Abstract
Heavy metal contamination in water, soil and crops has become an increasing health problem. The present study aimed to investigate potential health risks to public health by evaluating the mean metal concentrations in water, soil and Cucurbita maxima (pumpkin) samples collected from three different irrigation areas using canal water (CWI), groundwater (GWI) and municipal wastewater (MWI). Concentrations of copper (Cu), lead (Pb), iron (Fe), cobalt (Co), zinc (Zn), cadmium (Cd) and nickel (Ni) in collected soil and vegetable samples were estimated through atomic absorption spectrophotometer. Molybdenum (Mo) level in the specimens was analysed by graphite furnace and the flow injection hydride generation AAS was employed for arsenic (As) and the fluorometric method was used for determination of selenium (Se) contents. The metal concentrations in pumpkin samples were ranged from 7.75 to 9.01, 2.82 to 4.03, 0.43 to 0.74, 34.41 to 41.60, 16.76 to 20.15, 29.87 to 34.15, 6.86 to 8.99, 5.99 to 7.86, 3.55 to 3.91 and 0.13 to 0.16 mg/kg for Mo, As, Se, Fe, Cu, Zn, Ni, Pb, Cd and Co, respectively. Likewise, the concentrations of Pb, Mo and Cd in the pumpkin at all irrigation sites were estimated to be above the respective maximum permissible levels. Arsenic, at the MWI site, posed the highest risk threat (HRI = 77. 34) for public health. The highest pollution load index (PLI) was estimated for Ni (4.21) at the GWI site indicating soil contamination. According to the findings of this study, the amount of potentially toxic metals in all sample waters, especially municipal wastewater, is at a level that may pose a risk to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are a constant part of ecosystems, but their irregular amounts can be dangerous to ecosystems (Ugulu 2015). Plants are directly contaminated with these metals in the rhizosphere by the initial attachment of the metals to the outer tissue surfaces and their subsequent transportation into the internal tissues (Amadi et al. 2018). Different irrigational practices lead to the incorporation of toxic metallic components into the rhizosphere. Irrigational practices employing wastewater result in high depositions of poisonous metals in the soil interfering with its properties and posing serious threats to animals (Amlan et al. 2012; Ugulu et al. 2019). Crop productivity and quality are significantly affected by the accumulation of the heavy metals in soil which potentially gets absorbed into the plants. A high content of toxic metals in vegetables has serious impacts on the consumers in terms of health and food security (Tariq et al. 2021).
Heavy metals are primarily metallic elements that have a high concentration and are reported to be toxic at low concentrations (Karak et al. 2014). In generic terms, the group of elements having five times more atomic density than water or greater than 4 g/cm3 are called heavy metals. During chemical reactions, these metals release electrons to form simple cations and are usually required in some definite trace amounts among all living organisms (Khan et al. 2019). Living organisms are affected badly by both the deficiency and excess of these metals (Xiao et al. 2018). Inorganic metallic compounds characteristically possess the strongest toxicity due to their ability to penetrate internal organs via cellular membranes (Nawaz et al. 2021). Multiple factors like properties and composition of the soil, type of plant species and the kind of metallic element determine the efficacy of translocation of heavy metals (Balkhair and Ashraf 2016).
Pumpkin (Cucurbita maxima Duch.) is an extensively produced and highly consumed vegetable, belonging to the family Cucurbitaceae, due to its nutritional value. Additionally, the medicinal features of pumpkin make it important as a potent remedy for hypertension, diabetes, inflammation, bacterial infections and cancers. It is also known to promote immunological responses (Ugulu et al. 2009). C. maxima production and demand are usually met by wastewater irrigation in peri-urban agricultural lands, while irrigation with heavy metal polluted canal water is also in practice in some other areas. Metal accretion in soil and vegetable can occur through continuous wastewater irrigation contaminating human bodies consequently (Sahin et al. 2016).
In Pakistan, which is an agricultural country, most of the population is associated with agriculture and related sectors (Qadir et al. 2015). This situation makes the studies on agriculture and agricultural ecology in the region even more important. This study on the pumpkin, which is consumed in the country for many purposes, especially for nutritional and medicinal purposes, is also important in this respect. Most recently, no study has been conducted on heavy metal accretion in pumpkins and its effect on human health. Therefore, this study was designed to investigate the heavy metal content of C. maxima grown in irrigation waters, soil and multiple irrigation regimes. In addition, the correlation between plant and soil systems for metal accumulation and displacement was also evaluated. The health risk to general consumers was estimated through the enrichment coefficient of irrigated soils and the pollution load index.
Materials and methods
Study site
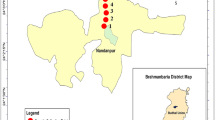
The study was conducted at different sites in Bhakkar District, Punjab, Pakistan, where C. maxima are cultivated. Both urban and peri-urban areas were chosen for sampling. Bhakkar city is located on the southern side of Punjab with distinct dunes and a drier climate. River Indus flows on the western side of Bhakkar city which favours most of the agricultural activities like cultivating cereal crops and vegetables. The average winter temperature varies between 5 and 23 °C and 35 and 49 °C in summer (Khan et al. 2020a).
Collection and preparation of samples
Chosen sampling sites were visited in late August to collect soil, water and pumpkin samples in triplicates from three sites irrigated with canal water (CWI), groundwater (GWI) and municipal wastewater (MWI) (Fig. 1). Water samples collected from each sampling site (100 mL) were stored in acid polypropylene washed vials and 1 mL of HNO3 was added to each storage vial for prevention of microbial growth. The samples were transferred to the laboratory for further analysis.
Soil samples were processed initially for the assessment of basic soil properties. Soil texture and organic content were estimated through Gee and Budaer method (Gee and Bauder 1986) and the Walkey-Black method (McLean 1982) respectively. Available nitrogen, phosphorous and potassium were determined through the method given by Mathieu and Pieltain (Mathieu and Pieltain 2003). pH and EC (electrical conductivity) were estimated by taking water to soil extract in a 2:1 ratio (Mathieu and Pieltain 2003).
Pre-wet acid digestion procedure involved the collection of soil samples at the depth of 20 cm. Fifteen soil samples were collected in total; each sample weighed up to 1 kg. The samples were dried in the open air and then placed in an oven for 4–5 days at 72 °C for moisture eradication. The samples were ground to a fine powder using a pestle and mortar. The powdered samples were passed through a fine sieve (2-mm mesh size). The samples were stored in labelled plastic bottles until further analysis for wet-acid digestion (Khan et al. 2019).
Fruits of C. maxima were collected from the three different irrigational sites and dried in the oven for 3 days at 72 °C. Dried fruits were ground to fine powder by pestle and mortar and stored until further analysis.
Wet digestion procedure
Powdered soil and vegetable samples (1 g each) and water samples (25 mL) were put in conical flasks with a 17-mL mixture of H2SO4, HNO3 and HClO4 (1:5:1) and heated on a hot plate at 80 °C about 1 h for complete digestion of the contents and attainment of transparent solution. The colourless solution, obtained after digestion, was allowed to cool for half an hour and filtered by a Whatman filter paper. A required amount of deionized water was added to each sample to achieve a 50-mL final solution.
Metal analysis and quality control
The digested samples were analysed for different metallic elements like cadmium (Cd), cobalt (Co), nickel (Ni), zinc (Zn), lead (Pb), iron (Fe) and copper (Cu) using atomic absorption spectrophotometer (model PGI 990). Molybdenum (Mo) was estimated through a graphite furnace (Perkin-Elmer Model 503) and a fluorometric method was employed for the evaluation of selenium (Se). Meanwhile, the quantitative evaluation of arsenic (As) was done through flow injection hydride generation AAS (Perkin Elmer Analyst 400). The detection limits of the AAS for Mo, As, Se, Fe, Cu, Zn, Ni, Pb, Cd and Co were defined as 5, 0.05, 0.05, 5, 15, 1.5, 0.05, 0.05, 0.8 and 9 mg/L, respectively. As and Se were determined with the very sensitive hydride technique with the operating conditions given in Table 1. Repetitive analysis was done, for all samples to ensure precision and accuracy, against standard reference material (SRM 2709) given by the National Institute of Standard Technology for all metals under study. SRM soil mean recoveries for Cd, Zn, As, Cu and Pb were 92%, 92%, 90%, 105% and 95% respectively whereas mean SRM recoveries for all C. maxima samples were 94%, 109%, 88%, 98% and 92% for Cd, Zn, As, Cu and Pb respectively.
Statistical analysis
SPSS (version 22) was employed for the analysis of statistical parameters. Descriptive analysis, one-way ANOVA and correlation were used for data interpretation. The difference in means was computed through one-way ANOVA keeping sampling locations as groups and heavy metals as the variables for which mean concentrations were evaluated. ANOVA runs on three basic assumptions of homogeneity of variance, random occurrences and normal distribution of responses for variables (metals) (Miller and Miller 2005). These assumptions were tested using the Runs test, Levene statistic and K-S (Kolmogorov–Smirnov) methods respectively. Homogeneity of variance for the variables was found to be significant (Levene statistic = 4.896, P < 0.001) providing validation for the statistical method used. Moreover, all the mean concentrations for all heavy metals under study followed a normal distribution passing the Kolmogorov–Smirnov normality test (P < 0.05). Relation between mean metal concentrations in soil and C. maxima samples was established through Pearson correlation keeping probability levels at 0.001, 0.01 and 0.05.
Transfer factor (TF)
The transfer factor provides a quantitative insight into the accumulation capability of metallic ions in the vegetable tissues. It is estimated as a ratio of the mean level of metal in vegetables to the mean level of metal in the corresponding soil depicting the transferability of a particular metal (Cui et al. 2004).
Health risk index (HRI)
The health risk index (HRI) involves identifying potential threats to the health of consumers by taking into account the oral reference dose (RfD) and estimated daily intake of the metal (DIM) (Cui et al. 2004).
The average DIM for an adult human was estimated at 0.345 kg of vegetable whereas the average body weight was considered to be 60 kg according to standard protocol (Wang et al. 2005). The oral reference doses for metals were taken from WHO (1996).
The values of RfD (oral reference dose) were for Co (0.043), As (3 × 10−4), Se (5 × 10−3), Ni (0.02), Fe (0.70), Cu (0.04), Zn (0.37), Mo (0.009), Cd (0.001) mg/kg/day and for Pb was 0.0035 mg/kg/day (Wang et al. 2005).
Enrichment coefficient (EC)
The enrichment coefficient (EC) was determined by following Buat-Menard and Chesselet (1979).
EC is computed based on control values (permissible metal concentrations in vegetables and reference values of metals in soil) and estimated metal concentrations in vegetables and soil. In this study, standard reference concentrations of Mo, As, Se, Fe, Cu, Zn, Ni, Pb, Cd and Co for soil were applied as 3, 29, 0.7, 56.9, 8.39, 8.15, 44.9, 9.06, 8.15, 1.49 and 9.1 mg kg−1, respectively, in line with the relevant literature (Dutch Standards 2000; Dosumu et al. 2005; Singh et al. 2010).
Results and discussion
All heavy metals showed a similar trend in mean levels among the three water regimes selected for metal evaluation. The examined metals were in the following order: Fe > Pb > Ni > Co > Se > Cu > Zn > As > Mo > Cd among all irrigation waters (Table 2). The metal concentrations differed non-significantly among water samples concerning the selected sites (Table 3) indicating the possibility of induction of metals in the irrigation waters through some contamination sources. All metals exceeded their respective maximum permissible limits (MPLs) defined by WWF (2007) for the three irrigation water except for Zn (Table 2). Metals could end up in water from multiple sources like aerosol particles, urban runoffs and soil erosion.
The soil was evaluated for the determination of basic physical properties at the three sites under study. The soil was mainly of loamy texture at all sites and the pH of the soil varied between 8.2 (GWI) and 8.8 (MWI). The highest organic matter was recorded for CWI as 0.93%. Almost similar concentrations of N, P and K were determined for soils at three sampling stations. Electrical conductivity was invariably similar at all sites with a mean value of 1.3 dS/m (Table 4). The findings of this study were lower for EC but higher for pH in comparison to a similar study (Murtaza et al. 2010).
Soil samples were further evaluated for the presence of heavy metals at the three different sampling sites. The metals had relatively higher mean levels in soils irrigated with municipal wastewater (Table 5); however, this difference was not significant (P > 0.05) except for Pb (P < 0.05) (Table 3). A similar study carried out in contaminated agricultural areas of Sargodha, Punjab, Pakistan (Ahmad et al. 2016a) reported lower mean concentrations for As (3.52–4.19 mg/kg) and Se (1.81–2.51) in comparison to current findings. A study conducted on the outskirts of main Khushab city, Punjab, Pakistan, reported relatively higher mean levels for Cd, Fe, As, Zn, Cu and Co (Ahmad et al. 2016b) than the mean levels reported in the current study. Contamination for brinjal was assessed by Ahmad et al. (2016c) for long-term wastewater irrigated lands at Sargodha and it was determined that Se, Co, As, Zn, Cu and Fe accumulated in much higher concentrations in the soil compared to the values estimated for these metals in the current study. Metals can build up in soils from different anthropogenic and natural sources and alter the soil composition of those areas (Ugulu et al. 2019).
Evaluated heavy metal concentrations were of the following order in C. maxima sampled from three irrigational sites: Fe > Zn > Cu > Mo > Ni > Pb > Cd > As > Se > Co. Mean concentrations of six heavy metals (Se, Ni, As, Fe, Zn and Pb) were relatively higher in vegetable samples collected from the MWI site (Table 5); however, only As (P < 0.05) and Ni (P < 0.01) showed significant differences in mean concentrations for C. maxima at all sites (Table 3). Mo, Pb and Cd showed higher mean levels in C. maxima collected from all sites compared to the respective MPLs given by WHO (1996). Zn was reported to be the most common metal in vegetables cultivated in wastewater irrigated areas in Nanjing city, China (Chao et al. 2007) which could probably reflect higher input of Zn in municipal wastewater; however, the current study reported lower mean values (below MPL) for Zn in all vegetable specimens. Ni, in the current study, had a higher value than the MPL described by WHO (1996). Differences in elemental concentrations in soils and plants depend upon soil features like organic content, pH and texture; the root penetration ability of a plant also affects the transfer of metals from soil to the plant (Khan et al. 2019). Accumulation properties of a plant may also affect the ability of a metal to get stored in plant tissues. Additionally, huge quantities of agrochemicals (fertilizers, pesticides, herbicides) make their way into the agricultural areas through direct application and indirect sources multiplying the level of certain compounds and ions in soils and waters (Uboh et al. 2011).
Transfer factor for C. maxima accumulation potential for metals from soil to vegetable was also determined. Zn had the highest value of TF at MWI indicating efficient translocation. This observation was also strengthened by the high mean level of Zn in C. maxima at the municipal wastewater irrigated site. The lowest TF was reported for Co at MWI strengthening the low mean concentration observed in C. maxima at the MWI site (Table 6). The bioaccumulation potential of metals also affects their transferability from soil to the living systems. TF greater than 1 indicates an efficient transfer mechanism in operation for metals even transporting them to aerial parts (Rai et al. 2019). In the present investigation, Cu, Zn, Fe, Mo and Cd showed TF values higher than 1. Age and type of plant, different edaphic and environmental factors are known to influence the metal uptake by plants (Karak et al. 2014).
Health risk index (HRI) was calculated for C. maxima consumers based on DIM and oral reference doses for each metal under study. Highest HRI was observed for As (77.34 mg/day) while the lowest was obtained for Co (0.01 mg/ day). HRI > 1 is considered an imminent threat to public health and Cd, Mo, Pb, As, Ni and Cu had HRIs much higher than 1 in the current study (Table 6). Uboh et al. (2011) computed high values for daily intake for Cu and lower values for Pb and Cd in comparison to the current investigation. Metal accumulation in food chains can be avoided through quality based standardized approaches for the evaluation of metallic contents in vegetables (Wajid et al. 2020).
Another parameter depicting the accumulation of metals in the vegetable is the enrichment coefficient (EC). EC values for C. maxima sampled from all irrigation sites were of the following order: Co < Ni < As < Cu < Mo < Fe < Cd < Zn < Pb (Table 7). Metal enrichment capability is manifested through EC values exceeding 1. The highest EC was obtained for Pb (10.02) at GWI in the current study; meanwhile, the lowest EC was observed for Co (0.004) at MWI. The root system of plants and biotransportation of metal to plants and its parts seem to influence the accumulation of metals in vegetables (Tariq et al. 2021).
Pearson correlation test, for establishing positive and/or negative relationships between different metals translocating between soil and C. maxima, was analysed in the present investigation. Only Fe displayed a significantly positive correlation (P < 0.05) whereas Mo, Se and Cu had a non-significant but positive correlation between soil and vegetable. A non-significant but negative correlation was obtained for As, Ni, Zn, Cd and Pb (Table 8). Metal availability determines the relationship between metal contents in soil and plants. Positive and negative results regarding the correlations of metal concentrations in vegetables (Luffa cylindrica (L.) Roem.) and soil in the region were also recorded and evaluated by Khan et al. (2020b). Khan et al. (2020b) determined a positive but non-significant correlation for As (0.414), Se (0.038), Zn (0.524), Ni (0.576), Pb (0.031) and Cd (0.515) and a non-significant but negative correlation for Mo (–0.408), Fe (–0.267), Cu (–0.072) and Co (–0.019). The differences between the results of the studies may be due to the various mechanisms of vegetables to uptake different metals and metalloids. The differences in correlation values for the metals under study also depict uneven concentrations of these metallic elements in the soil. Metal bioavailability and accessibility in soil affect the metal contents of vegetables grown over contaminated soils.
Conclusion
Nearly all metals displayed considerably higher mean concentrations in water at the MWI site in comparison to their respective MRLs in soil, water and C. maxima. Water resources used for crop irrigation and other agricultural purposes should be kept fairly clean and properly managed. Concentrations beyond MPLs were found for Pb, Cd and Mo in C. maxima at all sites. The quality of irrigation water and distance from pollution sources affect metal concentrations in vegetables and corresponding soils. High TF for Zn and HRI (> 1) for As, Mo, Cu, Ni, Cd and Pb signify potential health risks associated with the consumption of heavy metal-contaminated vegetables. Therefore, appropriately scientific and legal measures should be taken and implemented by local governments and agriculture authorities to ensure the safety of consumers.
Data availability
All data generated or analysed during this study are included in this published article.
References
Ahmad K, Ashfaq A, Khan ZI et al (2016a) Health risk assessment of heavy metals and metalloids via dietary intake of a potential vegetable grown in contaminated water irrigated agricultural sites of Sargodha. Pakistan Hum Ecol Risk Assess 22(3):597–610
Ahmad K, Khan ZI, Yasmin S et al (2016b) Contamination of soil and carrots irrigated with different sources of water in Punjab. Pakistan Environ Earth Sci 75:426
Ahmad K, Khan ZI, Ashfaq A et al (2016) Contamination and accumulation of heavy metals in brinjal (Solanum melongena L.) grown in a long-term wastewater-irrigated agricultural land of Sargodha. Pakistan. Fresen Environ Bull 25(7):2404–2410
Amadi BA, Akaninwor JO, Igwe FU, Amad EI (2018) Biochemical impact of sludge obtained from wastewater treatment plant on soil properties within Port Harcourt. J Environ Anal Toxicol 8(1):1–5
Amlan K, Ghosh MA, Bhatt HP (2012) Effect of long-term application of treated sewage water on heavy metal accumulation in vegetables grown in Northern India. Environ Monit Assess 184:1025–1036
Balkhair KS, Ashraf MA (2016) Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J Biol Sci 23:32–44
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42(3):399–411
Chao W, Xiao-Chen L, Li-Min Z, Pei-Fang W, Zhi-Yong G (2007) Pb, Cu, Zn and Ni concentrations in vegetables in relation to their extractable fractions in soils in suburban areas of Nanjing. China Polish J Environ Stud 16(2):199–207
Cui YL, Zhu YG, Zhai RH, Chen DY, Huang YZ, Qiu Y (2004) Transfer of metals from soils to vegetables in an area near a smelter in Nanning, China. Environ Int 30:785–791
Dosumu OO, Salam NA, Oguntoy NS (2005) Trace metals bioaccumulation by some Nigerian vegetables. Centrepoint 13(1):23–32
Dutch Standards (2000) Circular on target values and intervention values for soil remediation. Ministry of Housing, Spatial Planning and the Environment, Bilthoven, The Netherlands.
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (Ed.), Methods of soil analysis. American Society of Agronomy, Inc. & Soil Science Society of America, Inc., Madison WI, pp. 383–411.
Karak T, Paul RK, Sonar I, Sanyal S, Ahmed KZ, Boruah RK, Das DK, Dutta AK (2014) Chromium in soil and tea (Camellia sinensis L.) infusion: does soil amendment with municipal solid waste compost make sense? Food Res Int 64:114–124
Khan ZI, Ugulu I, Sahira S, Mehmood N, Ahmad K, Bashir H, Dogan Y (2020b) Human health risk assessment through the comparative analysis of diverse irrigation regimes for Luffa (Luffa cylindrica (L.) Roem.). Journal of Water, Sanitation and Hygiene for Development 10(2):249–261. https://doi.org/10.2166/washdev.2020b.132
Khan ZI, Nisar A, Ugulu I, Ahmad K, Wajid K, Bashir H, Dogan Y (2019) Determination of cadmium concentrations of vegetables grown in soil irrigated with wastewater: evaluation of health risk to the public. Egypt J Bot 59(3): 753–762. https://doi.org/10.21608/ejbo.2019.9969.1296
Khan ZI, Safdar H, Ahmad K, Wajid K, Bashir H, Ugulu I, Dogan Y (2020a) Copper bioaccumulation and translocation in forages grown in soil irrigated with sewage water. Pak J Bot 52 (1):111–119. https://doi.org/10.30848/PJB2020a-1(12)
Mathieu C, Pieltain F (2003) Analyse chimique des sols: Méthodes choisies. Lavoisier, Paris
McLean EO (1982) Soil pH and lime requirement. In: Page AL (Ed.), Methods of soil analysis. Second Edition, American Society of Agronomy, Inc. & Soil Science Society of America, Inc., Madison WI, pp 199–224.
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry, 5th edn. Pearson, London
Murtaza G, Ghafoor A, Qadir M, Owens G, Aziz MA, Zia MA, Saifullah A (2010) Disposal and use of sewage on agricultural land in Pakistan: a review. Pedosphere 20:23–34
Nawaz H, Anwar-ul-Haq M, Akhtar J, Arfan M (2021) Cadmium, chromium, nickel and nitrate accumulation in wheat (Triticum aestivum L.) using wastewater irrigation and health risks assessment. Ecotoxicol Environ Saf 208:111685
Qadir A, Amina Q, Kanwal R, Bano M, Yousaf B (2015) Accessing potential bioaccumulation of heavy metals in selective vegetables from Gujranwala District. Pakistan J Environ Earth Sci 5(8):58–70
Rai PK, Lee SS, Zhang M, Tsang YF, Kim K-H (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385
Sahin I, Akcicek E, Guner O et al (2016) An investigation on determining heavy metal accumulation in plants growing at Kumalar Mountain in Turkey. EurAsian J BioSci 10:22–29. https://doi.org/10.5053/ejobios.2016.10.0.3
Singh A, Sharma RK, Agarwal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs, from waste water irrigated site of dry tropical area of India. Food Chem Toxicol 48(2):611–619
Tariq F, Xiukang W, Saleem MH et al (2021) Risk assessment of heavy metals in Basmati rice: implications for public health. Sustainability 13:8513. https://doi.org/10.3390/su13158513
Uboh FE, Akpanabiatu MI, Edet EE, Okon IE (2011) Distribution of heavy metals in fluted pumpkin (Telfeiria occidentalis) leaves planted at different distances away from the traffic congested highways. Int J Adv Biotech Res 2(2):250–256
Ugulu I (2015) A quantitative investigation on recycling attitudes of gifted/talented students. Biotech Biotechnol Equip 29:20–26
Ugulu I, Khan ZI, Rehman S, Ahmad K, Munir M, Bashir H, Nawaz K (2019) Trace metal accumulation in Trigonella foenum-graecum irrigated with wastewater and human health risk of metal access through the consumption. Bull Environ Contam Toxicol 103:468–475
Ugulu I, Baslar S, Dogan Y, Aydin H (2009) The determination of colour intensity of Rubia tinctorum and Chrozophora tinctoria distributed in Western Anatolia. Biotech Biotechnol Equip 23(SE):410–413
Wajid K, Ahmad K, Khan ZI, Nadeem M, Bashir H, Chen F, Ugulu I (2020) Effect of organic manure and mineral fertilizers on bioaccumulation and translocation of trace metals in maize. Bull Environ Contam Toxicol 104:649–657. https://doi.org/10.1007/s00128-020-02841-w
Wang X, Sato T, Xing B, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
WHO (1996) Trace elements in human nutrition and health. Prepared in Collaboration with the FAO of the UN and the IAEA. World Health Organization, Geneva.
WWF (2007) Report on national surface water classification criteria, irrigation water quality guidelines for Pakistan, February–2007. Waste Water Forum, Pakistan.
Xiao W, Ye X, Zhang Q, Chen D, Hu J, Gao N (2018) Evaluation of cadmium transfer from soil to leafy vegetables: influencing factors, transfer models, and indication of soil threshold contents. Ecotoxicol Environ Saf 164:355–362
Author information
Authors and Affiliations
Contributions
SS and NM were responsible for conducting the experiments and the data analysis. IU, YD and ZIK were responsible for analyzing and interpreting the data and writing the manuscript. KA and ZIK supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The authors declare that the manuscript has not been published previously.
Consent to participate
All authors voluntarily participated in this research study.
Consent to publish
All authors consent to the publication of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Ugulu, I., Khan, Z.I., Sahira, S. et al. Determination of heavy metal accumulation in wastewater irrigated pumpkin (Cucurbita maxima Duch.) by spectroscopic method. Arab J Geosci 15, 1238 (2022). https://doi.org/10.1007/s12517-022-10519-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10519-2