinere ad Americam meridionalem collectis. (Fortsaettelse). [Part 2]. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Köbenhavn 1857:158–186
Grube AE (1858) Annulata Örstediana. Enumeratio Annulatorum, quae in itinere Indiam Occidentalem et Americam Centralem annis 1845-1848 suscepto legit cl. A.S. Oersted, adjectis speciebus nonnullis a cl. H. Kroyero in itinere ad Americam meridionalem collectis, [part 3], 105–120. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Köbenhavn 3:105–120
Hartman O (1948) The marine annelids erected by Kinberg. With some notes on some other types in the Swedish State Museum. Arkiv för Zoologi 42A:1–137
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Ipucha MC, Santos CSG, Lana PC, Sbalqueiro IJ (2007) Cytogenetic characterization of seven South American species of Nereididae (Annelida: Polychaeta): implications for the karyotypic evolution. J Basic Appl Genet 18:27–38
Jeldes F (1963) Un nuevo nereido de agua dulce para Chile. Gayana 9:4–10
Jolly MT, Viard F, Gentil F, Thiébaut E, Jollivet D (2006) Comparative phylogeography of two coastal polychaete tubeworms in the Northeast Atlantic supports shared history and vicariant events. Mol Ecol 15:1841–1855
Jouzel J, Masson-Delmotte V, Cattani O et al (2007) Orbital and millennial Antarctic climate variability over the past 800. 000 years Science 317:739–796
Kinberg JGH (1866) Annulata nova. Öfversigt af Königlich VetenskapsAkademiens Förhandlingar 22:167–179
Klautau M, Russo CAM, Lazoski C, Boury-Esnault N, Thorpe JP, Solé-Cava AM (1999) Does cosmopolitanism result from over conservative systematics? A case study using the marine sponge Chondrilla nucula. Evolution 53:1414–1422
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24:189–216
Knowlton N (2000) Molecular genetic analyses of species boundaries in the sea. Hydrobiologia 420:73–90
Lamarck JB (1818) Histoire naturelle des Animaux sans Vertèbres, préséntant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s'y rapportent; precedes d'une Introduction offrant la determination des caracteres essentiels de l`Animal, sa distinction du vegetal et desautres corps naturels, enfin, l'Exposition des Principes fondamentaux de la Zoologie. Paris, Deterville, pp 612
Lana PC (1984) Anelídeos Poliquetas Errantes do Litoral do Estado do Paraná. PhD thesis, Universidade de São Paulo
Lessa EP, Cook JA, Patton JL (2003) Genetic footprints of demographic expansion in North America, but not Amazonia during the Late Quaternary. Proc Nat Acad Sci 100:10331–10334
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Liu H, Liu M, Ge S, Wang Q, Yu D, Guan S (2012) Population structuring and historical demography of a common clam worm Perinereis aibuhitensis near the coasts of Shandong Peninsula. Biochem Syst Ecol 44:70–78
Mayr E, Ashlock PD (1991) Principles of systematic zoology, 2nd edn. McGraw-Hill, New York
Neiman M, Lively CM (2004) Pleistocene glaciation is implicated in the phylogeographical structure of Potamopyrgus antipodarum, a New Zealand snail. Mol Ecol 13:3085–3089
Nygren A, Eklöf J, Pleijel F (2010) Cryptic species of Notophyllum (Polychaeta: Phyllodocidae) in Scandinavian waters. Org Divers Evol 10:193–204
Nygren A, Pleijel F (2011) From one to ten in a single stroke—resolving the European Eumida sanguinea (Phyllodocidae, Annelida) species complex. Mol Phylogenet Evol 58:132–141
Nygren A (2014) Cryptic polychaete diversity: a review. Zool Scr 42:172–183
Örsted AS (1843) Annulatorum danicorum conspectus. Auctore A.S. Örsted. Fasc. I. Maricolæ. (Quæstio ab universitate Hafniensi ad solvendum proposita et proemio ornata)
Palacio FJ (1982) Revisíon Zoogeográfica marina del sur del Brasil. Bolm Inst Oceanogr 31:69–92
Pallas PS (1766) Miscellanea zoologica. Quibus novae imprimis atque obscurae animalium species describuntur et observationibus iconibusque illustrantur. Petrum van Cleef. Hagí Comitum., xii + 224 pp.;14 pls
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Evol Syst 25:547–572
Palumbi SR, Martin AP, Romano S, Mcmillan WO, Stice L, Grabowski G (1991) The simple fool’s guide to PCR. Department of Zoology Special Publication, University of Hawaii, Honolulu
Paradis E (2010) Pegas: a R package for populations genetics with an integrated modular approach. Bioinformatics 26:419–420
Park TS, Kim W (2007) A taxonomic study on Perinereis nuntia species group (Polychaeta: Nereididae) of Korea. Korean Journal of Systematic Zoology 23:75–85
Park T, Kim W (2017) Description of a new species for Asian populations of the “cosmopolitan” Perinereis cultrifera (Annelida: Nereididae). Zool Sci 34(3):252–260
R Development Core Team (2012) R: a language and environment for statistical computing [computer program]. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org
Read G, Glasby C (2017) World Polychaeta Database—Nereididae Blainville, 1818. Available from: http://www.marinespecies.org/polychaeta/aphia.php?p=taxdetails&id=22496 Accessed 20 April 2017
Rioja E (1960) Estudios anelidológicos. 24. Adiciones a la fauna de anélidos poliquetos de las costas orientales de México. A Inst Biol Univ Nac Aut Mex 31:289–316
Rodrigues AM, Pires A, Mendo S, Quintino V (2009) Diopatra neapolitana and Diopatra marocensis from the Portuguese coast: morphological and genetic comparison. Estuar Coast Shelf Sci 85:609–617
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rouse GW, Pleijel F (2001) Polychaetes. Oxford University Press, London
Russo CAM, Solé-Cava AM, Thorpe JP (1994) Population structure and genetic variation in two tropical sea anemones (Cnidaria, Actinidae) with different reproductive strategies. Mar Biol 119:267–276
Salazar-Vallejo SI, Jiménez-Cueto MS (1996) Neréididos (Polychaeta) del Caribe Mexicano con una clave para las especies del Gran Caribe. Rev Biol Trop 44:361–377
Salazar-Vallejo SI, Londoño-Mesa MH (2004) Lista de especies y bibliografía de poliquetos (Polychaeta) del Pacífico Oriental Tropical. Ann Inst Biol 75:9–97
Sampértegui S, Rozbaczylo N, Canales-Aguirre CB, Carrasco F, Hernández CE, Rodríguez-Serrano E (2013) Morphological and molecular characterization of Perinereis gualpensis (Polychaeta: Nereididae) and its phylogenetic relationships with other species of the genus off the Chilean coast, Southeast Pacific. Cah Biol Mar 54:27–40
Santos CSG, Pleijel F, Lana P, Rouse GW (2005) Phylogenetic relationships within Nereididae (Annelida: Phyllodocida). Invertebr Syst 19:557–576
Santos CSG, Steiner TM (2006) Família Nereididae. In: Amaral ACZ, Rizzo AE, Arruda EP (eds) Manual de Identificação dos Invertebrados Marinhos da Região Sudeste-Sul do Brasil, vol 1, 1st edn. Edusp, São Paulo, pp 121–133
Schulze SR, Rice SA, Simon JL, Karl SA (2000) Evolution of poecilogony and the biogeography of North American populations of the polychaete Streblospio. Evolution 54:1247–1259
Seixas VC, Zanol J, Magalhães WF, Paiva PC (2017) Genetic diversity of Timarete punctata (Annelida: Cirratulidae): detection of pseudo-cryptic species and a potential biological invader. Estuar Coastal Shelf Sci 197:214–220
Silva CF, Seixas, VC, Barroso R, DiDomenico M, Amaral ACZ, Paiva PC (2017) Demystifying the Capitella capitata complex (Annelida, Capitellidae) diversity by morphological and molecular data along the Brazilian coast. PLoS One https://doi.org/10.1371/journal.pone.0177760
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57:573–583
Struck TH, Feder JL, Bendiksby M, Birkeland S, Cerca J, Gusarov VI, Kistenich S, Larsson K-H, Liow LH, Michael D, Nowak MD, Stedje B, Bachmann L, Dimitrov D (2018) Finding evolutionary processes hidden in cryptic species. Trends Ecol Evol 33:153–163
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and Maximum Parsimony methods. Mol Biol Evol 28:2731–2739
Templeton AR, Boerwinkle E, Sing CF (1987) A cladistic analysis of phenotypic associations with haplotypes inferred form restriction endonuclease mapping. I. Basic theory and an analysis of alcohol dehydrogenase activity in Drosophila. Genetics 117:343–351
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Waters JM, Roy MS (2003) Marine biogeography of southern Australia: phylogeographical structure in a temperate sea-star. J Biogeogr 30:1787–1296
Virgilio M, Fauvelot C, Costantini F, Abbiati M, Backeljau T (2009) Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distributions. Mol Ecol 18:1980–1994
von Marenzeller E (1879) Südjapanische Anneliden. I. (Amphinomea, Aphroditea, Lycoridea, Phyllodocea, Hesionea, Syllidea, Eunicea, Glycerea, Sternaspidea, Chaetopterea, Cirratulea, Amphictenea.). Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Classe, Wien. 41:109–154 plates I–VI
Zanol J, Halanych KM, Struck TH, Fauchald K (2010) Phylogeny of the bristle worm family Eunicidae (Eunicida: Annelida) and the phylogenetic utility of noncongruent 16S, COI and 18S in combined analyses. Mol Phylogenet Evol 55:660–676
Acknowledgments
We thank André Santos (in memoriam), Ricardo Krul, Wilson Franklin, and José Roberto Botelho de Souza for the assistance with sample collection, and Cristiana Sette and João Miguel de Matos Nogueira for providing the specimens from Bahia and Paraíba, respectively. We sincerely thank two anonymous reviewers for their valuable and constructive comments to earlier versions of the manuscript. Permissions for sampling were provided by SISBIO—Brazilian Ministry of Environment (MMA), under the number 10238-1 to PCP.
Funding
A fellowship was provided by Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to BFM and a fellowship and grant were provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) to PCP (grants numbers: 443900/2014-0 and 303939/2014-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the corresponding author through SISBIO—Brazilian Ministry of Environment (MMA) under the number: 10238-1.
Additional information
Communicated by P. Lana
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the corresponding author through SISBIO—Brazilian Ministry of Environment (MMA) under the number: 10238-1.
Rights and permissions
About this article
Cite this article
Paiva, P.C., Mutaquilha, B.F., Coutinho, M.C.L. et al. Comparative phylogeography of two coastal species of Perinereis Kinberg, 1865 (Annelida, Polychaeta) in the South Atlantic. Mar Biodiv 49, 1537–1551 (2019). https://doi.org/10.1007/s12526-018-0927-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-018-0927-0