Abstract
Nemertesia ramosa (Lamarck, 1816) is a large eurybathic hydrozoan species. Its habitat and spatial distribution in the Azores archipelago (central North Atlantic) are described based on 22 new records collected in situ by scuba diving and by observations using a drop-down camera and remote operated vehicles (ROVs) between 2004 and 2011. N. ramosa grows on hard substrates in mono and multi-specific assemblages. In the Azores it is well known in the sublittoral at 15–158 m depth in the central group of islands, but historical records exist from seamounts down to bathyal grounds of nearly 1000 m. Scuba diving surveys generally reveal N. ramosa as an occasionally occurring and rare species in the infralittoral (1–9 colonies 10−3 m−2), although it may also be common and even abundant at some sites. A circalittoral aggregation was assessed in detail exhibiting densities of up to 2.82 colonies m−2 on rocky substrate and one order of magnitude lower on mixed bottom (0.25–0.55 colonies m−2). An aggregated spatial distribution is described, with aggregations being considered hydroid gardens (>1–9 10−1 m−2). Colonies measured on ROV imagery averaged 23.5 cm in height (STD = 4.1). Taller colonies (max = 36.7 cm) were registered on rocky outcrops protruding more than 15 cm. N. ramosa co-occurred mainly with brown and red algae, bryozoans, polychaetes, and other hydroids. A diverse ichthyofauna was associated to these habitats. Circalittoral communities of N. ramosa included hydrozoans, sponges, and fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The description of the assemblages consisting of sessile invertebrates is crucial for the understanding of benthic ecosystems by providing a baseline for the classification of habitats, which is an integral part of any nature information system (Greene et al. 1999; Connor et al. 2003; Davies et al. 2004). Hydrozoa are cnidarians, usually growing in colonial forms with the polyps connected to a hydrocauli. Sessile hydrozoans can reach considerable densities (Williams 1976), and tall colonies reach a few dozens of centimetres. They have been shown to characterize and dominate certain shelf habitats, standing out as habitat-building macrofauna (Strong et al. 2012; Tempera et al. 2013). As knowledge of circalittoral epibenthic assemblages grew in result of the increasing utilization of remote surveying techniques, these biotopes were assigned to their own habitat classes. Specifically, the EUNIS habitat classification (http://eunis.eea.europa.eu) has integrated the class “Facies with large hydrozoa”, whilst CMECS (Madden et al. 2009) integrated “Attached Hydroids” and “Soft Sediment Hydroids” communities.
Nemertesia ramosa (Lamarck, 1816) is a large plumulariid hydroid (Order: Leptothecata). Its distribution ranges from Iceland, the Faeroes and Scotland (Broch 1918), down to South Africa, including the southeast coast of Africa (Millard 1975). The species is eurybathic, with records ranging from sublittoral areas (Picton and Morrow 2010) to depths of 1,182 m off Morocco (Ramil and Vervoort 1992). It is a conspicuous element of animal turf communities on several locations in Britain and Ireland (Porter 2012), but habitats dominated by N. ramosa have never been reported in detail.
N. ramosa has been reported from shelf areas at <200 m depth around the islands (Rees and White 1966; Cornelius 1992), as well as from seamount samples collected at bathyal depths (Calder and Vervoort 1998). It was first reported in the Azores from two stations at Faial-Pico Channel during the surveys of Prince Albert 1st of Monaco in the late 1800s (Pictet and Bedot 1900). Cornelius (1992) compiled the existing records in 1989, including a previous collection by Patzner south of Faial Island, and added new observations close to Faial Island obtained by scuba divers from around 30 m depth and by a remote underwater video camera operated at depths of ca. 100 m for which no precise locality data is given. Calder and Vervoort (1998) collected two N. ramosa with a remote operated vehicle at 788 and 919 m depth, from seamounts in the Azores region. These records are, however, somewhat questionable as both are small and their hydrocladia are biserial instead of multiserial in arrangement (Calder and Vervoort 1998). Recently, further samples were collected from Faial-Pico Channel by fishing vessels (Moura et al. 2012).
N. ramosa is one of several species belonging to the genus Nemertesia that occur in the Azores. Other species include N. belini, N. norvegica, and at least three cryptic species sharing morphological affinities with N. antennina (Moura et al. 2011). N. ramosa is a conspicuous hydrozoan, and one of the tallest branching hydroid species in the Azores. Its appearance makes it is macroscopically distinguishable from regional congenerics (see the “Material and methods” section for more details), as well as from other large hydrozoan species such as Polyplumaria flabellata (fan-shaped) or Lytocarpia myriophyllum (tall feather-like plumes).
Samples collected by classical methods, like dredging, usually yield more than one colony, suggesting the existence of N. ramosa aggregations. In situ observations have previously reported aggregations of several colonies (e.g. Cornelius 1992), but no further details were given.
This paper provides 22 new records for the species, including several locations with a large number of N. ramosa colonies. Details of a deep circalittoral aggregation of N. ramosa is provided, along with a list of co-occurring species.
Material and methods
The Azores is an archipelago in the northeast Atlantic composed of nine islands situated at latitudes ranging between 37°N and 40°N (Santos et al. 1995). The islands spread across an extent of 617 km and are surrounded by narrow shelves and a rich submarine topography dominated by over 400 seamount-like features (Morato et al. 2008) and, more distally, abyssal plains extending to depths exceeding 5,000 m.
Surveys conducted by scuba diving, drop-down camera, and remote operated vehicles (ROVs) in the Azores archipelago between 2004 and 2011 were analyzed to investigate the habitat and distribution of N. ramosa. Densities of N. ramosa and co-occurring organisms were estimated using the SACFOR scale for species with sizes >15 cm (Hiscock 1996). Abundances recorded from ROV SP where estimated using the ROV field of view (Porteiro et al. 2013), as detailed below.
Identification of N. ramosa was based on phenotypic characters, using: i) the long, sparse, and irregularly branched main stems; ii) whorled three-dimensional side branches in groups of six angled upward; and iii) the close correspondence with the descriptions and illustrations of N. ramosa from (Ramil and Vervoort 1992; Porter 2012). Other phenotypic characters considered where (i) the species large size, (ii) its prominent branching, and (iii) the yellowish colouration. The two latter characters generally distinguish it from the N. aff. antennina group of species. Colonies were hand-collected during scuba dives and kept in the reference collection of the Dept. of Oceanography and Fisheries of the University of the Azores (COLETA).
Scuba dives between 15–40 m performed in the scope of projects MARÉ, OGAMP, and MARMAC recorded N. ramosa on coastal areas around the islands of Faial, São Miguel, and Santa Maria. Dives were geo-referenced using (i) a hand-held GPS deployed on the support boat or (ii) "transit marks" and site proxies as described in the scuba diving survey protocol of Holt and Sanderson (2001).
Underwater surveys were performed with a drop-down camera between 30–60 m comprising a Tritech MD4000 video camera mounted on a metal frame and suspended from M/V Jonas using a cable containing coaxial and LED light control elements. Approximate camera position was obtained from a hand-held GPS on deck (±50 m accuracy).
Several locations around Faial Island and Faial-Pico channel were also surveyed with a VideoRay Explorer Mini-ROV between 30–60 m, operating from the R/L Águas Vivas in 2004. The video survey was georeferenced using a hand-held GPS on deck (±80 m accuracy).
The ROV SP (SeaBotix LBV300S-6; IMAR-DOP/UAz), was operated between 80–255 m from the R/L Águas Vivas. The ROV was equipped with a colour camera (570 line/02 Lux, 4:3), with four lights installed externally on the four front corners (480 Lumen each) and a scaling two-point laser system (5 cm apart) that was used to estimate sizes. ROV SP was positioned with a Subsea Micron Nav USBL (Ultra Short Baseline) unit fitted onto the vehicle in the responder mode. The navigational data were manually filtered for outliers and smoothed with a moving average, to remove false loops. The moving average allowed adjusting the resulting dive track, which was aided by visualizing the ROV behaviour on the recorded video. Finally, the track was splined to obtain coordinates for every second.
The surveyed area was estimated by multiplying the distance travelled on the seafloor (x, y) with the average field of view. With a tilt range of 270° and panning achieved by moving the ROV itself, the camera orientation can change during the dive while the seafloor and its features are being explored. The average field of view was obtained by measuring the image width when the scaling lasers were projected on the seafloor. Measurements of video stills were repeated each 5 s of the dive, with the image analysis software ImageJ (1.46r). As the field of view can vary considerably, an average value was estimated for each transect as well as the surveyed area (total average field of view 1.96, SD = 0.4; see information on transects below).
The video was annotated for the presence of N. ramosa and other large megafauna (>10 cm), with the Customizable Observation Video Image Recorder - COVER (v0.7.2, Ifremer; Carré 2010). The seafloor geomorphology was classified in four categories: "Gravel and Sand", "Sand-Dominated Mixed", "Rock-Dominated Mixed", and "Hard Bottom". "Sand-Dominated Mixed" comprises unconsolidated sediments, i.e. gravel and sand, covering >50 % of seafloor (not taken into account if they consist of only a very thin layer). "Rock-Dominated Mixed" comprises areas with hard substrate (i.e. rocky bottom, boulders, cobbles, and pebbles; Wentworth 1922) representing >50 % of seafloor coverage. Hard bottom are areas of consolidated ground only.
The density of N. ramosa (colonies m−2) was estimated for several transects developed during one ROV dive (11CF_RSP018), and colony size was measured whenever possible. Dive sections were considered transects every time the ROV was transiting close to the seafloor with a good visibility and covering a minimum of 5 m2. Twenty transects were considered for analysis, including areas before and after the colonies were observed (Fig. 2). Surveyed areas by each transect varied between 5.3–38.2 m2 (average 16.8 m2, STD = 6.2), 00:30-01:17 min:s (average 00:48) and an average distance of 8.9 m (STD = 3.9).Colonies growing at seafloor level, or on outcrops protruding <15 cm from the seafloor, were compared with colonies growing on rocky outcrops protruding >15 cm from seafloor to evaluate possible differences in colony heights. The Wilcoxon–Mann–Whitney two-sample rank-sum test (two-tailed, p < 0.05) was used to test for differences in this parameter. The probability of N. ramosa having an equal abundance on different bottom types was tested with Kruskal-Wallis using transects T5-T17 (the first and last transect where the species was observed; see the “Results” section). Subsequently, the abundance of N. ramosa between different bottom types were evaluated using t-tests. All statistical analyses were performed using Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA). The coefficient of dispersion was calculated as CD = s2 μ−1, where s is the standard deviation and μ the observed average (Bliss 1958), to ascertain the type of distribution: random (CD = 1), uniform (CD < <1), or aggregated (CD> > 1).
Results
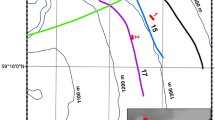
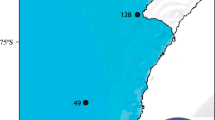
We report 22 new records of Nemertesia ramosa on different locations around Faial island and in the Faial-Pico channel as illustrated in Fig. 1. New records were compiled with a list of previous records after Cornelius (1992), and are provided in Table 1. This raises the total amount of records of N. ramosa to 31, ranging in depth mostly from the infralittoral (15 m) to the deep circalittoral (158 m).
Locations of Nemertesia ramosa records in the Azores Archipelago. Locations of historical samples are based on site descriptions and are not necessarily accurate, except for 8 and 13–31. The asterisk indicates the approximate locality for samples labelled as “Faial-Pico Channel” 1–3, 5, and 9. Bathymetric contours are spaced at 500 m depth intervals in the left figure, and 100 m in the right figure
During infralittoral scuba diving surveys, N. ramosa was observed around Faial Island and Faial-Pico Channel at three sites, at 15–36 m depth. Occurrences on the three locations were considered as occasional and rare.
The drop-down camera recorded a mono-specific facies of N. ramosa in the Faial-Pico Channel, east of the Espalamaca headland at 50–60 m depth (Table 1; Fig. 2). The seafloor was dominated by rocky outcrops mixed with gravelly sand. Colonies were recorded as soon as the DDC reached the bottom, during 11:15 min:s, until the DDC transect reached an area of continuous unconsolidated sediment. A total of 302 colonies were counted, being abundant on every rocky outcrop in the area (i.e., 1–9 colonies m−2). N. ramosa was also encountered in high densities on a communications cable lying on the seafloor.
Nemertesia ramosa from infralittoral and circalittoral depths in the Azores Archipelago. a–c small number of colonies recorded by Mini-ROV (a sample 5, b sample 16, c sample 29); d–f large number of colonies on hard substrate recorded by drop-down camera (DDC20); g–i circalittoral aggregation recorded by ROV SP, showing colonies on the edge of rocky outcrops, Anthias anthias and Serranus atricauda (g–h transect 9), and close-up view of tall colonies with an epibiont crinoidea (i); see Table 1 for sample numbers and information
The Mini-ROV recorded N. ramosa at 16 sites, at 30–60 m depth (Table 1; Fig. 2). Densities varied from occasional, where several colonies were observed, to rare, where only isolated or very few colonies were identified on the seabed.
Density estimates on infralittoral areas collected by scuba diving, drop-down-camera, and mini-ROV reveal mostly occasional species with colonies ranging between 1–9 10−3 m−2 (40 %), but also rare species in some locations (30 %) (Fig. 3). In the remaining 30 % of the sites N. ramosa was frequent, common or even abundant, reaching densities of 1–9 m−2.
Density estimates of Nemertesia ramosa using the SACFOR scale, on 20 infralittoral surveys from SCUBA diving (3), drop-down camera (1), and Mini-ROV (16) in the Azores Archipelago (see Table 1 for more details)
The infralittoral conspicuous biota occurring together with N. ramosa on rocky areas recorded during 16 Mini-ROV dives at 30–60 m depth included nearly 100 taxa (Tables 1, 2 in ESM). The most abundant sessile species were the brown algae Halopteris filicina, Dictyota spp., and Zonaria tournefortii, Delesseriacea red algae, and encrusting Corallinacea; several sponges, particularly Clathrina lacunosa, the bryozoan Smittina cervicornis, the polychaethes Hermodice carunculata, and Sabella spallanzanii. Several Hydrozoa also occurred frequently in the same location as N. ramosa, particularly Aglaophenia spp. and Sertularella sp., as well as scleractinian corals such as Antipathella spp. and Caryophyllia sp. The infralittoral ichthyofauna was diverse (Table 2, ESM), with the labrids Coris julis and Bodianus scrofa as the most frequently observed species followed by Boops boops. Mullus surmulentus, Scorpaena scrofa, Diplodus sargus, and Muraena helena were also frequently observed in the area. In the drop-down camera survey fewer species were identified, with Zonaria tournefortii, Caryophillia sp. and a purple encrusting sponge as the only sessile invertebrates identified from imagery. The fish species most frequently observed were Serranus atricauda and the micronectivorous Anthias anthias and Thalassoma pavo.
Exploratory dives in the circalittoral with ROV SP on the southern sector of the Faial-Pico Channel yielded records of two facies with N. ramosa at 118–158 m depth (see Table 1 and Table 3 in ESM for more details).
On dive 11CF_RSP018 a total of 62 colonies were identified in eight transects (T5, T7-11, T13, and T17) (Table 3 in ESM; Figs. 2 and 4), comprising a surveyed area of 109 m2. A strong tidal current was reflected on the colonies, as seen in Fig. 2i. Colony height averaged 23.5 cm (STD = 4.1). The seafloor was composed of a mixture of outcrops of consolidated sedimentary rock and unconsolidated sediment covered by bioclastic material (e.g. made by bryozoans). The rocky layer showed several crevices, protruding from the seafloor level up to ca. 40 cm in some locations. Small and medium boulders occurred throughout, appearing to be broken sections of the rocky underground. The sedimentary origin of the hard substrate and heavy colonization by encrusting sponges and other fauna gave it a very rough appearance. The "Sand-dominated mixed" area was composed mostly of small boulders, pebbles, shells, and other bioclasts, alternated with gravel.
Hydroids growing on rocky outcrops protruding more than 15 cm from the surrounding seafloor averaged 25.3 cm height (min., 16.2; max., 36.7 cm; STD = 5.1; N = 11). Colonies growing near the seafloor averaged 22.2 cm height (18.7–26.7 cm; STD = 2.5; N = 15). The difference between colonies at both locations is marginally significant (Mann–Whitney U = 126, p = 0.0257; two-tailed test). Before crossing the area colonized by N. ramosa, the ROV SP traversed ca. 40 m over a seabed composed of unconsolidated sediment, pebbles, and small boulders. The area traversed after the hydroid aggregation was identical, suggesting that N. ramosa colonies occurred in a rocky area surrounded by unconsolidated sediment and smaller rocks.
Densities of N. ramosa were different according to the geological setting of each transect (p < 0.05; Kruskal-Wallis). The largest density was recorded on a small area of (strictly) rocky bottom, with an average density of 2.82 m−2 (min.: 2.6, max.:3.1; corresponding to Abundant in the SACFOR scale). In areas of mixed seafloor types, N. ramosa were common, with densities of 0.25 m−2 for sand-dominated mixed and 0.55 m−2 for rock-dominated mixed substrates (Fig. 5). The differences were actually not significant (T-test; p = 0.12), except for the rocky area (p < 0.05). The coefficient of dispersion CD = 1.45 implies an aggregated spatial distribution that could be described by a negative binomial distribution (Bliss 1958). However the small sample size hampered further investigations (Heip 1976). In general, many of the colonies observed (but not all), were close to other conspecific colonies, forming small patches on the rocks, being often closer than each other’s height. In the occasions the ROV approached the seabed, several colonies were observed growing in close proximity (i.e., < 5 cm in some cases; Fig. 2).
On dive 11CF_RSP023, five colonies were identified in a step-like large outcrop. The step ‘face’ was ca. 2 m tall, protruding from unconsolidated sediment, and hosted a great diversity of sessile invertebrate fauna. A small area of the outcrop was inspected by the ROV, with the transect developed perpendicularly to the outcrop (step). N. ramosa, including large colonies, were observed mostly on the edge of the outcrop, but also on the vertical wall. Colony size and density were not estimated. Two fishing lines were observed in the vicinity.
In both locations several co-occurring species were identified with the ROV SP. These were mostly sponges, such as Haliclona implexa, Auletta sessilis, Axinella vasonuda, cf. Pseudotrachya histrix, Petrosia sp. (N = 1), and several encrusting species. Other hydrozoans include Polyplumaria flabellata, Lytocarpia myriophyllum, some observations of Aglaophenia sp., cf. Sertularella gayi, and several other small-sized, undetermined species. Alcyonacean corals included Alcyonium sp. and Viminella flagellum. Several unidentified bryozoans were present, comprising a great portion of the bioclastic material. An unidentified crinoid was observed perched on the colonies tall branches (Fig. 2i).
The ichthyofauna at dive 11CF_RSP018 was characterized by several schools of Callanthias ruber (N > 50) and Anthias anthias (N = 20–30). Several Lappanella fasciata and Serranus atricauda were observed (N < 5), also Conger conger (N = 1), Pontinus kuhlii (N = 1), Scorpaena scrofa (N = 3), and Scorpaena sp. (N = 2) were seen in the vicinity of the hydroids. Serranus atricauda was observed chasing Callanthias ruber on one occasion, an agonistic behaviour with possible predatory purposes. On the gravel and sandy bottom, an Aulopus filamentosus was observed. At dive 11CF_RSP023 S. atricauda and Anthias anthias where the most frequently observed species.
Discussion
Nemertesia ramosa colonies show an aggregated spatial distribution in the Azores, occurring mainly in low densities throughout infralittoral and circalittoral rocky bottoms. Aggregated distribution is a common distribution pattern of many sessile marine invertebrates (Underwood and Chapman 1996), often resulting from a compromise between energy uptake and the necessity for proximity for reproductive purposes (Heip 1975). Nemertesia species release short-lived planulae, like many deep-water hydroids (Henry et al. 2008), which represent the only mobile stage of these species (Hughes 1977). Williams (1976) found that settled larvae form aggregated clusters between neighbouring individuals. Close proximity is required to achieve routine cross-fertilisation, and once planulae are released, long-distance dispersal away from the natal conspecific colonies should be highly limited (Williams 1976). Similar spatial dispersion patterns in deep-water coral ecosystems were also associated to philopatric larval settlement of planuae releasing species, such as with Eudendrium hydroids (Henry et al. 2013).
The preference of N. ramosa for rocky bottom shown in this work agrees with previous observations from the coasts of Britain and Ireland (Porter 2012). In addition, it was seen that colonies growing on outcrops and boulders protruding more than 15 cm from the surrounding seafloor were taller than colonies growing in smaller rocky outcrops. It is known that roughness of the substrate increases turbulent transport, which combined with reduced flow close to the seabed (Frechette et al. 1989) may drive benthic suspension feeders to settle and develop in more protruding locales.
A diverse number of deep-water coral taxa aggregations are now termed “coral gardens” (a classification derived originally from the resemblance of gorgonian-dominated habitats to “trees” in the cold-water environment, Andrews et al. 2002; OSPAR 2008). Consequently, the term hydroid gardens can be applied to aggregations of large hydrozoa. Like corals, hydroids also form three-dimensional habitats (Porter 2012; Tempera et al. 2013), and support associated species (Hughes 1986). Recent studies proposed that such classification should be applied only to areas where the density of coral colonies is ten times higher than a defined threshold value (Bullimore et al. 2013). N. ramosa was considered a rare and occasional species (1–9 10−3 m−2), which can be used as a threshold value for the species in the Azores. However, based on the imagery analysed in this study and the circalittoral community studied in detail, we propose that colony densities of 1–9 m−2 or higher should be considered hydroid gardens of N. ramosa.
Patchy and aggregated communities are more sensitive to anthropogenic impacts such as physical disturbance from dredge deposits, fishing or anchoring activities (Thrush et al. 1998) since localized impacts may affect entire gene pools (Sommer 1992). Large hydroids (here considered colonies >15 cm height) are more sensitive to physical disturbance than small hydroid species that are often reported growing on marine litter or other substrate available (Harms 1990; Gomes-Pereira et al. 2012). In turn, in comparison to corals, hydroids have faster growth rates and shorter life spans (Hughes 1986), than have been reported for scleractinians or stylasterid hydrocorals corals (Andrews et al. 2002). These aspects, as well as reproductive strategies should be addressed in future works, as these might translate into different susceptibilities to human impact and the subsequent need of conservation measures.
Further records of N. ramosa are expected from other island shelves, slopes, and seamounts. Recent studies have revealed a great diversity of hydroids in deep-water coral habitats (Henry et al. 2008). As these habitats extend throughout Atlantic seamounts and are being increasingly studied (Tempera et al. 2012, 2013), knowledge on their diversity and on eurybatic hydroid species is expected to increase. The hydroid gardens reported here were used by a diverse ichthyofauna, and epibiont species have been previously recorded, such as Aglaophenia tubulifera off Faial Island (Cornelius 1992). However, a quantification of the importance of N. ramosa as a habitat-building species remains unaddressed. The ecological processes related to its settlement require further research. Improved sampling methodologies should be utilized to provide more data on the spatial distribution of this species and the extent of communities dominated by large hydrozoans.
References
Andrews AH, Cordes EE, Mahoney MM, Munk K, Coale KH, Cailliet GM, Heifetz J (2002) Age, growth and radiometric age validation of a deep-sea, habitat-forming gorgonian (Primnoa resedaeformis) from the Gulf of Alaska. Hydrobiologia 471:101–110
Bliss C (1958) The analysis of insect counts as negative binomial distributions. Proc 10th Int Congr Entomol, pp 1015–1032
Broch H (1918) Hydroida. (Part II) Danish Ingolf-expedition, vol 5. Zoological Museum of the University, Copenhagen
Bullimore RD, Foster NL, Howell KL (2013) Coral-characterized benthic assemblages of the deep Northeast Atlantic: defining “Coral Gardens” to support future habitat mapping efforts. ICES J Mar Sci 70:511–523
Calder DR, Vervoort W (1998) Some hydroids (Cnidaria: Hydrozoa) from the Mid-Atlantic Ridge, in the North Atlantic Ocean. Zool Verh Leiden 319:1–65
Carré C (2010) COVER - Customizable Observation Video Image Record. User manual v0.8.4, Ifremer
Connor DW, Allen JH, Golding N, Lieberknecht LM, Northen KO, Reker JB (2003) The national marine habitat classification for Britain and Ireland. Joint Nature Conservation Committee, Peterborough
Cornelius PF (1992) The Azores hydroid fauna and its origin, with discussion of rafting and medusa suppression. Arquipel Life Mar Sci 10:75–99
Davies CE, Moss D, Hill MO (2004) EUNIS habitat classification. Revision 2004. European Environment Agency. European Topic Centre on Nature Protection and Biodiversity, Copenhagen
Frechette M, Butman CA, Geyer WR (1989) The importance of boundary-layer flow in supplying phytoplankton to the benthic suspension feeder, Mytilus edulis L. Limnol Oceanogr 34:19–36
Gomes-Pereira JN, Tempera F, Ribeiro P, Porteiro FM (2012) Notes on fauna associated with an opportunistic artificial reef near cold-water corals. Arquipel Life Mar Sci 29:69–75
Greene HG, Yoklavich MM, Starr RM, O’Connell VM, Wakefield WW, Sullivan DE, McRea JE Jr, Cailliet GM (1999) A classification scheme for deep seafloor habitats. Oceanol Acta 22:663–678
Harms J (1990) Marine plastic litter as an artificial hard bottom fouling ground. Helgolander Meeresun 44:503–506
Heip C (1975) On the significance of aggregation in some benthic marine invertebrates. In: Barnes H (ed) Proc. 9th Europ Mar Biol Symp. Aberdeen University Press, Aberdeen, pp 527–538
Heip C (1976) The spatial pattern of Cyprideis torosa (Jones, 1850) (Crustacea: Ostracoda). J Mar Biol Assoc UK 56:179–189
Henry L-A, Nizinski MS, Ross SW (2008) Occurrence and biogeography of hydroids (Cnidaria: Hydrozoa) from deep-water coral habitats off the southeastern United States. Deep-Sea Res I Oceanogr Res Pap 55:788–800
Henry L-A, Moreno Navas J, Roberts J (2013) Multi-scale interactions between local hydrography, seabed topography, and community assembly on cold-water coral reefs. Biogeosciences 10:17885–17912
Hiscock K (1996) Marine Nature Conservation Review: rationale and methods. Joint Nature Conservation Committee, Peterborough
Holt R, Sanderson W (2001) Procedural guideline 3-5: identifying biotopes using video recordings. In: Davies J et al (eds) Natura 2000 marine monitoring handbook, UK Marine SACs Project. Joint Nature Conservation Committee, Peterborough
Hughes R (1977) Aspects of the biology and life-history of Nemertesia antennina (L.) (Hydrozoa: Plumulariidae). J Mar Biol Assoc UK 57:641–657
Hughes R (1986) Differences in the growth, form and life history of Plumularia setacea (Ellis and Solander)(Hydrozoa: Plumulariidae) in two contrasting habitats. Proc R Soc Lond B Biol Sci 228:113–125
Madden C, Goodin K, Allee R, Cicchetti G, Moses C, Finkbeiner M, Bamford D (2009) Coastal and marine ecological classification standard. NOAA and Nature Serv. Available online: http://www.csc.noaa.gov/benthic/cmecs/, 107 pp
Millard NAH (1975) Monograph on the Hydroida of southern Africa. Ann S Afr Mus 68:1–513
Morato T, Machete M, Kitchingman A, Tempera F, Lai S, Menezes G, Pitcher TJ, Santos RS (2008) Abundance and distribution of seamounts in the Azores. Mar Ecol Prog Ser 357:17–21
Moura CJ, Cunha MR, Porteiro FM, Yesson C, Rogers AD (2011) Evolution of Nemertesia hydroids (Cnidaria: Hydrozoa, Plumulariidae) from the shallow and deep waters of the NE Atlantic and western Mediterranean. Zool Scr 41:79–96
Moura CJ, Cunha MR, Porteiro FM, Yesson C, Rogers AD (2012) Evolution of Nemertesia hydroids (Cnidaria: Hydrozoa, Plumulariidae) from the shallow and deep waters of the NE Atlantic and Western Mediterranean. Zoologica Scripta 41(1):79–96. doi:10.1111/j.1463-6409.2011.00503.x
OSPAR (2008) OSPAR list of threatened and/or declining species and habitats. OSPAR Agreement. 2008-06
Pictet C, Bedot M (1900) Hydraires provenant des campagnes de l’Hirondelle, 1886–1888. Résult Camp Scient Prince de Monaco 18:1–59
Picton BE, Morrow CC (2010) Encyclopedia of marine life of Britain and Ireland. http://www.habitas.org.uk/marinelife/species.asp?item=D599. Accessed 23 Oct 2013
Porteiro FM, Gomes-Pereira J, Pham CK, Tempera F, Santos RS (2013) Distribution and habitat association of benthic fish on the Condor seamount (NE Atlantic, Azores) from in situ observations. Deep-Sea Res II Top Stud Oceanogr 98:114–128
Porter J (2012) Seasearch guide to bryozoans and hydrozoans of Britain and Ireland. Marine Conservation Society, UK
Ramil F, Vervoort W (1992) Report on the Hydroida collected by the “ BALGIM ” expedition in and around the Strait of Gibraltar. Zool Verh Leiden 277:3–262
Rees W, White E (1966) New records and fauna list of hydroids from the Azores. Ann Mag Nat Hist 9:271–284
Santos RS, Hawkins S, Monteiro LR, Alves M, Isidro EJ (1995) Marine research, resources and conservation in the Azores. Aquat Conserv 5:311–354
Sommer C (1992) Larval biology and dispersal of Eudendrium racemosum (Hydrozoa, Eudendriidae). Sci Mar 56:205–211
Strong JA, Service M, Plets R, Clements A, Quinn R, Breen J, Edwards H (2012) Marine substratum and biotope maps of the Maidens/Klondyke bedrock outcrops, Northern Ireland. J Maps 8:129–135
Tempera F, Pereira JN, Henriques AB, Porteiro F, Morato T, Matos V, Souto M, Guillaumont B, Santos RS (2012) Cataloguing deep-sea biological facies of the Azores. Rev Investig Mar 19:36–38
Tempera F, Atchoi E, Amorim P, Gomes-Pereira J, Gonçalves J (2013) Atlantic area marine habitats. Adding new Macaronesian habitat types from the Azores to the EUNIS Habitat Classification. Tech Rep No. 4/2013 - MeshAtlantic, IMAR/DOP-UAç, Horta
Thrush S, Hewitt JE, Cummings VJ, Dayton PK, Cryer M, Turner SJ, Funnell GA, Budd RG, Milburn CJ, Wilkinson MR (1998) Disturbance of the marine benthic habitat by commercial fishing: impacts at the scale of the fishery. Ecol Appl 8:866–879
Underwood A, Chapman M (1996) Scales of spatial patterns of distribution of intertidal invertebrates. Oecologia 107:212–224
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Williams G (1976) Aggregation during settlement as a factor in the establishment of coelenterate colonies. Ophelia 15:57–64
Acknowledgments
We acknowledge: Ricardo Serrão Santos, Filipe Porteiro, and Telmo Morato (IMAR-DOP/UAz) for their coordination and support throughout the projects mentioned below; Jorge Fontes, Frederico Cardigos, Vanessa Santos, Jaen Nieto Amat, Ricardo Delgado, and Pedro Frade for their collaboration in the scuba-diving surveys; David Abecasis and Manuela Ramos for their help in the ROV and drop-down camera surveys; Renato Bettencourt for his proficiency in piloting ROV SP; the crews of RV Arquipélago and RL Águas Vivas for their competence during the surveys; the dedicated editorial work by B. W. Hoeksema and detailed revisions from Lea-Anne Henry and one anonymous referee that contributed to improve this manuscript. Data collection and analysis was supported by projects MARÉ (Life-Nature B4-3200/98/509), MAROV (PDCTM/P/MAR/15249/1999), MAYA (AdI/POSI/2003), OGAMP (INTERREG IIIb - MAC/4.2/A2 2001), MARMAC (INTERREG IIIb - 03/MAC/4.2/A2 2004), CORALFISH (FP7 ENV/2007/1/21314 4), CORAZON (FCT/PTDC/MAR/72169/2006) and MeshAtlantic (AA-10/1218525/BF). Frederico Dias as coordinator of Project M@rbis (EMEPC). IMAR-DOP/UAz is Research and Development Unit no. 531 funded by the Portuguese Foundation for Science and Technology (FCT) through pluriannual and programmatic funding schemes (OE, FEDER, POCI2001, FSE) and by the Azores Directorate for Science and Technology (DRCT). JNGP was supported by the doctoral grant from the Regional Directorate for Science, Technology and Communications (DRCTC) of the Regional Government of the Azores (M3.1.2/F/062/2011). Fernando Tempera was supported by a post-doc grant from the Portuguese Foundation for Science and Technology (ref. SFRH/BPD/79801/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. W. Hoeksema
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(DOCX 35 kb)
ESM Table 2
(DOCX 23.1 kb)
ESM Table 3
(DOCX 24.5 kb)
Rights and permissions
About this article
Cite this article
Gomes-Pereira, J.N., Tempera, F. Hydroid gardens of Nemertesia ramosa (Lamarck, 1816) in the central North Atlantic. Mar Biodiv 46, 85–94 (2016). https://doi.org/10.1007/s12526-015-0325-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-015-0325-9