Abstract
Two new species of Otoplana (Proseriata: Otoplanidae) from the Canary Islands are here described: Otoplana norenburgi sp. nov. and Otoplana didomenicoi sp. nov. These new species are distinguished from their congeners by unique features of the sclerotized structures of the copulatory organ, in particular of the aculeus, swollen proximally in O. norenburgi sp. nov. and lanceolate in shape in O. didomenicoi sp. nov. Specimens of the latter species were also found in South Portugal. Canarian and Portuguese specimens show poor genetic distinction based on the markers used, hinting to a remarkable dispersal power for a mesopsammic organism. New information is given on the “accessory male canal”, a putative autapomorphy for the genus Otoplana. Its functions and connections with genital systems are discussed, in the light of new data on the molecular phylogeny of the family Otoplanidae presented. The Mediterranean specimens of Otoplana sequenced, morphologically attributed to O. truncaspina, O. bosporana, O. falcataspina, and O. labronica, did not show genetic distinction, urging for a reconsideration of the status of the Mediterranean taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Otoplana Du Plessis, 1889 has a particular relevance for the taxonomic history of Platyhelminthes Proseriata. It is, in fact, one the first genera described in the whole order Proseriata, and the eponym of the family to which it belongs, Otoplanidae Hallez, 1892. The genus is often taken as representative of the Proseriata in textbooks (i.e., Ruppert et al. 2004) and is well known to marine meiobenthologists as, once again, eponymous of the interstitial community occurring in the mid to lower reach of the swash zone (the Otoplanen-zone of Remane 1933), presently widely known as Otoplana-zone (Brown and McLachlan 2010).
It may, thus, come as a surprise that, contrarily to many genera of Proseriata, which have wide if not cosmopolitan distributions, the genus Otoplana, in the strict sense (see discussion below), is limited to the Mediterranean. Furthermore, its peculiar autapomorphy, i.e., the presence of an accessory male pore, makes its morphology quite aberrant for the family (see Miller and Faubel 2003). In addition, its present taxonomy is particularly problematic, as the seven species unquestionably ascribed to it (Lanfranchi and Melai 2007, 2010; Meini 2013) appear very similar, and their validity should be more thoroughly assessed.
Recent research in the Canary Islands, during a workshop on the meiofauna of Lanzarote (held in October 2011), and in southern Portugal revealed individuals clearly referable to the genus Otoplana on morphological grounds, and which are the first findings of the genus outside the Mediterranean. In this contribution, we describe two new species, following an integrative taxonomic approach (Padial et al. 2010). Furthermore, we reconstruct the phylogenetic relationships within the family Otoplanidae and the genus Otoplana, and attempt a critical evaluation of the crucial morphological features of the genus.
Molecular analyses have been performed by means of the most trusted markers on the taxon Proseriata, the rDNA 18S and 28S genes, which have been extensively used to both reconstruct their phylogeny and detect species boundaries (Litvaitis et al. 1996; Littlewood et al. 2000; Curini-Galletti et al. 2010; Casu et al. 2011, 2014; Girstmair et al. 2014; Scarpa et al. 2016a, 2017a, b).
Materials and methods
Sampling
Samples were collected in the Canary Islands (Lanzarote, Gran Canaria, La Palma) in October 2011 and near Faro (Portugal) in May 2013, scooping up the superficial layer of sediment in the swash zone. Neither specific permits for collecting small amounts of marine sediments were required nor were the animals being the object of this study protected or endangered.
Specimens from La Maddalena Archipelago (Italy) were collected during a faunal survey carried out in September 2010, and permits were issued by Mauro Gargiulo, Director of the National Park “Arcipelago di La Maddalena”.
Extraction of the animals from the sediment was accomplished using MgCl2 decantation (Schockaert 1996). Once isolated, individuals were first studied alive by slight squeezing under the coverslip. Specimens were then retrieved and processed. For morphological analysis, after relaxation in an isotonic MgCl2 solution, specimens were fixed in cold Bouin’s fluid and embedded in Paraplast at 56 °C. Serial sections were made 3 to 4 μm thick, stained in Mayer’s hematoxylin and eosin, and mounted in Depex.
Whenever possible, the rear part of the organism, where most of the critical features allowing identification are located, was cut and mounted in lactophenol or treated with a 5% acetic acid solution, in order to dissolve tissues and make observations of details of the sclerotized structure possible, and preserved as part of the type series or as voucher. The front part was stored in ethanol 95% for molecular studies.
The nomenclatural acts have been registered in ZooBank, the online registration system for the International Commission on Zoological Nomenclature (ICZN). The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is urn:lsid:zoobank.org:pub:1B6FF6EB-22FE-4844-9C61-3F55AA879FFE.
The authorship of the new taxa reflects the actual involvement of the participants of this research in the taxonomic description. Types have been deposited at the Swedish Museum of Natural History (SMNH) (Stockholm, Sweden) and in the collections of the Zoological Museum (CZM), University of Sassari (Italy).
Abbreviations used in figures
a: common genital atrium; ac: aculeus; ag: adhesive glands; amc: “accessory male canal”; ap: “accessory male canal” pore; aso: anterior sense organ; br: brain; cf.: ciliated furrow; cgp: common genital pore; co: copulatory organ; ed: ejaculatory duct; fd: female duct; fg: female glands; fp: female pore; fsv: false seminal vesicle; g: gut; ov: ovary; pg: precerebral gut; ph: pharynx; pso: posterior sense organ; s: statocyst; sd: spermiduct; sg: “shell glands”; sv: seminal vesicle; t: testis; vi: vitellaria.
DNA extraction, amplification, and sequencing
Genomic DNA was extracted using the Macherey Nagel NucleoSpin Tissue (MACHEREY-NAGEL GmbH & Co. KG), according to the supplier’s instructions. After extraction, DNA was stored as a solution at 4 °C. Complete 18S and partial 28S (spanning variable domains D1–D6) were analyzed for a total of 128 individuals, 28 of which were newly sequenced for this work and 100 were taken from GenBank, 95 of which have already been sequenced by us for previous papers (for details about specimens and sampling localities, see Supplementary Material S1). Overall, the molecular dataset includes two specimens belonging to the suborder Unguiphora and 126 to the Lithophora. Among these, several families were represented: Calviriidae (2), Monocelididae (8), Archimonocelididae (20), Yorkniidae (21), and Otoplanidae (75), the latter including 28 specimens belonging to the genus Otoplana. Polymerase chain reaction (PCR) assays for the 18S and 28S regions were carried out using the following primers: 18S: A (forward) GCG AAT GGC TCA TTA AAT CAG and B (reverse) CTT GTT ACG ACT TTT ACT TCC (Littlewood and Olson 2001); 28S: for (forward) GCG GAG GAA ARG AAA CTA ACA AGG A and rev (reverse) AAC TCT TCC GGG AAC CAT CGC CGA C (Scarpa et al. 2016b); 28S D1–D6: LSU5 (forward) TAG GTC GAC CCG CTG AAY TTA AGC A and LSUD6-3 (reverse) GGA ACC CTT CTC CAC TTC AGT C (Littlewood et al. 2000). PCRs were carried out in a total volume of 25 μL containing 1 ng/μL (quantified using NanoDrop™ Lite by Thermo Scientific) of total genomic DNA, on average, 1.0 U of Taq DNA Polymerase (EuroClone), 1× reaction buffer, 3.5 mM of MgCl2, 0.32 μM of each primer, and 200 μM of each dNTP. PCR amplifications were performed in an MJ PTC-200 Thermal Cycler (Bio-Rad), programmed as follows: 1 cycle of 2 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 54 °C (18S/28S D1–D6 primers’ annealing temperature), and 90 s at 72 °C. At the end, a post-treatment for 5 min at 72 °C and a final cooling at 4 °C were carried out. Both positive and negative controls were used to test the effectiveness of the PCR reagents and the absence of possible contaminations. Electrophoresis was carried out on 2% agarose gels, prepared using 1× SBA buffer (sodium boric acid, pH 8.2) and stained with a 1 μL/20 mL ethidium bromide solution. PCR products were purified by ExoSAP-IT (USB Corporation) and sequenced for both forward and reverse 18S and 28S D1–D6 strands (by means of the same primers used for PCR), using an external sequencing core service (Macrogen Europe).
Phylogeny and species delimitation
The 18S and 28S D1–D6 sequences were aligned separately using the algorithm Q-INS-I implemented in Mafft 7.187 (Katoh and Standley 2013). In order to test the phylogenetic signal, the likelihood-mapping analysis of 10,000 random quartets was performed for both genes using TREE-PUZZLE (Schmidt et al. 2002). The best probabilistic model of sequence evolution was determined independently for each gene using jModeltest 2.1.3 (Daribba et al. 2012), with a maximum likelihood optimized search, and both the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Both criterions selected the GTR + I + G (Tavaré 1986) as the best fitting model for both 18S and 28S D1–D6 datasets.
Phylogenetic relationships among taxa were investigated using both maximum likelihood (ML) and Bayesian inference (BI) on the concatenated 18S and 28S D1–D6 sequences. ML analysis was carried out using the software raxmlGUI version 1.3 (Silvestro and Michalak 2012), setting the analysis option to “ML+ thorough bootstrap”. Analysis was carried out with 10 runs and 1000 bootstrapping replicates using the GTRGAMMAI model. The consensus tree and the bootstrap support values were visualized by means of the software FigTree 1.4.0 (available at http://tree.bio.ed.ac.uk/software/figtree/). BI was carried out using the software MrBayes 3.2.6 (Ronquist et al. 2012), specifying a partitioned model in which 18S and 28S genes were deemed as distinct partitions. Setting as model parameters NST = 6, rates = invgamma, ngammacat = 4, we allowed each partition to have its own set of parameters and a potentially different overall evolutionary rate. Two independent runs, each consisting of four Metropolis-coupled Markov chain Monte Carlo (MCMCMC) chains (one cold and three heated chains), were run simultaneously for 5,000,000 generations, sampling trees every 1000 generations. The first 25% of the 10,000 sampled trees was discarded as burn-in. Runs have been carried out at the CIPRES Phylogenetic Portal (Miller et al. 2010). Convergence of chains has been tested following Ronquist et al. (2012) and Gelman and Rubin (1992). Nodes with a percentage of posterior probability lower than 95% were considered as not highly supported. The phylogenetic tree was visualized using FigTree 1.4.0 (available at http://tree.bio.ed.ac.uk/software/figtree/).
In order to verify the taxonomic assessment of the specimens belonging to the genus Otoplana, several methods of species delimitation were used. We applied the ST-GMYC (Single Threshold-Generalized Mixed Yule Coalescent) method by Pons et al. (2006), the PTP (Poisson Tree Processes) model and its Bayesian implementation, the bPTP (Zhang et al. 2013), and the ABGD (Automatic Barcode Gap Discovery) (Puillandre et al. 2012) method on the combined dataset (18S + 28S D1–D6) using the settings described by Scarpa et al. (2016a, 2017a, b).
In addition, here, we also used the NDT (Nucleotide Divergence Threshold) on each gene separately, by means of a customized script (see Supplementary Material S2) written in the R statistical environment (available at https://cran.r-project.org/). The script ranks taxa into entities applying the fixed threshold (0.16% and 0.52% for the 18S and 28S genes, respectively) obtained by Scarpa et al. (2015) using a pairwise Kimura two-parameter model (K2P) (Kimura 1980) genetic distances matrix.
Finally, for the new species Otoplana didomenicoi sp. nov. and Otoplana norenburgi sp. nov., molecular pure diagnostic characters (see Jörger and Schrödl 2013), based on the 18S and 28S genes, were detected within the family Otoplanidae by means of the SPIDER package (SPecies IDentity and Evolution in R) (Brown et al. 2012) implemented in the R statistical environment (available at https://r-forge.r-project.org/projects/spider/). This analysis allowed us to obtain for each tested taxon a list of the diagnostic nucleotides in each marker (i.e., those nucleotides that are fixed within species and different from all other species within their family) (Brown et al. 2012).
Alignments and phylogenetic tree files were deposited and made available in TreeBase with the accession number TB2:S21114.
Results
Molecular analyses
After the alignment, 1595- and 1594-bp-long sequences were obtained for the 18S and 28S D1–D6 regions, respectively (see Supplementary Material S1 for the GenBank accession numbers). The likelihood map (Fig. 1) indicated a very strong phylogenetic signal, with a percentage of points in the network-like areas (Schmidt et al. 2002) of 0.4% for both 18S (Fig. 1a) and 28S (Fig. 1b). Accordingly, both datasets were reliable for phylogenetic and taxonomic inferences (Schmidt and von Haeseler 2012). Both ML and BI analyses converged on the same topology; thus, only the Bayesian tree was reported (Fig. 2).
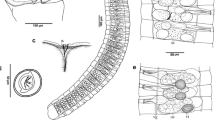
Likelihood mapping of the 18S (a) and 28S D1–D6 (b) genes. The likelihood-mapping method (Strimmer and von Haeseler 1996) partitions the area of the equilateral triangle into seven regions. The three trapezoids at the corners represent the areas supporting strictly bifurcating trees; that is, the presence of a tree-like phylogenetic signal. The three rectangles on the sides represent regions where the decision between two trees is not obvious. The center of the triangle represents sets of points P (posterior probabilities of the unrooted trees) where all three trees are equally supported
Phylogenetic tree. Tree obtained by Bayesian inference showing the interrelationships among species based on combined 18S + 28S D1–D6. The branch length scale refers to the number of substitutions per site. Nodal supports are indicated for both maximum likelihood (bootstrap values, BV) and Bayesian inference (posterior probability, PP). The family Otoplanidae (terminal A) is depicted in Fig. 3
Phylogenetic tree. Part of the tree in Fig. 2, therein abbreviated as terminal A, shows phylogenetic relationship within the family Otoplanidae. Taxon names belonging to the genus Otoplana are in bold
The species delimitation methods yielded slightly different results. The ST-GMYC model identified 56 entities (confidence interval [CI] = 41–63), 35 of which were represented by singletons and 21 by clusters (P < 0.001). The PTP/bPTP model found 59 entities (CI = 56–62), 39 of which were represented by singletons and 20 by clusters. The ABGD method, checked at the prior maximal distance (P = 0.001), identified 58 entities. The NDT method detected 62 and 57 entities for the 18S and 28S datasets, respectively. Further details on the species delimitation results are given in Supplementary Material S1.
Taxonomic account
Order Proseriata Meixner, 1938.
Family Otoplanidae Hallez, 1892.
GenusOtoplana Du Plessis, 1889
Otoplana norenburgi Curini-Galletti, Scarpa & Casu sp. nov.
urn:lsid: zoobank.org :act:20B02528-27A3-460E-A8F8-D9863F457C77
(Figs. 4a, c, d, f and 5b, d, f–h; Supplementary Material S3)
a, c, d, fOtoplana norenburgi sp. nov.: habitus of a live animal (a); sclerotized copulatory structures (c); cephalic area (d); reconstructions of the genital organs from sagittal sections, as seen from the left, with the “accessory male canal” (amc) drawn in the background (f). b, eOtoplana didomenicoi sp. nov.: habitus of a live animal (b); sclerotized copulatory structures (e)
a, c, eOtoplana didomenicoi sp. nov.: sagittal section of the genital area (a); sclerotized copulatory structures: aculeus (c), girdle (e). b, d, f–h: Otoplana norenburgi sp. nov.: sagittal section of the genital area (b); sagittal section of the “accessory male canal” (d); sclerotized copulatory structures of specimens from Gran Canaria (f) and La Palma (g; h: detail): Scale bars = 10 μm
Holotype. Gran Canaria (Canary Islands): Bahia de Santa Agueda, intertidal in medium sand (October 2011) (Lat: 27.750529°; Long: −15.642610°), karyological slide made permanent with lactophenol with four specimens, one of which marked and chosen as holotype (SMNH: Type-8941).
Other material. Same locality, three specimens sagittally sectioned (paratypes, SMNH: Type-8942; CZM 724–725).
Other locality. La Palma (Canary Islands): Puerto de Naos, intertidal in medium sand (October 2011) (Lat: 28.586027°; Long: −17.911162°), three karyological slides made permanent with lactophenol (CZM 726–728), five specimens sagittally sectioned (CZM 729–733).
Etymology. Species named in honor of Jon L. Norenburg (Smithsonian’s National Museum of Natural History, Washington DC), who collected the specimens in La Palma, and gave MC-G the unrivaled chance to join meiofauna workshops around the world.
Description. A remarkably large and slender Otoplana (Fig. 4a): fixed specimens are up to 5 mm in length. Cephalic end truncated, with numerous tactile bristles, and two pairs of sensory organs, symmetrically arranged in front of the statocyst (Fig. 4d). They consist of numerous, very fine, tightly packed sensory bristles, up to 35 μm long, retractable into wide pockets, and connected basally to nerve cords, which extend anteriorly from the brain. At the level of the two sensory organs, a wide furrow, ventrally and laterally ciliated, is present (cf. Fig. 4d). Posterior end pointed. Body flattened, ciliated ventrally in two distinct patches, one running from the cephalic, ciliated furrow to in front of the pharynx, the other from the mouth to just in front of the genital pore. Cilia are about 10 μm long. Epithelium intranucleated in unciliated areas, infranucleated in ciliated areas. Rhabdoids rod-shaped, 10–12 μm long, arranged in longitudinal rows in the unciliated parts of body, and particularly dense cephalically. With very numerous adhesive glands in the unciliated parts of body, also present on the dorsal surface. Brain encapsulated, irregularly prismatic in shape in living specimens, abutting the statocist anteriorly. Brain lining poorly evident in sections, particularly anteriorly, where nerve cords departs, and most nerve cell nuclei are located. With a precerebral extension of the gut, solid, without an inner lumen (Fig. 4d, pg). Body musculature consisting of an exceedingly thin outer layer of circular musculature, barely detectable ventrally, and not noticeable dorsally. Inner layer of longitudinal musculature extremely well developed, with few strong, parallel fibers, extending the whole length of the animal, and particularly strong ventrally.
Pharynx in the second half of body elongate, horizontally oriented, about 300 μm in length. It is ciliated externally (cilia length: 2 μm) and internally (cilia length: 4–5 μm). Pharynx musculature with thin outer longitudinal fibers and very thick inner circular fibers. Pharyngeal glands extending very shortly outside the pharynx, and discharging through the unciliated tip of the pharynx. With acidophilic (with slightly coarser content) and basophilic pharyngeal glands. A distinct esophagus is lacking. Buccal cavity unciliated, lined by flattened, intranucleated epithelial cells. The gut forms a narrow canal, dorsal to the pharynx; no evidence of a muscular septum could be detected.
Male genital system. With numerous testes (about 60) arranged in two lateral ventral rows in front of the pharynx. Copulatory organ with one seminal vesicle, a prostate vesicle, surrounded by the sclerotized apparatus (Fig. 4f). Before entering the seminal vesicle, the seminal ducts form two symmetrical, large sperm reservoirs (“false seminal vesicles”) (Fig. 4a, fsv), lined by a ciliated epithelium (even if the large amount of sperm present makes observation problematic). The somewhat pyriform seminal vesicle is lined with a flat epithelium. The ejaculatory duct, just outside the seminal vesicle, receives the common inlet of the “false seminal vesicles” (Fig. 5b, sd); then enters the ovoid-elongate prostate vesicle, where only the distalmost portion of the cellular lining appears to have some glandular function. The ejaculatory duct is surrounded by a layer of circular muscles: externally to this, the intracellular sclerotized structures are present, consisting of an aculeus, placed dorsally to a girdle of needles of variable shape and length (Figs. 4c and 5f, g). The straight, rod-shaped aculeus, ranging from 70 to 88 μm long (n = 4) in the population from La Palma and from 72 to 87 μm in Gran Canaria (n = 3), has a swollen proximal basis [up to 7.5 μm wide (La Palma) to 8.5 μm in specimens from Gran Canaria] and tapers progressively along its length. The shape of its distal tip appears linked to the degree of sclerotization, and varies from obtuse to acutely pointed. The girdle consists of 18–23 (specimens from La Palma)/19–24 needles (specimens from Gran Canaria). The longest needles, up to 55 μm (La Palma)/67 μm (Gran Canaria), are placed dorsally, beneath the aculeus, and decrease in size ventrally to 28–46 μm, depending on the degree of sclerotization: in mature, fully formed specimens, differences in size among the needles of the girdle are less marked. The needles have a stem of about 1.5 μm wide, and a sickle-shaped distal tip. The apophysis is placed at 3.5–5 μm from the tip, and varies in morphology from obtuse, poorly developed in dorsal needles, to long (up to 2.5 μm), and, in most instances, characteristically truncated distally in the ventral ones. In specimens from Gran Canaria, apophyses are generally more developed, up to 2.5 μm in the longest needles, and up to 4.5 μm in the shortest, where they may be truncated to bifid or even multifid. Needles are arranged with the apophyses placed externally, to which thin bundles of muscles are attached. The copulatory organ opens into the anterior, dorsal portion of the common atrium.
Laterally to the seminal vesicle, a large, bursa-like structure, filled with sperm, and lined with a resorbiens epithelium, is present (Figs. 4f, amc and 5f). This structure opens to the outside with a pore (the so-called “male accessory pore”) at the right side of the genital pore. The bursa-like structure corresponds to what Ax (1956) considered as an “accessory male canal”. To the best of our ability and based on the power of resolution allowed by the available sections, it was impossible to ascertain its connections with either the male or the female genital systems (see discussion below).
Female genital system. With two ovaries just in front of pharynx (Fig. 4a). With numerous (about 160) vitellarian follicles, arranged into two irregular rows at each side of the animal, alongside the testes. Most of the follicles are prepharyngeal; a few lateral to pharynx, in a single line per side, and very few (about four per side) are post pharyngeal. The female duct is traceable from the level of the posteriormost vitellarian follicles; it is ciliated and opens into a female pore, placed just below the male pore, in the common genital atrium, and surrounded by numerous, coarse-grained eosinophilic female glands (Fig. 4f).
The common atrium is large, irregularly shaped, and lined by an unciliated, intranucleated epithelium, and opens ventrally to the outside via the common genital pore, surrounded by fine-grained eosinophilic “shell” glands.
Diagnosis. A large and slender species of Otoplana, with about 60 testes. With a straight aculeus, 70–88 μm long, swollen proximally to 8.5 μm in diameter. With a girdle of 18–24 needles, ranging from 28 to 67 μm in length. Apophyses of shorter needles distinctly truncated to bifid.
Molecular diagnostic pure characters (sensu Sarkar et al. 2008) have been detected for both analyzed genes (see Supplementary Material S3 for details).
Otoplana didomenicoi Curini-Galletti, Scarpa & Casu sp. nov.
urn:lsid: zoobank.org :act:C9390A37-76E4-446C-82C4-F1969208D09C
(Figs. 4b, e and 5a, c, e; Supplementary Material S3)
Holotype. Lanzarote (Canary Islands): Costa de Papagayo, intertidal in medium-coarse sand (October 2011) (Lat: 28.844193°; Long: −13.789200°), karyological slide made permanent with lactophenol with five specimens, one of which marked and chosen as holotype (SMNH: Type-8944).
Other material. Same locality, five specimens sagittally sectioned (paratypes, SMNH: Type-8943; CZM 734–737).
Other locality. Ilha de Culatra (Algarve, Portugal), intertidal in coarse sand (May 2013) (Lat: 36.984715°; Long: −7.838506°), three karyological slides made permanent with lactophenol (CZM 738–740).
Etymology. Species named in honor of Maikon di Domenico (Universidade Federal do Paraná, Brazil), who collected the Papagayo specimens, and whose friendly and calm organization greatly contributed to the success of the meiofauna workshops around the world.
Description. Similar in general habitus to the previous species, but markedly stouter and shorter (Fig. 4b): fixed specimens are only about 1.5 mm in length. Head as the previous species. Ventral ciliation running from the cephalic ciliated furrow to in front of pharynx; a ciliated area in front of the genital pore is lacking. Cilia about 9 μm long.
Rhabdoids rod-shaped, 5–6 μm long, arranged in longitudinal rows in the unciliated parts of body. With numerous adhesive glands in the unciliated parts of body. Brain encapsulated, ovoid, abutting the statocist anteriorly, with most nerve cell nuclei located posteriorly. Brain lining evident. With a comparatively short precerebral extension of the gut.
Body musculature consisting of a very thin outer layer of circular musculature, only noticeable ventrally. Inner layer of longitudinal musculature extremely well developed ventrally, with few but very strong, parallel fibers, extending the whole length of the animal.
Pharynx in the second half of body, elongate, horizontally oriented, 100–130 μm in length. It is ciliated externally (cilia length: 2 μm) and internally (cilia length: 4–5 μm). Pharynx musculature with well developed outer longitudinal fibers and inner circular fibers. Pharyngeal glands extending very shortly outside the pharynx. A distinct esophagus is lacking. Buccal cavity unciliated. The gut runs through a very narrow canal, dorsal to the pharynx; no septum observed.
Male genital system. With about 30 testes arranged in two lateral rows in front of the pharynx, internally to the vitellaries. Spermiducts enlarged into “false seminal vesicles” and connected to the ejaculatory duct in the same way as in the previous species. Copulatory organ with one seminal vesicle and a prostate vesicle, surrounded by the sclerotized apparatus. The seminal vesicle is lined with a flat epithelium. With a narrow, elongate prostate vesicle; glandular tissue poorly developed, undetectable in many sections. The intracellular sclerotized structures consist of an aculeus, placed dorsally to a girdle of needles, markedly different in length (Figs. 4e and 5c, e). The aculeus ranges from 72 to 97 μm long (n = 4) in specimens from Lanzarote and from 84 to 105 μm in specimens from Portugal (n = 3). It consists of a central axis, 3.5–4 μm wide proximally and tapering into a sharp point distally. At a short distance from the basis (12–15 μm), a thin lamina emerges at both sides, widens progressively till about half the length of the aculeus (to a maximum width of about 16 μm across), and then narrows again distally till it merges with the aculeus, at a short distance from its distal tip, resulting in a shape strongly reminiscent of a lanceolate leaf. The outer edges of the lamina are strengthened, up to 2.5 μm thick.
Girdle consisting of 9–14 needles in specimens from Lanzarote. The pair of needles ventral to the aculeus (Pair I) is distinctly longer (42–51 μm) than the others, with a falcate distal tip, and a narrowly elongate (up to 4 μm in length) apophysis placed at about 3.5 μm from the tip. Pair II, lateral to the previous pair, is similar in shape but shorter, 30–39 μm long. The remaining needles are markedly shorter (ranging from 14 to 21 μm), with narrow, long apophyses (to 6.5 μm) parallel to the stem of the needle. The shape and thickness of the apophysis varies with the degree of sclerotization and may even fuse with the stem. Similarly, the shortest needles have poorly sclerotized, evanescent bases, so that the measured length may not reflect the actual size attained at full maturity.
Specimens from Portugal had an aculeus ranging from 84 to 105 μm long (n = 3), and up 25 um broad. Needles were more numerous, ranging from 16 to 26, with a comparable size pattern: one pair of long needles (Pair I: 65–78 μm long) ventral to the aculeus, and one to two pairs intermediate in size (50–70 μm long) flanking them. The remaining pairs are markedly smaller in two specimens (ranging from 18 to 21 μm) and, thus, comparable to specimens from Lanzarote. In the third specimen, however, they were much longer (45 μm). This specimen also had the longest aculeus, and the bases of the needles were well sclerotized: this may be taken as a sign of full maturity. Morphology of needles was identical to those from Lanzarote, with long and thin apophyses, which may be fused to the main axis of the stem.
The copulatory organ opens into the anterior part of the common genital atrium. An “accessory male genital canal” is present lateral to the copulatory organ. It is shaped as in the previous species, and opens to the outside with the “male accessory pore” at the right side of the common genital pore. Also in this species, no clear connections with either the male or the female genital systems could be detected.
Female genital system. With two ovaries just in front of pharynx. With numerous vitellarian follicles, arranged into two irregular rows at each side of the animal, external to the testes, and extending posteriorly to the level of the copulatory organ. The ciliated female duct opens into a female pore, placed just below the male pore, in the common genital atrium, and surrounded by numerous, coarse-grained eosinophilic female glands.
The common atrium opens ventrally through the common genital pore, surrounded by fine-grained eosinophilic “shell” glands.
Diagnosis.A small and stout Otoplana, with about 30 testes. With a lanceolate aculeus, 72–107 μm long, and a girdle of 9–26 needles, with one pair distinctly longer than the others and one to two pairs intermediate in size.
Molecular diagnostic pure character have been detected for both analyzed genes (see Supplementary Material S3 for details).
Discussion
Species justification
The taxonomy of the genus Otoplana had a troubled history since its very start. The original description of Otoplana intermedia (Du Plessis, 1889; type locality: Plage de La Reserve, Nice, France) contained little information on the sclerotized structures of the copulatory organ, which have been considered as the main discriminatory features among species of Otoplana by all authors who later dealt with the genus. Rather, Du Plessis (1889) and, later, Wilhelmi (1908) gave emphasis to external features. Particularly, cephalic sensory organs and gut morphology gave credits for the ascription of the genus to the Alleocoela Graff, 1882, which, since then, has been reported as a non-monophyletic taxon (Tyler et al. 2006–2016).
The first thorough morphological description of Otoplana intermedia was then given by von Hofsten (1921). However, his redescription was not based on specimens from the type locality, which, according to the author, was unsuccessfully searched for. It was based on slides of specimens from the Gulf of Naples and from Faro (Sicily), sent to him by Wilhelmi.
Ax (1956) attributed to the taxon O. intermedia specimens from the Gulf of Lyons (Banyuls-sur-Mer). Otoplana intermedia sensu Ax (1956) is a comparatively very large species, with living individuals attaining 8 mm in length, and with sclerotized structures consisting of a girdle of 22–24 needles, ranging from 80 to 90 μm in length, without an aculeus (Ax 1956, figs 24, 25, p 172). This is what has been taken as the “true” O. intermedia by all subsequent authors (e.g., Lanfranchi and Melai 2010). However, it is worth mentioning that the sclerotized structures described by Ax (1956) are markedly different from von Hofsten’s drawings (von Hofsten 1921: p 41, fig 11).
Later, two additional smaller species were described: O. bosporana Ax, 1959, with a pointed aculeus with 30–33 needles, and O. truncaspina Lanfranchi, 1969, with an obtuse-tip aculeus and 23 needles, respectively from the eastern (Bosporus) and western (Ligurian Sea) Mediterranean (Ax 1959; Lanfranchi 1969). In recent years, four more species were described from the same small stretch of the Ligurian Sea coast: O. oxyspina Lanfranchi & Melai, 2007; O. proxima Lanfranchi & Melai, 2010; O. falcataspina Melai, 2013; O. labronica Melai, 2013. These species are distinguished either on the basis of a lack of aculeus (O. proxima) or number, shape, and size of needles of the girdle (Lanfranchi and Melai 2007, 2010; Meini 2013). While this high number of closely similar species in a small stretch of coastline may give rise to some interesting evolutionary hypotheses, it is fair to say that the species descriptions above are based on a few specimens of single populations, and no indication of intrapopulation (not to mention interpopulation) variability has been given. When observing large numbers of specimens of the genus Otoplana, from various Mediterranean areas, a puzzling array of situations, which are intermediate among the (somewhat) clear-cut descriptions available in the literature, is apparent. In addition, it is equally evident that there is a high degree of intrapopulation variability, as well as differential morphologies according to the degree of maturity of individuals. Furthermore, the presence of an aculeus is not easily appreciable in whole mounts, whose tissues have not been previously dissolved.
Mediterranean specimens from northern Sardinia, sequenced in the present study, present characters compatible with some of the species above, and have been tentatively assigned to them (see Supplementary Material S1). They do not show any hint of genetic separation, and appear to belong to a single entity according to all the species delimitation methods adopted (see Supplementary Material S1). This is not the place nor is it our intention to discuss exhaustively the taxonomic problems related to the Mediterranean species of Otoplana, which need a thorough examination of material from type localities. It is suffice to say here, that the Mediterranean specimens sequenced (all of which have an aculeus) are well separated from the lineage, including the two Canarian species here described. In any case, no species of Otoplana has a lanceolate aculeus, which places O. didomenicoi sp. nov. immediately apart. Otoplana norenburgi sp. nov. is characterized by the bulbous proximal basis of its aculeus, unlike the other species described, whose aculeus has the same diameter from the basis till the distal tip. Furthermore, the shorter needles of the girdle show a peculiar shape of the apophyses.
Phylogenetic relationships
The phylogenetic tree (Fig. 2) retrieved the main configuration obtained in a previous study (Scarpa et al. 2017a), and confirmed the sister taxon relationships between Otoplanidae and the newly described Yorkniidae Curini-Galletti, Casu & Scarpa, 2017. Species morphologically ascribed to the genus Otoplana cluster together, and the monophyly of the genus appears thus supported (see Fig. 3). It is remarkable that our molecular phylogeny pinpoints species of the genera Kata Marcus, 1949 and Xenotoplana Ax, Weidemann & Ehlers, 1978 as the closer taxa to species of Otoplana. Otoplanidae, as a rule, have a single genital opening, the common genital pore. The only genera of Otoplanidae with a second genital opening represented in our tree are, indeed, Otoplana, Kata, and Xenotoplana. The close relationships of these genera need further investigations, as it may have implications on reconstructing the ancestral character states of the family Otoplanidae. However, in contrast to the genus Otoplana, the second opening is linked to the female genital system in the genera Kata and Xenotoplana, but see below for further discussion on this point.
The genus Otoplana appears much more differentiated outside the Mediterranean than hitherto considered. Two geographically separated lineages can be recognized: Atlantic and Mediterranean, which overlap in southern Portugal. In fact, one specimen from Algarve (Otoplana sp. in Fig. 3) appears to be the sister taxon of all Sardinian specimens. Remarkably, all the species delimitation methods adopted in the present study rank this specimen as a well defined, distinct entity (see Supplementary Material S1). In the light of the confused taxonomic scenario of the Mediterranean taxa depicted above, and the exceedingly limited samples available, any taxonomic decision on the status of the Algarve specimen is postponed to future studies.
The “accessory male canal” problem
von Hofsten (1921) reconstructed in detail the morphology of his O. intermedia. In particular, he was the first to describe the connection of the “accessory male canal” with the right spermiduct (p 44, fig 12). Ax (1956, p 174, fig 34) supported this interpretation, and his reconstruction of the species’ anatomy is very similar to von Hofsten’s drawings, and apparently based on it. From then on, the connection of the accessory canal with the right spermiduct has been taken for granted (e.g., Miller and Faubel 2003; Tajika 1983). While this structure and its outlet to the right of the common genital pore are easy to see in both living individuals and sections, we are unable to either confirm or dismiss previous observations on its connections. von Hofsten (1921) and Ax (1956) described the structure as a mere canal, connecting the right spermiduct to the outside through the accessory male pore. As evident from our descriptions of the new species, the “accessory male canal” is more complex, and resembles a veritable bursa of the resorbiens type (Fig. 5d). Sections of Mediterranean specimens of Otoplana revealed a similar morphology (unpubl. obs.).
The accessory male canal is a most unusual structure, as no other Proseriata has a similar connection of the male system with the exterior, whose function, according to von Hofsten (1921), would be to eliminate the “excess” sperm. In this regard, it is noteworthy that our molecular phylogeny revealed a sister taxon relationship between the genera Kata and Otoplana (Fig. 3). Kata is a prevalently southern hemisphere genus, whose species present an organ comparable to Otoplana’s male accessory canal, similarly caudal to the copulatory organ, and opening to the outside via one or two pores (Marcus 1949, 1950; Curini-Galletti 2014). However, in Kata, this structure is considered as a post-penial bursa, provided with resorbiens tissue and with vagina(e), and, thus, part of the female genital system. To make things more complex, Zygotoplana Tajika 1983 from Japan, which is morphologically similar to Otoplana (Miller and Faubel 2003), has an organ nearly identical in morphology and position to the post-penial bursa of Kata, and similarly opening to the outside with two pores. However, this structure is considered to be part of the male system, with the same function as the accessory male canal of Otoplana (Tajika 1983).
To complete the picture, it is worth remembering the existence of species of Orthoplana Steinböck, 1932 and Paradoxoplana Ax,1956, both considered closely related to Otoplana (Miller and Faubel 2003; Ax 1956), which lack a bursa and accessory male canal altogether. Moreover, several otoplanid taxa have a post-penial bursa connected with neither male nor female genital systems (e.g., species of Paraplana Ax, 1956).
Even theoretically allowing that structures with the same morphology and position, presumably homologous, may have shifted totally different functions in lineages derived from a common ancestor, as is apparently the case at least of the sister genera Kata and Otoplana, it needs to be acknowledged that, in this group of species, it is extremely difficult to follow the course of genital ducts. In fact, not only is the post-pharyngeal area very small, but it is occupied for most of its volume by a large seminal vesicle and by the posterior widening of the gut. Trying to follow the course of post-pharyngeal genital ducts is, thus, particularly challenging. The median, post-pharyngeal duct of Paradoxoplana, whose connections could not be resolved, and which has been aptly labeled by Ax (1956, p 192 (690), fig 80) as “ductus problematicus”, may be taken as exemplificative in this respect. Therefore, any functional and evolutionary inference on the accessory male organ should be preceded by a more thorough check of the anatomy of species involved.
It is worth noting a further unusual character present in species of Otoplana which has received little attention so far. In Proseriata, the spermiducts are connected to the seminal vesicle(s) (see Curini-Galletti 1998, fig 1, p 475). Species of Otoplana appear to be the only ones in which the spermiducts are connected to the ejaculatory duct, at its exit from the seminal vesicle (Fig. 4f): an autapomorphy for the genus, adding to its peculiarities.
A Canarian paradox?
Proseriates have no dispersal stages (Curini-Galletti 2001). However, individuals of species that occur in high energy habitats have chances to be suspended in the water column; this may ensure gene flow among neighboring populations (Casu and Curini-Galletti 2006).
Species of Otoplana occur in a quite energetic environment, which may account for the very limited genetic differentiation between populations of the species considered here. The case of O. didomenicoi sp. nov. is particularly striking, as populations of Lanzarote and Algarve, at least 1600 km apart along the coastline, show poor genetic differentiation, based on the markers used. Although variability among different populations belonging to the same species should be better surveyed with less conserved markers than 18S and 28S genes, it is worth mentioning that these same genes discriminated populations belonging to the Monocelis lineata species complex, occurring in low energy, brackish water habitats (Scarpa et al. 2016a). Given this potential for dispersal, it is, therefore, surprising that the range of the two Canarian species does not overlap. Rather, O. norenburgi sp. nov. appears limited to outer islands, while O. didomenicoi sp. nov. is present on one of the islands closer to the continent. Ecological preferences for different textures of the substrate may be responsible, although our samplings in the intertidal area, at least in Lanzarote, included sediments with a wide range of granulometry. Sandy beaches are few and limited in extension in the Canary Islands: in such a fragmented habitat, repeated processes of extinction/recolonization may, thus, add an element of randomness to the pattern. However, no conclusions on the actual range of the species involved, nor on the possible endemicity to the Canary Islands of O. norenburgi sp. nov., can be advanced, due to the lack of knowledge on the proseriate fauna of the other Atlantic Archipelagoes, as well as of West Africa. Extension of sampling in this exceedingly poorly known area of the Eastern Atlantic may, thus, be highly desirable.
References
Ax P (1956) Monographie der Otoplanidae (Turbellaria) Morphologie und systematik. Abhandlungen der mathematisch-naturwissenschaftlichen klasse 13:3–298
Ax P (1959) Zur Systematik, Ökologie und Tiergeographie der Turbellarienfauna in den ponto-kaspischen Brackwassergebieten. Zool Jahrb Abt Syst Oekol Geogr Tiere 87:43–184
Brown AC, McLachlan A (2010) The ecology of sandy shores, 2nd edn. Academic Press, San Diego, CA
Brown SDJ, Collins RA, Boyer S, Lefort M-C, Malumbres-Olarte JA, Vink CJ, Cruickshank RH (2012) Spider: an R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol Ecol Resour 12:562–565
Casu M, Curini-Galletti M (2006) Genetic evidence for the existence of cryptic species in the mesopsammic flatworm Pseudomonocelis ophiocephala (Rhabditophora: Proseriata). Biol J Linn Soc 87:553–576
Casu M, Cossu P, Sanna D, Lai T, Scarpa F, Curini-Galletti M (2011) A reappraisal of the monophyly of the genus Pseudomonocelis Meixner, 1943 (Platyhelminthes: Proseriata), with the description of a new species from the Mediterranean. Zootaxa 3011:59–68
Casu M, Scarpa F, Delogu V, Cossu P, Lai T, Sanna D, Curini-Galletti M (2014) Biodiversity patterns in interstitial marine microturbellaria: a case study within the genus Parotoplana (Platyhelminthes: Rhabditophora) with the description of four new species. J Zool Syst Evol Res 52:190–202
Curini-Galletti M (1998) The genus Polystyliphora Ax, 1958 (Platyhelminthes: Proseriata) in eastern Australia. J Nat Hist 32:473–499
Curini-Galletti M (2001) The Proseriata. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 41–48
Curini-Galletti M (2014) Contribution to the knowledge of the Proseriata (Platyhelminthes: Rhabditophora) from southeast Brazil. Mar Biodivers 44:287–312
Curini-Galletti M, Webster BL, Huyse T, Casu M, Schockaert ER, Artois TJ, Littlewood DTJ (2010) New insights on the phylogenetic relationships of the Proseriata (Platyhelminthes), with proposal of a new genus of the family Coelogynoporidae. Zootaxa 2537:1–18
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Du Plessis G (1889) Note sur l’Otoplana intermedia. Zoolog Anz 12 Jahrg Leipzig 1889:339–342
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472
Girstmair J, Schnegg R, Telford MJ, Egger B (2014) Cellular dynamics during regeneration of the flatworm Monocelis sp. (Proseriata, Platyhelminthes). EvoDevo 5:37
Jörger KM, Schrödl M (2013) How to describe a cryptic species? Practical challenges of molecular taxonomy. Front Zool 10:59
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lanfranchi A (1969) Nuovi otoplanidi (Turbellaria, Proseriata) delle coste della Liguria e della Toscana. Bollettino di Zoologia 36:167–188
Lanfranchi A, Melai M (2007) Morphology and taxonomy of a new species of Otoplanid (Plathelminthes, Rhabditophora, Proseriata) from the Ligurian Sea. Ital J Zool 74:209–214
Lanfranchi A, Melai M (2010) A new marine flatworm (Plathelminthes: Rhabditophora: Otoplanidae) from the Ligurian coast. J Mar Biol Assoc UK 90:423–427
Littlewood DTJ, Curini-Galletti M, Herniou EA (2000) The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol Phylogenet Evol 16:449–466
Littlewood DTJ, Olson PD (2001) Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 262–278
Litvaitis MK, Curini-Galletti MC, Martens PM, Kocher TD (1996) A reappraisal of the systematics of the Monocelididae (Platyhelminthes, Proseriata): inferences from rDNA sequences. Mol Phylogenet Evol 6:150–156
Marcus E (1949) Turbellaria Brasileiros (7). Bol Fac Fil Ci Letr Univ Sao Paulo, Zool 14:7–156
Marcus E (1950) Turbellaria Brasileiros (8). Bol Fac Fil Ci Letr Univ Sao Paulo, Zool 15:5–192
Meini G (2013) Two new marine flatworms (Platyhelminthes: Rhabditophora: Proseriata) of the genus Otoplana Du Plessis, 1889 from the upper Tuscany sandy shores (Italy). Zootaxa 3608:575–586
Miller W, Faubel A (2003) Six new species of Proseriata (Plathelminthes) from eastern Australia. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 100:27–57
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, November 2010
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Front Zool 7:16
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S, Sumlin WD, Vogler AP (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol 55:595–609
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21:1864–1877
Remane A (1933) Verteilung und Organisation der benthonischen Mikrofauna der Kieler Bucht. Wissenschaftlichen Untersuchung der Deutschen Meere 21:161–221
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Ruppert EE, Fox RS, Barnes RD (2004) Invertebrate zoology: a functional evolutionary approach, 7th edn. Brooks/Cole Thomson Learning, Belmont, CA, 1008 pp
Sarkar IN, Planet PJ, DeSalle RO (2008) CAOS software for use in character-based DNA barcoding. Mol Ecol Resour 8:1256–1259
Scarpa F, Cossu P, Sanna D, Lai T, Norenburg JL, Curini-Galletti M, Casu M (2015) An 18S and 28S-based clock calibration for marine Proseriata (Platyhelminthes). J Exp Mar Biol Ecol 463:22–31
Scarpa F, Cossu P, Lai T, Sanna D, Curini-Galletti M, Casu M (2016a) Meiofaunal cryptic species challenge species delimitation: the case of the Monocelis lineata (Platyhelminthes: Proseriata) species complex. Contrib Zool 85:123–145
Scarpa F, Sanna D, Lai T, Cossu P, Curini-Galletti M, Casu M (2016b) New set of nuclear primers for the ribosomal regions in Proseriata (Platyhelminthes). Conserv Genet Resour 8:411–413
Scarpa F, Cossu P, Delogu V, Lai T, Sanna D, Leasi F, Norenburg JL, Curini-Galletti M, Casu M (2017a) Molecular support for morphology-based family-rank taxa: the contrasting cases of two families of Proseriata (Platyhelminthes). Zool Scr. https://doi.org/10.1111/zsc.12251
Scarpa F, Sanna D, Cossu P, Lai T, Curini-Galletti M, Casu M (2017b) A molecular approach to the reconstruction of the pre-Lessepsian fauna of the Isthmus of Suez: the case of the interstitial flatworm Monocelis lineata sensu lato (Platyhelminthes: Proseriata). J Exp Mar Biol Ecol. https://doi.org/10.1016/j.jembe.2017.08.011
Schmidt HA, Strimmer K, Vingron M, von Haeseler A (2002) TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502–504
Schmidt HA, von Haeseler A (2012) Phylogenetic inference using maximum likelihood methods. In: Lemey P, Salemi M, Vandamme AM (eds) The phylogenetic handbook, 5th edn. Cambridge University Press, Cambridge, pp 181–209
Schockaert ER (1996) Turbellarians. In: Hall GS (ed) Methods for the examination of organismal diversity in soils and sediments. CAB International, Wallingford, pp 211–225
Silvestro D, Michalak I (2012) raxmlGUI: a graphical frontend for RAxML. Org Divers Evol 12:335–337
Strimmer K, von Haeseler A (1996) Quartet puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol Biol Evol 13:964–969
Tajika K-I (1983) Zwei neue Otoplaniden (Turbellaria, Proseriata) aus Hokkaido, Japan. Annot Zool Jap 56:100–110
Tavaré S (1986) Some probabilistic and statistical problems in the analysis of DNA sequences. In: Miura RM (ed) Some mathematical questions in biology: DNA sequence analysis. American Mathematical Society, Providence, RI, pp 57–86
Tyler S, Schilling S, Hooge M, Bush LF (comp.) (2006–2016) Turbellarian taxonomic database. Version 1.7. http://turbellaria.umaine.edu
von Hofsten N (1921) Anatomie, Histologie und systematische Stellüng von Otoplana intermedia. Du Plessis Zoologiska Bidrag från Uppsala 7:1–74
Wilhelmi J (1908) Über einige Alloiocoelen des Mittelmeeres. Mitt Zool Stat Neapel 18:644–650
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29:2869–2876
Acknowledgements
We thank Prof. Dr. Katrine Worsaae and Dr. Alejandro Martinez for organizing the workshop in Lanzarote in the autumn of 2011. Dr. Valentina Delogu is thanked for her skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Martínez García
This article is a contribution to the Topical Collection Interstitial and Cave Diversity in Atlantic Oceanic Islands
Rights and permissions
About this article
Cite this article
Scarpa, F., Cossu, P., Sanna, D. et al. New insights on the genus Otoplana Du Plessis, 1889 (Platyhelminthes: Proseriata), with description of two new species from the Canary Islands. Mar Biodiv 49, 2075–2087 (2019). https://doi.org/10.1007/s12526-017-0785-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0785-1