Abstract
Sampling campaign took place in October 2011 and included 7 locations and 16 stations along the eastern coast of the island of Lanzarote (Spain). Samples yielded 61 species for a total of 96 records. Thirty-six species (27 genera and 11 families) belong to Macrodasyida while 25 species (18 genera, 7 families) to Chaetonotida. Thirty-two are known species while 29 appear to be undescribed taxa or putatively so. The finding at Lanzarote of some of the known species bear particular significance: Oregodasys cirratus and Tetranchyroderma canariense are recorded for the second time ever, while Musellifer delamarei and Urodasys acanthostylis were previously known only from the Mediterranean, and Urodadys mirabilis was acknowledged only for northern Europe. Furthermore, the presence of Chaetonotus apechochaetus, C. apolemmus, C. siciliensis, Heterolepidoderma loricatum, Lepidodasys unicarenatus, Musellifer delamarei, Thaumastoderma mediterraneum, and Urodasys acanthostylis strongly suggest them to be part of the temperate/warm fauna that invaded the Mediterranean basin after the Missinian crisis during the different climate eras. Of the new species, one is described as its characteristics substantially widen our knowledge of the entire genus. Urodasys completus sp. nov. is unique in that it possesses, among others, two testes and a sclerotic stylet. Results of a phylogenetic analysis indicated that the sequence of the evolutionary transformation that have occurred in the reproductive system of the species of Urodasys are likely dissimilar from the ones proposed thus far. The overall results testify the need to continue the exploration in the Canary Islands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrotricha are microscopic invertebrates (0.08–3.0 mm in total body length) constituting a phylum likely sister to the Platyhelminthes (e.g., Struck et al. 2014; Egger et al. 2015). The group is cosmopolitan and includes, as of April 2017, 826 species, 497 of which are marine and 329 freshwater (WoRMS 2017). The phylum is divided into two orders: Macrodasyida (M), mostly marine, and Chaetonotida (C), common in both freshwater and marine ecosystems (taxa/habitat distribution detailed in Todaro 2017 and Todaro and Tongiorgi 2017; see also Kieneke and Schmidt-Rhaesa 2014).
Gastrotrichs are mainly interstitial in marine habitats, whereas in freshwater they are also found as ubiquitous components of the periphyton and benthos (Balsamo and Todaro 2002). Some freshwater genera have adapted to a semi-planktonic life style, e.g., Haltidytes, Stylochaeta, Kijanebalola and Neogossea (e.g., Kånneby and Todaro 2015; Todaro et al. 2013).
Marine species live both intertidally and subtidally, being most abundant in fine- to medium-grained sediments in crystalline waters of coastal areas (e.g., Garraffoni et al. 2016a; Todaro and Rocha 2004); only some species of the genus Musellifer are known to inhabit deep-sea muddy substrata (Hummon 1969; Leasi and Todaro 2010). In marine sandy bottoms, gastrotrichs typically rank third in abundance among the meiofaunal taxa behind the Nematoda and the harpacticoid Copepoda (e.g., density up to 364 ind./10 cm2; Todaro 1998), although in several instances, they have been found to be the first or the second most abundant meiofauna taxon (e.g., Coull 1985; Gray 1971; Hochberg 1999; Todaro et al. 1995).
Gastrotrichs feed on microalgae, bacteria and occasionally on protists such as flagellates or foraminiferans (Kieneke and Schmidt-Rhaesa 2014, and unpublished). Like most other meiobenthic organisms they have a short life cycle and lack larval stages useful for dispersal; consequently, marine gastrotrichs spend their entire existence within the sediments. Despite this life history, many species are not restricted to confined areas; on the contrary, they seem to be widely distributed, with some species being amphi-Atlantic or cosmopolitan (e.g., Todaro et al. 1995; Todaro and Rocha 2004; see also Artois et al. 2011). In the last decade, the widespread availability of novel techniques, such as high-resolution microscopy and gene sequencing, allows for a better comparison at morphological and/or genetic levels of specimens from distant areas, and have stimulated interest in the taxonomy, phylogeny and biogeography of these marine worms (e.g., Curini-Galletti et al. 2012; Hochberg et al. 2014; Hummon 2011; Kieneke et al. 2012, 2013a; Kolicka et al. 2015; Leasi and Todaro 2009; Schuster et al. 2017; Todaro et al. 2015a). Increasing information comes especially from areas of the world new or relatively new with regard to the gastrotrich faunistic investigation (e.g., Pacific US: Hummon 2010a; Brazil: Araujo et al. 2014; Hochberg 2014; Todaro 2012, 2013; Garraffoni et al. 2016b; Caribbean: Araujo et al. 2015; Atherton 2014; Hummon 2010b; Kieneke et al. 2013b, 2015, Kånneby et al. 2014, Todaro and Leasi 2013, Todaro et al. 2014; South Africa: Todaro et al. 2011, 2015b; Near East: Hummon 2011), but, even from the well-studied Europe, recent studies have brought about important novelties (e.g., Sweden: Willems et al. 2009; Poland: Hummon 2008, Kolicka et al. 2014; Britain, Ireland, France and the Azores: Hummon 2008; Italy: Dal Zotto et al. 2010; Hummon and Todaro 2009).
In this framework of exciting new data, it is pity that information on the gastrotrich fauna from some biogeographic crucial regions remains very scarce. In Europe, for example, prior to investigation in Lanzarote, only 7 species have been reported in print from Spain (Giere 1979; Marotta et al. 2008; Rothe and Schmidt-Rhaesa 2010; Todaro et al. 2003a, b). Spain, including the Canary archipelago, hosts an extreme varied fauna made up of cold, temperate-cold and warm-subtropical elements (e.g., Marina et al. 2015). Consequently, a better knowledge of the Spanish gastrotrich fauna could shed some light for example on the origin and evolution of the Mediterranean gastrotrich assemblage, which currently appears to be one of the most diversified in the world (Todaro et al. 2003a). The Mediterranean Sea is the largest and deepest enclosed basin on Earth and is a marine biodiversity hot spot (Coll et al. 2010). It has had a multifaceted geological history, including isolation from the world oceans, which led to its near drying out during the Messinian crisis and to severe changes in sea level and salinity (Hsü 1983). The recent marine biota of the Mediterranean Sea is primarily derived from the Atlantic Ocean, through the Strait of Gibraltar, and includes the persistent descendants of the cold, temperate, and subtropical immigrants that invaded the basin over the various climate eras (Coll et al. 2010). The knowledge of the gastrotrichs of Spain (at large) could shed light on which of the different species that currently inhabit the Mediterranean basin belong to these three components (cold, temperate or subtropical).
The aim of this research is to assess the diversity and distribution of gastrotrich species along the coasts of the Canary island of Lanzarote (Spain). The study is part of the 1st International Workshop on Marine and Anchialine Meiofauna held in Lanzarote in October 2011 and represents the most extensive investigation on the Gastrotricha from Spain to date. A short account of the results has been proposed in Riera and Todaro (2012).
In a larger framework, additional information about the poorly understood segment of the fauna from the Macaronesia region should contribute to future debate on the global biogeography of the Gastrotricha and in particular of the Mediterranean fauna. Descriptions of clearly new or putative new taxa are beyond the scope of the present article, and will be presented in forthcoming papers. However, since one of the new species bears a special relevance, e.g., for the ongoing debate on the phylogeny of the peculiar genus Urodasys, we provide its description here and the formal taxonomic affiliation.
Materials and methods
Sampling collection and processing
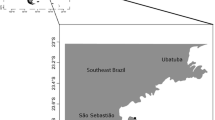
The meiofauna sampling campaign took place in October 2011 and included several locations and stations along the different sides of Lanzarote (Canary Islands, Spain). The gastrotrich species reported here were found in littoral and sublittoral samples collected from seven locations and 16 stations along the eastern coast of the island (Fig. 1). For the purpose of the present article, a location is a locality with a name on a map and/or identifiable with geographic coordinates, while stations are the specific points of a location where the samples were taken, in general differentiated by water depth. Littoral samples were collected during low tide, by digging several 3- cm-deep holes at the mid-water mark, and transferring the sand with a spoon to a 500-ml plastic jar. Bulk sublittoral samples were taken at 1.5–35 m depth using 0.5- to 2.0-L jars or plastic bags. Samples below 5 m were taken by scuba diving, and the shallower samples by skin diving. In general, no special permission/permits were needed to collect these animals as gastrotrichs are microscopic, non-pathogenic organisms; field studies did not involve endangered species and sampling was carried out on public beaches. A permit to collect at site number 5, inside a submarine lava tube, was granted by the Government of the Canary Islands. Additional details regarding collecting dates, sampling procedures and characteristics of the investigated microhabitats will be provided in a forthcoming general paper; to avoid confusion, here the numbering of locations and stations is maintained in accordance with the information reported in the general paper.
Locations (and stations) along the east coast of Lanzarote (Canary Islands, Spain) where gastrotrichs were found. Playa del Caleton Blanco (1); Punta Jameos de Agua (5, 36, 37); Playa de Orzola (7, 27); Mala (10, 13, 16, 17, 30); Arrieta, playa de la Garita, (18); Playa del Papagayo (29); Puerto del Carmen, playa Chica (32, 34, 35)
After each collecting trip, the sandy material was brought as soon as possible to the field laboratory (Aula de Naturaleza de Máguez, in Lanzarote) and processed within 1 week from collection; from each sampling station, up to 300 ml of sediment were processed for the gastrotrich study, in many cases sediment derived from containers shared with colleagues studying other meiobenthic organisms. Gastrotrichs were extracted from the sediment by the narcotization–decantation technique, using a sea-water isosmotic (7%) magnesium chloride solution. The fauna-containing supernatant was then poured directly into a 5-cm-diameter Petri dish and scanned for specimens under a Wild M3 dissecting microscope (Leasi and Todaro 2009).
Species examination and identification
When located, gastrotrichs were picked out with a micropipette, mounted on glass slides, and observed in vivo with Nomarski differential interference contrast optics using a Leitz Dialux 20 microscope equipped with a DS–5 M Nikon digital camera. During observation, the animals were photographed with a DS-Fi1 Nikon digital camera and measured with the Nikon NIS-F v.4.0 software. Specimens were affiliated to: (1) known species, if their morpho-metric characteristics matched those reported in the literature for the taxon, (2) new species (n. sp.) if their anatomical traits appear to be clearly unique, and (3) putative new species (sp1, sp2, sp3) if the data gathered so far are not sufficient to grant their affiliation to species already described (see Tables 1, 2). As the finding of a new species of Urodasys brings important novelties to the vivid debate on the phylogeny of the genus, we decided to anticipate here the description and the formal affiliation of this taxon. The description of the new species follows the convention of Hummon et al. (1993), whereas the position of some morphological characteristics along the body are given in percentage units (U) of total body length measured from anterior to posterior. The rationale for the key to the ecological characteristics of the species, according to Hummon et al. (1992), is as follows: frequency of a species from among a sample series (i.e., frequency of a species in samples collected in any given sampling trip): sparse, found in less than 10% of samples; occasional, found in 10–30% of samples; common, found in 30–60% of samples; usual, found in more than 60% of samples. Abundance of a species among other species of a sample: rare, less than 1% of a sample; scarce, 3–5% of a samples; numerous, 10–20% of a sample (often a sub-dominant); prevalent, more than 30% of a sample (usually dominant or co-dominant).
Granulometric analysis
The study was performed on 50–100 g of sediment for each sample following the general procedure of Buchanan (1984). Dried samples (for 24 h at 60 °C) were sieved through an 8-sieve column in an Octagon D200 test sieve shaker for 1 h. Granulometric parameters were obtained with the RYSGRAN package for R (Camargo 2006) following the method of McCammon (1962). Sediment size and sorting classes are based on Wentworth tables (Wentworth 1922).
Phylogenetic analysis
To shed light on the position of the new species within the Urodasys phylogenetic branch, seven traits regarding the reproductive apparatus organs composition and layout, and the reproductive condition were coded in 17 taxa (Tables 3, 4). Sixteen species of Urodasys constituted the in-group whereas Macrodasys meristocytalis Evans, 1994 was used as the out-group in order to determine character transformation within the Urodasys evolutionary lines. Urodasys bucinastylis Fregni, Faienza, Grimaldi, Tongiorgi & Balsamo, 1999, U. uncinostylis Fregni, Tongiorgi & Faienza, 1998 and U. toxostylus Hummon, 2011 were assumed to conform to the other stylet-bearing species with regard to the coded traits (see Fregni et al. 1998, 1999). Phylogenetic analysis was carried out using PAUP* (Swofford 2002; v.4.0a150 for 32-bit Microsoft Windows) using parsimony as the optimal criterion. Parsimony analyses were run using a full heuristic search strategy; the characters, all unordered, were equally weighted. Nodal support was assessed by a bootstrap analysis performed using 1000 replicates (heuristic search) and summarized in a 50% majority-rule consensus tree. We limited the morphological matrix to reproductive structures because they are the most conspicuous features of the group and have been the base of the previous hypothesis regarding relationships among the species of the genus (see Fregni et al. 1999; Atherton and Hochberg 2014). On the other hand, we would like to stress that other somatic characters (e.g., regarding the external anatomy) in Urodasys are difficult to interpret, and either seem parsimoniously uninformative or concern a lot of meristic features demanding specific analyses, as well as the examination of many specimens, usually not available. Macrodasys meristocytalis was chosen as the outgroup because it summarizes the reproductive characteristics of the genus Macrodasys, which is currently systematized with Urodasys in the family Macrodasyidae.
Results and discussion
Gastrotrich diversity
Collection from 7 locations (16 stations/sites) along the east coast of Lanzarote yielded 61 species for a total of 96 records (species × site; Tables 3, 4). These species belonged to 27 genera and 11 families within the orders Macrodasyida (36 species, 18 genera, 7 families) and Chaetonotida (25 species, 9 genera, 4 families). Locations such as Mala and Playa de Caletón Blanco displayed the highest species richness with 31 and 17 species, respectively.
Thanks to our survey the particularly minute paucitubulate gastrotrichs are reported for the first time from the Canary islands. The suborder Paucitubulatina is cosmopolitan and comprises half of all marine gastrotrichs; thus, to find some of its representatives in Lanzarote should not come as a surprise. In our samples, paucitubulate chaetonotidans account for some 39% of the total species (Table 2). In this, Lanzarote shares some similarities with areas of the world where gastrotrichs have been studied to a good extent and where members of the suborder account for about 40% of the total gastrotrich fauna, e.g., Italy 154 spp., 59% M and 41% C (Todaro et al. 2008), Belgian coast (37 spp., 62% M, 38% C; Jouk et al. 1992) and Sweden (54 spp., 66% M and 34% C; Willems et al. 2009; Todaro et al. 2010 and unpublished). A higher percentage of chaetonotidans is reported for the Gulf of Mexico (45 spp., 53% M and 47% C, Todaro et al. 1995) and the Caribbean island of St John (70 spp., 50% M and 50% C, Hummon et al. 2010). So far only in Greece do the chaetonotidans outnumber macrodasyidans (63 spp., 21 M and 42 C; Hummon and Roidou 1995).
Most of the species found during the current survey have been recorded only once or twice and consequently appear to have a restricted distribution in Lanzarote; exceptions are two chaetonotidans, Aspidiophorus marinus and Chaetonotus apolemmus, found from north to the south of the investigated coastline.
The investigation carried out at Lanzarote increased tremendously the number of gastrotrich species and genera previously reported from Spain, from 7 to 61 species and from 7 to 27 genera respectively. Thirty-two are known species while 29 appear to be new species or putatively so (Tables 1, 2). One such new species is described here (see below), while the formal affiliation of the others will be made at the end of the ongoing taxonomical survey and published in forthcoming papers along with a description of the most common species.
Of the 32 known species, Oregodasys cirratus and Tetranchyroderma canariense were both described from Tenerife (Rothe and Schmidt-Rhaesa 2010; Todaro et al. 2003b) and so far appear to be endemic to the Canary archipelago, while the other 30 species are also present in other nearby geographic areas, e.g., the Mediterranean Sea (Todaro et al. 2003a) and/or the North European coasts (e.g., Dewarumez et al. 2002; Hummon and Warwick 1990; Jouk et al. 1992; Remane 1927; Willems et al. 2009). More specifically, 28 species found in Lanzarote are in common with the Mediterranean (Urodasys mirabilis and Aspidiophorus marinus are missing from the Mediterranean basin) and 22 are shared with the North European coasts (Lepidodasys unicarenatus, Thaumastoderma mediterraneum, Urodasys acanthostylis, Chaetonotus apechochaetus, C. apolemmus, C. siciliensis, Heterolepidoderma loricatum, and Musellifer delamarei are missing from the North European coasts).
From a species distribution point of view, only a few records really stand out, as the finding in Lanzarote extends the geographic range of some species. For instance, Musellifer delamarei and Urodasys acanthostylis were so far known only from the Mediterranea Sea (Italian coasts), while Urodadys mirabilis was known for the North Sea and the Atlantic coast of France. However, in a biogeographic framework involving the origin of the Mediterranean gastrotrich fauna, the contingent of species found also in Lanzarote but missing from the northern European regions assumes particular relevance since it makes most likely the hypothesis that sees them as part of the temperate/warm fauna that invaded the Mediterranean basin after the Missinian crisis. Further investigations should support or falsify such a hypothesis
Taxonomy
Order Macrodasyida Remane, 1925 [Rao and Clausen, 1970]
Family Macrodasyidae Remane, 1924
Genus Urodasys Remane, 1926
Urodasys completus sp. nov.
urn:lsid:zoobank.org:act:CD495226-8801-4662-AB01-9E470A3EFB5C
Examined material. Morphological data of Urodasys completus sp. nov. is derived from six adult specimens observed under DIC optics. The holotype, LT = 297 μm excluding the tail, is illustrated in Figs. 3, 4 (International Code of Zoological Nomenclature, Articles 73.1.1, 73.1.4); all the six physical specimens are no longer extant. Five further identified specimens were fixed in alcohol and are kept in the author’s collection for future DNA analyses.
Type locality. The sediment sample was collected on 14 October 2011 from off Playa Chica, Puerto del Carmen (Lanzarote; 28°50′ 37″N, 13°46′ 53″W). The sediment, made up of shell fragments mixed with small amounts of mud, was collected by A. Martínez and M. Curini Galletti at 29–31 m depth inside the marine cave La Catedral (site 32, Fig. 1), filling a plastic bag by hand.
Etymology. The specific epithet completus (Latin word for “complete”, “perfect”) alludes to the reproductive system composed of all the structures/organs known to occur among species of the genus Urodasys.
Diagnosis. Body elongate, up to 297 μm in length (tail excluded), and rather narrow, up to 45 μm in width, flattened ventrally and vaulted dorsally; epidermic glands barely visible. Cuticular covering smooth, devoided of scales and/or spines. Head bluntly ovate, with sparse circumcephalic cilia but deprived of pestle organs. A pair of peculiar, flexible, rod-like organs, about 23 μm in length and 2.5–3 μm in diameter, are visible along the dorsolateral sides of the pharyngeal region. Body width relatively uniform, narrowing gradually in the hind-gut region toward the elongate tail. Numerous sensory hairs of different length occur around the head, others arise in 2–3 columns on each lateral and dorsolateral side of the body. Ventral locomotor ciliature forms a continuous field from under the head to the pharyngo-intestinal junction and then continuing back to the posterior trunk as paired bands. Anterior adhesive tubes (TbA), 3–4 per side, forming diagonal columns, which insert directly on the body surface at some distance from the mouth. Ventral adhesive tubes (TbV), absent; ventrolaterla adhesive tubes (TbVL), 9 per side, one along the pharyngeal region and the remaining along the intestinal region. Lateral adhesive tubes (TbL), three per side, two along the pharyngeal region and one along the intestinal region. Dorsal adhesive tubes (TbD), and dorsolateral adhesive tubes (TbDL) absent. Numerous, additional adhesive tubes are distributed asymmetrically along the tail. Mouth terminal, quite narrow, leading to a shallow and slightly cuticularized buccal cavity; pharynx up to 40 μm long and 12 μm wide; pharyngeal pores at some distance from the pharynx base, with ventrolateral openings. Pharyngo-intestinal junction (PhIJ) at about U47. Intestine is broadest ateriorly, narrowing to the rear; anus missing. Testes bilateral. Each testis starts behind the PhIJ and extends posteriorly into the sperm duct. At the rear of the frontal organ, both sperm ducts fused on the mid-ventral plane, opening externally into a common pore, with anteriormost region posterior to the PhIJ and extending as sperm ducts back to the rear of the frontal organ where they fuse on the mid-ventral plane to empty externally via a common pore. Mature sperm cells are about 20 μm long, with the anterior portion corkscrew-shaped and the posterior portion rod-like. Female gonads probably paired; left ovary with oocytes maturing in a caudo-cephalic direction with largest egg dorsal to the mid intestine. Frontal organ, sac-like, dorsal to the intestine at about U79; rather small, ovoidal in shape and completely filled with spermatozoa in individuals after copulation, but elongate and with a mass of spermatozoa agglutinated in form of a golf-club (a spermatophore?) in individuals soon after copulation. A clear anatomical-functional compartmentalization (i.e. subdivided into spermatheca and seminal receptacle regions) was not observed, neither the internal nor the external pore being seen. Caudal organ appearing as an oval capsule that encloses a hyaline elongated bulblet on the left side and a sclerotic stylet on the right side. The stylet resembles a narrow and elongated mouth funnel proximally, and a curved syringe needle, distally. The proximal portion of the stylet is anatomically located more posterior to its distal portion.
Description. Based mostly on the adult specimen with a total body length of 297 μm, excluding the tail, shown in Fig. 3a. Body elongate and rather narrow, flattened ventrally and vaulted dorsally; cuticular covering smooth, devoid of scales and/or spines (Figs. 2a, b, 3a). Body width relatively uniform from the pharynx to the anterior trunk region, increasing slightly in the mid-gut, and then narrowing gradually in the hind-gut region to the elongate tail (Figs. 2a, b, 3a). Head bluntly ovate, with sparse circumcephalic cilia but deprived of pestle organs. A pair of peculiar, flexible, rod-like organs, about 23 μm in length and 2.5–3 μm in diameter, are visible along the pharyngeal region originating from the dorsolateral sides, at U25 (Figs. 2a, b, 3a–c). Widths of head\mid-pharyngeal region\PhIJ\mid-trunk\at confluence with the tail are as follows: 29\26\31\34\18 μm at U06\U28\U47\U63\U96, respectively. Epidermal glands barely visible.
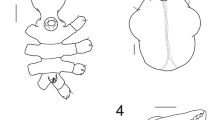
Illustrations of Urodasys completus sp. nov.; for clarity, the long tail has been omitted: a habitus as seen from the dorsal side, showing the internal anatomy with the maturing oocytes, testes, frontal- and caudal organ; b habitus as seen from the ventral side. Bc buccal cavity; CO caudal organ; Eg egg; FO frontal organ; Mo mouth; Ph pharynx; PhIJ pharyngo intestinal junction; PhP pharyngeal pores; RO rod-like organs; RMS round masses of secretory material; St stylet; TbA anterior adhesive tubes; TbL lateral adhesive tubes; TbVL ventrolateral adhesive tubes
Ciliation: Numerous sensory hairs up to 18 μm in length occur around the head, others, up to 25 μm, arise in 2–3 columns on each lateral and dorsolateral side of the body. Ventral locomotor ciliature forms a continuous field from TbA (U05) to the PhIJ (U47) then continuing back to the rear of the caudal organ (U95) as paired bands (Fig. 2b).
Adhesive tubes: TbA, 3–4 per side, 6–18 μm long, forming diagonal columns, which insert directly on the body surface, at some distance from the oral opening from U04 to U07 (Fig. 2b); TbV, absent; TbVL, 9 per side, 8–10 μm long; one along the pharyngeal region at U12 and the remaining eight more or less evenly spaced along the intestinal region (Fig. 2b); TbL, 3 per side, 6–9 μm long, two along the pharyngeal region (at U21 and U39 respectively) and one along the intestinal region at U77. TbD and TbDL apparently absent. Numerous, additional adhesive tubes are distributed asymmetrically along the tail.
Digestive tract: Mouth terminal, quite narrow, 11 μm in diameter, leading to a shallow (12 μm in length) and slightly cuticularized buccal cavity (Figs. 2, 3a); pharynx is 140 μm long, measured from the frontal edge, slightly increasing in width from anterior (9 μm) to posterior (12 μm); pharyngeal pores, sub-basal at U41, with ventrolateral openings (Fig. 2a). Pharyngo-intestinal junction at about U47 (Fig. 2a). Intestine is broadest in front, narrowing to the rear, but lacks an anus (Figs. 2a, 3a).
Reproductive tract: hermaphroditic; bilateral testes with their anterior-most region just passed the PhIJ and extending as sperm ducts back to the rear toward to the frontal organ where they fuse on the mid-ventral plane and apparently empty externally via a common pore (Figs. 2a, 3d, 4; see remarks below). The right testis is slightly smaller and begins slightly posterior to the left one. Mature sperm are about 20 μm long, its anterior portion is corkscrew-shaped while the posterior portion is rod-like (Fig. 4d). Female gonads probably paired, though right ovary was not seen; left ovary showing oocytes maturing in a caudo-cephalic direction with largest egg, 42 μm long and 16 μm wide, dorsal to the mid-intestine, centered at U67 (Figs. 2a, 3a). Frontal organ dorsal to the intestine (Figs. 2a, 4b), centered at U79; in the holotypic specimens it appeared rather small, sac-like (i.e. the wall not muscolarised), about 18 μm long and 15 μm wide; it was completely filled with spermatozoa and without a clear anatomical-functional compartmentalization (i.e. not subdivided into spermatheca and seminal receptacle regions); neither the internal nor the external pore were observed. Caudal organ (at U91) appearing as an oval capsule (25 μm long and 16 μm wide) that encloses a hyaline elongated bulblet on the left side and a sclerotic stylet on the right side (Figs. 2a, 4, 5). The bulblet is wider in front and narrower in the back; it is filled with globular masses of refringent material and bears a luminal continuity with the proximal portion of the sclerotized stylet (Figs. 2a, 5). The stylet has the proximal portion in the form of a narrow and elongated funnel while the distal portion resembles a curved syringe needle (Figs. 2a, 4, 5). Surprising, the proximal portion of the stylet is anatomically located more posterior to its distal portion. Anterior to the caudal organ are two round masses of secretory material connected at the ventral mid-line; the appearance of this material is similar to the refringent droplets seen inside the bulblet of the caudal organ and may explain the origin of the latter; however, a luminal connection between the two masses and the caudal organ has not been observed.
Urodasys completus sp. nov. from Lanzarote. DIC microscopy: a, b internal anatomy of the posterior body region at different focal planes showing the paired testes (arrows) and the caudal organ (arrowhead); c close-up of the frontal organ filled with allosperm; d close-up of a single auto spermatozoon
Urodasys completus sp. nov. from Lanzarote. DIC microscopy: a, b internal anatomy of the posterior body region at different focal planes, showing the caudal organ (arrow) and its internals structures; c close-up of the caudal organ showing the stylet (arrowhead) and bulblet (arrow); d composite reconstruction of the sclerotic stylet (arrowhead); white lines indicate portions of the figures (taken at different focal planes) that contributed to the reconstruction of the entire stylet
Ecology. Frequency of occurrence: occasional in coarse sublittoral sediment (20%); abundance: prevalent (> 30% of a sample, dominant); in intertidal at a water depth of 29–31 m in coarse (0.8 phi), poorly sorted (1.49 phi) carbonate sand (kurtosis = 1.94; skewness = −0.05). Values of salinity and temperature of the interstitial water at the time of sampling were 33‰ and 18 °C respectively.
Variability and remarks on general morphology. The total body length of the measured adult specimens (i.e. showing a large egg and/or a stylet) ranged from 245 μm to 297 μm (mean = 275 μm ± 19 SD, n = 6); maximum body width varied from 29 μm to 45 μm (mean = 35 μm ± 6 SD, n = 6). The number and, to a lesser extent, the arrangement of the adhesive tubes belonging to the different series varied among the observed specimens; in particular, two of the animals showed four TbA per side where the number of TbVL ranged from 6 to 10 and TbL 6 to 10 per side. In general, the number of these adhesive tubes was not strictly related to the body length, e.g., the longest specimen showed only 3 TbA and 9 TbVL per side, whilst the shortest had 4 TbA and 6 TbVL per side; moreover, in some specimens, the lateral tubes actually seemed to originate dorsolaterally instead. Some differences were also noted with regard to the reproductive structures. More specifically, a 285-μm-long specimen showed a frontal organ whose morphology and content (Fig. 6a) were rather different from the ones described for the holotypic and most of the other adult specimens. In particular, in this specimen, the frontal organ appeared rather large and elongated in place of the small and roundish structure seen in other specimens; furthermore, in place of the homogeneously distributed allosperm, inside it was a mass of spermatozoa agglutinated in form of a golf-club, whose head was located in the posterior region of the organ and the shaft obliquely oriented from the body midline to the left side (cf. Fig. 4c vs. Fig. 6a). Regardless of the shape, the bundle of spermatozoa recorded in the frontal organ of this specimen may be considered homologous to the spermatophore recently described for a potential new Urodasys species from Florida (Atherton and Hochberg 2014). In another specimen, 240 μm in body length, the stylet showed additional outlets/chambers along the distal portion (Fig. 6b). Very likely, the observed disparities represent normal morphological variation and/or normal, time-variable aspects of the functional reproductive biology of the species, which are visible only at a certain life stage and for a short period of time. In this hypothetical framework, the spermatozoa of a specimen, for example, may be injected in the frontal organ of the partner as a spermatophore, like the one seen in Fig. 6a, but later on they become free from each other, hence occupying the entire lumen of the frontal organ, as shown, e.g., in Fig. 4c. Most of the sperm-carrying specimens possessed also a mature egg dorsal to the mid intestine; but, unfortunately, their ovaries were not seen. All the observed specimens carried the paired, flexible rod-like organs along the pharyngeal region, a feature that appears to be unique among Gastrotricha. Very likely, these structures represent sensorial organs, perhaps similar in function to the drum-stick organs found in the cephalodasyid Pleurodasys helgolandicus Remane, 1927 for which a sensorial function as graviceptors has recently been demonstrated (Marotta et al. 2008).
Urodasys completus sp. nov. from Lanzarote. DIC microscopy: a internal anatomy of the posterior region of a mature specimen showing the stylet (arrowhead) and the frontal organ containing a golf-club shaped bundle of allosperm interpretable as a spermatophore (arrow); b internal anatomy of the posterior region of a different specimen showing the stylet with an additional chamber (arrowhead) and the frontal organ filled with homogenously distributed allosperm. Variation borne by the stylet and the frontal organ may be interpreted as normal morphological variation and/or normal, time-variable aspects of the functional reproductive biology of the species (see text for details)
Taxonomic remarks
The genus Urodasys currently includes 15 described species (Atherton 2014; Hummon 2011; Hummon and Todaro 2010). These species can easily be subdivided into three groups based on their reproductive condition and presence/absence of various reproductive organs; in short: Group 1, including hermaphroditic species lacking a sclerotic stylet (4 spp); Group 2, formed by hermaphroditic species possessing a sclerotic stylet, and Group 3, formed by Urodasys viviparus. Specimens of this last species possess ovaries but lack testicles and accessory reproductive structures; they reproduce by parthenogenesis and, unique among Gastrotricha, give birth by ovoviviparity (see also Kieneke and Schmidt-Rhaesa 2014).
Urodasys completus sp. nov., in virtue of its hermaphroditic condition and possession of a sclerotic stylet, is most similar to the ten species that make up the Group 2. The ten stylet-bearing species are: U. acanthostylis Fregni, Tongiorgi and Faienza 1998; U. bucinastylis Fregni, Faienza, Grimaldi, Tongiorgi and Balsamo 1999; U. calicostylis Schöpfer-Sterrer, 1974; U. cornustylis Schöpfer-Sterrer, 1974; U. nodostylis Schöpfer-Sterrer, 1974; U. poculostylis Atherton, 2014; U. remostylis Schöpfer-Sterrer, 1974; U. spirostylis Schöpfer-Sterrer, 1974; U. toxostylus Hummon 2011; and U. uncinostylis Fregni, Tongiorgi and Faienza 1998 (see Tables 3 and 4). From all of them the new species can be easily discerned because it bears two testes, while the others have either a single testis or none altogether (U. bucinastylis and U. toxostylus). The peculiar shape of the stylet and the number and distribution of the adhesive tubes may further separate U. completus sp. nov. from the above mentioned taxa. Furthermore, another important diagnostic feature distinguishes the new species from all of its congeners: the paired flexible, rod-like organs present along the pharyngeal region (e.g., Fig. 3a–c). Never before organs like these were reported among species of the genus Urodasys, nor among species of the entire phylum, making the new species even more unique.
Phylogeny
Our analysis found two most parsimonious trees, each of a length of 11 steps, whose topologies appeared extremely similar, and similar or equal to the topology of the consensus tree shown in Fig. 7. In both original trees, the species involved in the analysis appeared distributed into two main clades according the possession/lacking of a sclerotic stylet (see Tables 3 and 4 for additional characters and character states). The two trees also agreed in showing Urodasys completus sp. nov. as an early divergent line along the evolutionary branch of the stylet-bearing species, and in finding U. viviparus allied with the species lacking a sclerotic stylet. However, one of the trees found U. viviparus as an early divergent line along the evolutionary branch of the stylet-lacking species while the other tree found U. viviparus unresolved within this clade. Topology of the latter tree was equal to that of the consensus tree shown in Fig. 7.
Consensus tree (50% Majority-Rule) of 16 Urodasys species (ingroup) and 1 Macrodasys species (outgroup) based on 7 characters regarding the reproductive apparatus organs composition and layout, and the reproductive condition. Values at nodes represent bootstrap percentages based on 1000 replications (tree length = 11 steps; consistency index = 1.0; retention index = 1.0). U. completus sp. nov. is shown as an early divergent line of the branch that includes the hermaphroditic species bearing a sclerotic stylet (group 2). The parthenogenetic Urodasys viviparus is shown in alliance with the hermaphroditic species lacking a sclerotic stylet (group 1). The full heuristic serch found two most parsimonious trees of equal length (11 steps), which were very similar in topology. One of the trees had the same topology as the consensus tree while the other showed U. viviparus as an early divergent line of the branch that included the species lacking a stylet.
The phylogenetic relationships among species of the genus Urodasys have recently been investigated based on molecular data (Atherton and Hochberg 2014). In that study, the 33 investigated specimens, belonging to eight potential species, were found to be distributed in two main groups. Group 1 (named clade I), including species with paired testes and ovaries but lacking accessory sexual organs (e.g., a stylet), and Group 2, comprising the remaining species. Species belonging to Group 2 were subdivided into two subclades (named clade II and clade III) according to their reproductive condition and reproductive system organization. The hermaphroditic, stylet-bearing species formed clade II, while the parthenogenetic U. viviparus Wilke, 1954, represented by several specimens collected in different islands of the Caribbean Sea and in Brazil, formed clade III (see fig. 3 in Atherton and Hochberg 2014).
The phylogenetic results based on a molecular marker by Atherton and Hochberg (2014) were congruent with a previous hypothesis by Fregni et al. (1999), who, based on the recognizable organization/composition of the reproductive system of the species known at that time, envisioned an empirical evolutionary scenario according to which the genus Urodasys could be divided into two evolutionary lines. In short, an evolutionary line that includes species with paired gonads, but without accessory sexual organs, while the other line includes species bearing a stylet. Hypotheses about the reduction and/or loss of organs occurred among the species included in each of the two lines were put forward in the evolutionary scenario proposed by Fregni et al. (1999). Furthermore, it was acknowledged that, in the depicted framework, the parthenogenetic Urodasys viviparus could have originated from either one of the two lineages (see Fregni et al. 1999). The study by Atherton and Hochberg (2014) recovered the two evolutionary lines envisioned by Fregni et al. (1999), but Urodasys viviparus was found in a sister-group relationships with the stylet-bearing species.
The two aforementioned studies differ at least in another important point, i.e., the plesiomorphic organization of the reproductive system in Urodasys. According to Fregni et al. (1999), the ancestor of the extant Urodasys had paired male and female gonads, a frontal organ and a caudal organ furnished with a stylet. Atherton and Hochberg (2014) agreed on this general set-up except that, in their hypothesized evolution of the reproductive system within Urodasys, the hypothetical ancestor of the extant species had a caudal organ lacking a stylet (see Fig. 4; Atherton and Hochberg 2014).
Urodasys completus sp. nov., in possessing two testes and a caudal organ supplied with a sclerotic stylet, seems to match the characteristics of the Urodasys ancestor hypothesized by Fregni et al. (1999). However, the phylogenetic analysis performed in the present study clearly shows U. completus sp. nov. in a more derived position (Fig. 7). More specifically, the new species appears as an early divergent line along the evolutionary branch of the stylet-bearing taxa. On the other hand, a quick thought at the organization of the reproductive system of the new species makes it very unlikely that it could occupy the basal-most position along the evolutionary tree of the genus Urodasys, at least in the way this taxon is currently recognized. In any case, regardless of the position that the new species could occupy along the genus phylogenetic tree, its traits indicate that the sequence of the evolutionary transformations that have occurred in the reproductive system of the species of Urodasys is likely dissimilar from the ones proposed by different authors thus far. Finally, we acknowledge that, in contrast to the results of Atherton and Hochberg (2014), in our tree, U. viviparus appears in alliance with the stylet-lacking species. The inclusion of the new species in future cladistics analysis based on molecular traits should better inform on the transformation events concerning the reproductive system that took place during the evolution of these fascinating animals.
Conclusion
Lanzarote hosts a rich and diversified gastrotrich fauna as testified by the fact that more than 60 species were found in about 10 working days. In such a short period of time, not all the potentially gastrotrich rich locations could be investigated. Consequently, we anticipate that a higher number of species may be discovered in the island if, in future, additional localities, especially those on the western coast, will be investigated.
At this time, 15% of the species found in Lanzarote appear new to science; this percentage could rise to 45% if all the specimens still under investigation prove to belong to undescribed taxa. We speculate that the final figure of new species from Lanzarote will be about 25–30% of the total. While these statistics appear well below the percentage of putative new species found in remote areas investigated for the first time (e.g., up to 80% of new species in Brazil and Kuwait; see Todaro in Appeltans et al. 2012), the magnitude of new species found in this small island is equally impressive considered the relatively short distance of Lanzarote from the well-studied areas such as the Mediterranean Sea and the North European shores (e.g., Hummon 2008; Todaro et al. 2003b).
Whether new to science or already known from other geographical regions, many of the species found in Lanzarote bear a significance beyond their simple contribution to the global biodiversity of the island. For instance, Urodasys completus sp. nov. also has a phylogenetic relevance, as its complete set of reproductive structures allows for better inferences about the ancestral character pattern, and the possible transformations of these traits occurred during the evolution of this cosmopolitan and easy to identify gastrotrich genus. On the other hand, in a biogeographic framework involving the origin of the Mediterranean gastrotrich fauna, assumed relevant species include Chaetonotus apechochaetus, C. apolemmus, C. siciliensis, Heterolepidoderma loricatum, Lepidodasys unicarenatus, Musellifer delamarei, Thaumastoderma mediterraneum, and Urodasys acanthostylis. The finding at Lanzarote of these species lets us hypothesize that they are part of the temperate/warm fauna that invaded the Mediterranean basin after the Missinian crisis, during the periods in which the planet experienced a generalized increase of temperatures (see “Introduction”).
Beside the supplementary information obtained so far on the morphology and/or biology of some species, additional data are expected to emerge during the taxonomic survey still ongoing and when the specimens stored for ultrastructural and DNA analyses are studied. In this regard, it is worth mentioning that the recent ultrastructural study conducted on specimens of Megadasys sterreri found at Lanzarote contributed to the resystematization of the genus Megadasys, allowing its transfer to the family Planodasyidae from the original Cephalodasyidae (Guidi et al. 2014). Finally, it should be emphasized that many species were recovered from deep sediments (below 10 m water depth), usually neglected in studies on Gastrotricha, a clear indication for the future faunistic researches regarding these fascinating little creatures.
References
Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N et al (2012) The magnitude of global marine species diversity. Curr Biol 22:2189–2202
Araujo TQ, Atherton S, Hochberg R (2015) First record and a description of a new species of Oregodasys (Gastrotricha: Macrodasyida: Thaumastodermatidae) from Tobago. Proc Biol Soc Wash 128:176–185
Araujo TQ, Balsamo M, Garraffoni ARS (2014) A new species of Pseudostomella (Gastrotricha, Thaumastodermatidae) from Brazil. Mar Biodiv 44:243–248
Artois T, Fontaneto D, Hummon WD, McInnes SJ, Todaro MA, Sørensen MV, Zullini A (2011) Ubiquity of microscopic animals? Evidence from the morphological approach in species identification. In: Fontaneto D (ed) Biogeography of microscopic organisms: is everything small everywhere? Cambridge University Press, Cambridge, pp 244–283
Atherton S (2014) Urodasys poculostylis sp. nov., a new stylet-bearing gastrotrich (Macrodasyida) from Capron shoal, Florida. Mar Biol Res 5:530–536
Atherton S, Hochberg R (2014) The evolution of the reproductive system of Urodasys (Gastrotricha: Macrodasyida). Invert Biol 133:314–323
Balsamo M, Todaro MA (2002) Gastrotricha. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater Meiofauna: Biology and Ecology. Backhuys, Leiden, pp 45–61
Buchanan JB (1984) Sediment analysis. In: Holme NA, McIntyre AD (eds) Methods for the study the marine benthos, vol 16. Blackwell, London, pp 41–65
Camargo MG (2006) Sysgran: um sistema de codigo aberto para analises granulometricas do sedimento. Rev Bras Geosci 345:345–352
Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, Estrada M, Froglia C, Galil BS, Gasol JM, Gertwagen R, Gil J, Guilhaumon F, Kesner-Reyes K, Kitsos MS, Koukouras A, Lampadariou N, Laxamana E, Carlos M, de la Cuadra L, Lotze HK, Martin D, Mouillot D, Oro D, Raicevich S, Rius-Barile J, Saiz-Salinas JI, San Vicente C, Somot S, Templado J, Turon X, Vafidis D, Villanueva R, Voultsiadou E (2010) The biodiversity of the Mediterranean Sea: estimates, Patterns, and threats. PLoS ONE 5(8):e11842
Coull BC (1985) Long-term variability of estuarine meiobenthos: an 11 year study. Mar Ecol Prog Ser 24:205–218
Curini-Galletti M, Artois T, Delogu V, De Smet WH, Fontaneto D, Jondelius U, Leasi F, Martinez A, Meyer-Wachsmuth I, Nilsson KS, Tongiorgi P, Worsaae K, Todaro MA (2012) Patterns of diversity in soft-bodied Meiofauna: dispersal ability and Body size matter. PLoS ONE 7(3):e33801
Dal Zotto M, Ghiviriga S, Todaro MA (2010) A new Tetranchyroderma (Gastrotricha, Thaumastodermatidae) with triancres from the Mediterranean Sea. Meiofauna Mar 18:41–48
Dewarumez JM, d’Hondt JL, Hummon WD (2002) Faune et Flore du littoral du Pas de Calais et de la manche orientale. Mise àjour de la liste des espèces de Gastrotriches et de Céphalorhynques. Rev Trav Stat Mar Wimereux 24:7–9
Egger B, Lapraz F, Müller S, Dessimoz C, Girstmair J, Skunca N, Rawlinson KA, Cameron CB, Beli E, Todaro MA, Gammoudi M, Noreña C, Telford MJ (2015) A transcriptomic-Phylogenomic analysis of the evolutionary relationships of flatworms. Curr Biol 25:1–7
Fregni E, Tongiorgi P, Faienza MG (1998) Two new species of Urodasys (Gastrotricha, Macrodasyidae) with cuticular stylet. Ital J Zool 65:377–380
Fregni E, Faienza MG, Grimaldi-De Zio S, Tongiorgi P, Balsamo M (1999) Marine gastrotrichs from the Tremiti archipelago in the southern Adriatic Sea, with the description of two new species of Urodasys. Ital J Zool 66:183–194
Garraffoni ARS, Di Domenico M, Amaral ACZ (2016a) Patterns of diversity in marine Gastrotricha from southeastern Brazilian coast is predicted by sediment textures. Hydrobiologia 773:105–116
Garraffoni ARS, Di Domenico M, Hochberg R (2016b) New records of marine Gastrotricha from São Sebastião Island (Brazil) and the description of a new species. Mar Biodivers. doi:10.1007/s12526-016-0486-1
Giere O (1979) The impact of oil pollution on intertidal meiofauna. Field studies after the la Coruña-spill, may 1976. Cah Biol Mar 20:231–251
Gray JS (1971) The effects of pollution on sand meiofauna communities. In: Zavodnik D (ed) Proceedings of the Sixth European Symposium on Marine Biology, Rovinj, Croatia, Yugoslavia, September 27–October 2, 1971. Thalass Jugosl 7:79–86
Guidi L, Todaro MA, Ferraguti M, Balsamo M (2014) Reproductive system and spermatozoa ultrastructure support the phylogenetic proximity of Megadasys and Crasiella (Gastrotricha, Macrodasyida). Contr Zool 83:119–131
Hochberg R (1999) Spatiotemporal size-class distribution of Turbanella mustela (Gastrotricha: Macrodasyida) on a northern California beach and its effect on tidal suspension. Pacific Sci 53:50–60
Hochberg R (2014) Crasiella fonseci, a new species of Gastrotricha (Macrodasyida, Planodasyidae) from São Paulo, Brazil. Mar Biodivers 44:237–242
Hochberg R, Atherton S, Kieneke A (2014) Marine Gastrotricha of little Cayman Island with the description of one new species and an initial assessment of meiofaunal diversity. Mar Biodivers 44:89–113
Hsü KJ (1983) The Mediterranean was a desert. Princeton University Press, Princeton
Hummon WD (1969) Musellifer sublitoralis, a new genus and species of Gastrotricha from the San Juan archipelago, Washington. Trans Am Microsc Soc 88:282–286
Hummon WD (2008) Gastrotricha of the North Atlantic Ocean: 1. Twenty four new and two redescribed species of Macrodasyida. Meiofauna Mar 16:117–174
Hummon WD (2010a) Marine Gastrotricha of San Juan Island, Washington, USA, with notes on some species from Oregon and California. Meiofauna Mar 18:11–40
Hummon WD (2010b) Marine Gastrotricha of the Caribbean Sea: a review and new descriptions. Bull Mar Sci 86:661–708
Hummon WD (2011) Marine Gastrotricha of the near east: 1. Fourteen new species of Macrodasyida and a redescription of Dactylopodola agadasys Hochberg, 2003. Zookeys 94:1–59
Hummon WD, Roidou E (1995) Marine Gastrotricha of Greece: a preliminary report. Biol Gallo-Hellen 22:279–289
Hummon WD, Todaro MA (2009) Italian marine Gastrotricha: VI. Seven New Species Macrodasyida. Zootaxa 2278:47–68
Hummon WD, Todaro MA (2010) Analytic taxonomy and notes on marine, brackish-water and estuarine Gastrotricha. Zootaxa 2392:1–32
Hummon WD, Warwick RM (1990) The marine flora and fauna of the isles of Scilly - Gastrotricha. J Nat Hist 24:519–525
Hummon WD, Balsamo M, Todaro MA (1992) Italian marine Gastrotricha: I. Six new and one redescribed species of Chaetonotida. Boll Zool 59:499–516
Hummon WD, Todaro MA, Tongiorgi P (1993) Italian marine Gastrotricha: II. One new genus and ten new species of Macrodasyida. Boll Zool 60:109–127
Hummon WD, Todaro MA, Kånneby T, Hochberg R (2010) Marine Gastrotricha of the Caribbean Sea. Proc. 14th International Meiofauna Conference, Gent
Jouk PEH, Hummon WD, Hummon MR, Roidou E (1992) Marine Gastrotricha from the Belgian coast: species list and distribution. Bull Inst R Sci Nat 62:87–93
Kånneby T, Todaro MA (2015) The phylogenetic position of Neogosseidae (Gastrotricha:Chaetonotida) and the origin of planktonic Gastrotricha. Org Div Evol 15:459–469
Kånneby T, Atherton S, Hochberg R (2014) Two new species of Musellifer (Gastrotricha: Chaetonotida) from Florida and Tobago and the systematic placement of the genus within Paucitubulatina. Mar Biol Res 10:983–995
,Kieneke A, Schmidt-Rhaesa A (2014) Gastrotricha. In: Schmidt-Rhaesa A (ed) Handbook of zoology. Vol.3, Gastrotricha and Gnathifera. De Gruyter, Berlinn
Kieneke A, Martinez Arbizu P, Fontaneto D (2012) Spatially structured populations with a low level of cryptic diversity in European marine Gastrotricha. Mol Ecol 21:1239–1254
Kieneke A, Rothe BH, Schmidt-Rhaesa A (2013a) Record and description of Anandrodasys agadasys (Gastrotricha:Redudasyidae) from lee Stocking Island (Bahamas), with remarks on populations from different geographic areas. Meiofauna Mar 20:39–48
Kieneke A, Narkus S, Hochberg R, Schmidt-Rhaesa A (2013b) Diplodasys rothei n. Sp. (Gastrotricha, Macrodasyida), a new marine gastrotrich species from the Bahamas. Meiofauna Mar 20:49–61
Kieneke A, Schmidt-Rhaesa A, Hochberg R (2015) A new species of Cephalodasys (Gastrotricha, Macrodasyida) from the Caribbean Sea with a determination key to species of the genus. Zootaxa 3947:367–385
Kolicka M, Kisielewski J, Kotwicki L, Zawierucha K, Grzelak K (2014) Checklist of Gastrotricha of the polish Baltic Sea with the first reports of Heterolepidoderma joermungandri Kånneby, 2011, and Turbanella hyalina Schultze, 1853. Zootaxa 3869:101–130
Kolicka M, Jankowska E, Kotwicki L (2015) Baltic Sea Gastrotricha-one new species and one new record of Chaetonotida from Poland. Zootaxa 4027:487–508
Leasi F, Todaro MA (2009) Meiofaunal cryptic species revealed by confocal microscopy: the case of Xenotrichula intermedia (Gastrotricha). Mar Biol 156:1335–1346
Leasi F, Todaro MA (2010) The gastrotrich community of a north Adriatic Sea site, with a redescription of Musellifer profundus (Chaetonotida: Muselliferidae). J Mar Biol Assoc UK 90:645–653
Marina P, Rueda JL, Urral J, Salas C, Gofas S, García Raso JE, Moya F, García T, Lóopez-González N, Laiz-Carrión BJ (2015) Sublittoral soft bottom assemblages within a marine protected area of the northern Alboran Sea. J Mar Biol Assoc U K 95:871–884
Marotta R, Todaro MA, Ferraguti M (2008) The unique gravireceptor organs of Pleurodasys helgolandicus (Gastrotricha: Macrodasyida). Zoomorphology 127:111–119
McCammon RB (1962) Efficiencies of percentile measures for describing the mean size and sorting of sedimentary particles. J Geol 70:453–465
Remane A (1927) Gastrotricha. In: Grimpe G (ed) Die Tierwelt der Nord- und Ostsee. Akademische Verlag, Leipzig
Riera R, Todaro MA (2012) Check list of gastrotrichs from the Canary Islands. Rev Acad Canar Cienc 24:81–88
Rothe BH, Schmidt-Rhaesa A (2010) Oregodasys cirratus, a new species of Gastrotricha (Macrodasyida) from Tenerife (Canary Islands), with a description of the muscular and nervous system. Meiofauna Mar 18:49–66
Schuster J, Atherton S, Todaro MA, Schmidt-Rhaesa A, Hochberg R (2017) Redescription of Xenodasys riedli (Gastrotricha:Macrodasyida) based on SEM analysis, with first report of population density data. Mar Biodivers. doi:10.1007/s12526-017-0667-6
Struck TH, Wey-Fabrizius AR, Golombek A et al (2014) Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Mol Biol Evol 31:1833–1849
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer, Sunderland
Todaro MA (1998) Meiofauna from the Meloria shoals: Gastrotricha, biodiversity and seasonal dynamics. Biol Mar Medit 5:587–590
Todaro MA (2012) A new marine gastrotrich from the State of São Paulo (Brazil), with a key to species of Pseudostomella (Gastrotricha, Thaumastodermatidae). ZooKeys 223:39–51
Todaro MA (2013) A new non-naked species of Ptychostomella (Gastrotricha) from Brazil. Zookeys 289:13–24
Todaro MA (2017) Marine. In: Todaro MA (ed) Gastrotricha World Portal. “http://www.gastrotricha.unimore.it/marine.htm” Accessed on 14 February 2017
Todaro MA, Leasi F (2013) A new eye-bearing Macrodasys (Gastrotricha:Macrodasyida) from Jamaica. Meiofauna Mar 20:33–38
Todaro MA, Rocha CEF (2004) Diversity and distribution of marine Gastrotricha along the northern beaches of the state of São Paulo (Brazil), with a description of Macrodasys fornerisae sp. nov. (Macrodasyida, Macrodasyidae). J Nat Hist 38:1605–1634
Todaro MA, Tongiorgi P (2017) Freshwater. In: Todaro MA (ed) Gastrotricha World Portal. http://www.gastrotricha.unimore.it/freshwater.htm. Accessed on 14 February 2017
Todaro MA, Balsamo M, Tongiorgi P (2008) Gastrotricha. In: G Relini (ed) Checklist della flora e della fauna dei mari italiani. Biol Mar Medit 15(suppl.):159–168
Todaro MA, Fleeger JW, Hummon WD (1995) Marine gastrotrichs from the sand beaches of the northern Gulf of Mexico:species list and distribution. Hydrobiologia 310:107–117
Todaro MA, Ancona P, Marzano A, Gallo D’Addabbo M, De Zio GS (2003a) A new Tetranchyroderma species (Gastrotricha, Macrodasyida, Thaumastodermatidae) from the Canary Islands (Spain). Cah Biol Mar 44:191–197
Todaro MA, Matinato L, Balsamo M, Tongiorgi P (2003b) Faunistics and zoogeographical overview of the Mediterranean and Black Sea marine Gastrotricha. Biogeographia 24:131–160
Todaro MA, Kånneby T, Jondelius U (2010) Marine Gastrotricha from Sweden. Proc. 14th International Meiofauna Conference, Gent
Todaro MA, Dal Zotto M, Perissinotto R, Bownes SJ (2011) First records of Gastrotricha from South Africa, with description of a new species of Halichaetonotus (Chaetonotida, Chaetonotidae). Zookeys 142:1–13
Todaro MA, Perissinotto R, Bownes SJ (2013) Neogosseidae (Gastrotricha, Chaetonotida) from the iSimangaliso Wetland Park, KwaZulu-Natal, South Africa. Zookeys 315:77–94
Todaro MA, Leasi F, Hochberg R (2014) A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Syst Biodivers 12:473–488
Todaro MA, Perissinotto R, Bownes SJ (2015a) Two new marine Gastrotricha from the Indian Ocean coast of South Africa. Zootaxa 3905:193–208
Todaro MA, Dal Zotto M, Leasi F (2015b) An integrated morphological and molecular approach to the description and systematisation of a novel genus and species of Macrodasyida (Gastrotricha). PLoS ONE 10(7):e0130278
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Willems WR, Curini-Galletti MC, Ferrero TJ, Fontaneto D, Heiner I, Huys R, Ivanenko VN, Kristensen RM, Kånneby T, MacNaughton MO, Martínez Arbizu P, Todaro MA, Sterrer W, Jondelius U (2009) Meiofauna of the Koster-area, results from a workshop at the Sven Lovén Centre for Marine Sciences (Tjärnö, Sweden). Meiofauna Mar 17:1–34
WoRMS (2017) Statistics in: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=stats. Accessed 01 March 2017
Acknowledgements
Many thanks to the colleagues and students who made extremely enjoyable the stay on the Island. Special appreciations go to the chief collectors: Marco Curini Galletti, Alejandro Martínez, Maikon Di Domenico and Tom Artois. The senior author expresses sincere gratitude to Katrine Worsaae and Alejandro Martínez who invited him to the workshop, which was superbly organized. Additional funding was provided by UNIMORE (TIOME project) to MA Todaro. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Finally, we thank two anonymous reviewers for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Martínez García
This article is a contribution to the Topical Collection Interstitial and Cave Diversity in Atlantic Oceanic Islands
This article is registered in ZooBank under urn:lsid:zoobank.org:pub:14F6D378-BB90-413E-B674-39B017C9D7D0.
Rights and permissions
About this article
Cite this article
Todaro, M.A., Cesaretti, A. & Dal Zotto, M. Marine gastrotrichs from Lanzarote, with a description of a phylogenetically relevant species of Urodasys (Gastrotricha, Macrodasyida). Mar Biodiv 49, 2109–2123 (2019). https://doi.org/10.1007/s12526-017-0747-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0747-7