Abstract
Quality assurance of groundwater before drinking water supply is a major concern in South and South East Asia due to widespread presence of inorganic contaminants and their health implications. To investigate the groundwater quality and associated health risk, 65 groundwater samples were collected and analyzed for major ions along with Fe, As, and Mn. With 72.3% of the samples, Ca-HCO3 is found as the major water type followed by Na-HCO3 and Mg-HCO3 water type. Silicate weathering, ion exchange, and dissolution and precipitation of aquifer minerals are found as the dominant factors responsible for groundwater quality. Of the samples, 88% and 65% are oversaturated with calcite and dolomite, respectively; however, all samples are under-saturated with respect to anhydrite, gypsum, halite, and fluorite minerals. Exceeding concentrations of F−, Fe, and Mn above WHO guidelines in 15.4%, 52.3%, and 24.6% samples, respectively, pose a significant public health concern. High F− is found along older alluvium with Na-HCO3 water type and low Ca2+ suggests dissolution of fluorite contributing F− ions in groundwater. F− is found as the major contribution on health indices (HI) followed by As > Mn > Fe. High HI > 1 that is found in 26.15% and 50.76% samples for adults and children indicates significant potential health risk. Higher HI value for children suggests higher health risk due to relatively lesser body weight. Proper management and quality assurance of groundwater is needed before its use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is one of the most reliable sources of drinking and domestic water supply on earth. Millions of wells have been installed across the globe to fulfill the increasing demand of freshwater, as groundwater is considered safe, in term of microbial contamination. In recent past, the rapid demographic growth along with the widespread occurrence of inorganic contaminants has raised a concern over public health especially in South and South East Asia, as the shallow aquifers in this region are widely contaminated with elevated concentration of inorganic contaminants, i.e., arsenic (As), fluoride (F−), nitrate (NO3−), and other heavy metals and metalloids (Smedley and Kinniburgh 2002; Lafthouhi et al. 2003; Li et al. 2015; Zhang et al. 2018; Podgorski et al. 2018; van Geen et al. 2019; Kumar et al. 2020). These contaminants have both acute and toxic health implications; however, their effects largely depend on the exposure dose, frequency, and the nourishment of the exposed population (Singh et al. 2017; Kumar and Singh 2020; Singh et al., 2021). For instance, elevated As above WHO guideline (> 10 µg/L) may cause skin pigmentation; however, the long-term exposure may cause cardiovascular disease, cancers of lungs, skin, liver, and bladder (Rahman et al. 2009; Parvez et al. 2011; Zhang et al. 2019). It may also reduce intellectual and motor function in children and cause infant mortality (Wasserman et al. 2004). Elevated levels of NO3 (> 45 mg/L) in drinking water can cause methemoglobinemia, gastric cancer, goiter, birth malformations, hypertension, and low blood pressure (Crandall et al. 2013; Drozd et al. 2016). However, up to a certain level F− is essential for humans and its deficiency may cause dental caries and osteoporosis but, chronic excess intake of F−, above the WHO guideline of 1.5 mg/L, can cause dental and skeletal fluorosis (Edmunds and Smedley 2013; Zhang et al. 2017; Yadav et al. 2019; Pant et al., 2021). Similarly, a certain concentration of trace elements, i.e., Fe, Mn, and other heavy metals, is essential for growth and development of human life; however, their elevated concentration above the permissible limits may have severe health implications (Pietrangelo 2010; Fleming and Ponka 2012; Rahman et al. 2017).
Both natural processes and anthropogenic activities may be responsible for groundwater contamination. In natural condition, the quality of groundwater is mostly influenced by the aquifer minerals. When groundwater flows, geochemical processes such as weathering, dissolution, precipitation, ion exchange, or oxidation–reduction significantly control the interaction between groundwater and aquifer minerals (Belkhiri et al. 2010; Xiao et al. 2015). Apart from these geochemical process, other factors such as residence time, climatic condition, soil characteristics, flow path, topography, chemistry of recharge zone, regional geology, and intermixing of water also play vital role in determining the ionic species in groundwater (L-Ruiz et al. 2015; Singh et al. 2017; Su et al. 2019). In addition, anthropogenic activities such as leaching of fertilizers, sewages, industrial discharge, and overexploitation of groundwater may also alter its quality (Ayoob and Gupta 2006; Li et al. 2015; Lapworth et al. 2017).
Chemical evolution of groundwater and its suitability for drinking and domestic use has been widely assessed across the globe. Chemometric analysis, ionic ratio, conventional plots, and graphical methods (Yidana and Yidana 2010; Singh et al. 2017; Kumar et al. 2020), stable isotopes (Barbieri et al. 2005; He et al. 2013; Su et al. 2019), mineral phase equilibrium, structural equation modeling, and hydro-geochemical modeling (Belkhiri and Narany 2015; Liu et al. 2017; El-Alfy et al. 2017) are extensively used to understand the evolution of ionic species in groundwater. However, in recent years an index approach is extensively used to assess the risk of exposed population through contaminated groundwater. Depending on the exposure pathways, both ingestion and dermal health risk has been assessed using by calculating chronic daily intake (CDI), hazardous quotient (HQ), and hazardous index (HI) with reference to the US EPA standards (Avigliano and Schenone 2015; Saini et al. 2016; Magesh et al. 2018; Adimalla 2019; Kumar and Singh 2020). The outcomes from these studies have been often integrated with Geographical Information System (GIS) for proper representation of water quality and health risk to provide management strategies and reduce exposure through contaminated drinking water.
The study area is the part of middle Gangetic floodplain under the Sone subbasin, where groundwater from shallow aquifers is extensively used for drinking and domestic purpose. Quality of groundwater is a major concern in this region as high concentrations of As, Fe, F, NO3, and other heavy metals are reported exceeding the WHO guidelines (CGWB 2013; Donselaar et al. 2017; Maurya et al. 2020; Bhatt et al. 2021). With above background, this study is been designed to explore the hydro-geochemical evolution of groundwater quality in middle Gangetic floodplain using conventional graphical plots, ionic ratio, and mineral phases. This study also evaluates the spatial distribution of major contaminants and public health risk concern. Further, an index approach is used to assess the health risk on two different age groups of population residing into the study region and the spatial distribution is represented as map. The outcomes of this study may help in prioritizing contaminated areas to adopt proper mitigation strategies to reduce exposure.
Material and methods
Study area
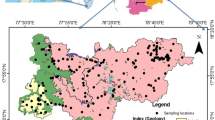
This study is conducted in a part of central Bihar floodplain which is situated at the south of the river Ganga and comes under the Sone-Punpun subbasin (Fig. 1). On administrative division the study region is under Arwal and Jehanabad district of Bihar where river Sone acts as a western boundary and separates it from Bhojpur district where As in groundwater above the WHO guidelines is a major public health concern (Saha et al. 2010; Donselar et al. 2016). Apart from these two perennial streams, i.e., Sone and Punpun, small seasonal channels such as Dardha, Phalgu, Jamuna, and Morhar also pass through the study area; however, most of the time these are dry except rainy season (Fig. 1b).
These rivers flow through South to North along the slope and further meet the main stream of river Ganga. Broadly, the climate of the study region can be classified into Summer, Winter, and Monsoon seasons. It experiences extreme climatic condition as in winters temperature drops to 3–4 °C; however, in summers it reaches up to 45 °C. The average rainfall is 1052 mm out of which more than 60% of the rainfall occurs in monsoon, i.e., July to August months. The study area is a part of fertile alluvial floodplain and it is under extensive agriculture. Soil has dominant percentage of loam with small proportion of clay and sand; however, high concentration of nutrients such as calcium and nitrogen makes it suitable for agriculture.
Geology and hydrogeology
Geologically the study area is underlined by the vast tract of Indo-Gangetic Quaternary alluvium, deposited by the river Ganga and its tributaries (Bhatt et al., 2021). Further this quaternary sequence is subdivided into newer alluvium, i.e., Holocene age, and older alluvium, i.e., Pleistocene age (Acharya 2005; Sahu et al. 2010). The older Holocene sediment is mostly found along the eastern region of the study area; however, the central and western part has dominance of Pleistocene deposits (Fig. 1b). The Holocene alluvium are argillaceous sediments with high organic matter and it is mostly found in entrenched channels and active floodplain while the Pleistocene sediment are reorganized by the yellow–brown color sediments with profuse calcareous and ferruginous concretions (Ganguli and Singh 2016). Apart from the floodplain there are few inselbergs located at the south-eastern region; Barabar hills is the most prominent one with 312-m height from mean sea level (CGWB 2013). The alluvial aquifers are composed of quaternary age sediment and it is composed of mainly of gravel, sand, and clay. Gravel and coarse-medium sand are the major ground water repository.
Alluvial formation is dominant in the study area; however, in some patches clay is prominently observed (CGWB 2013). The result of exploratory drilling up to the 135-m depth conducted by CGWB suggests alteration of clay, sand, sandy clay, and silt while the variation in the basement depth is reported from 120 to 150 m below the land surface (Ganguli and Singh 2016; CGWB 2013). The discharge from the aquifers is very good and it is reported as 20 m3/h in shallow aquifers and 50 m3/h in deep aquifers. However, a significant variation, i.e., 2–5 m in the groundwater table, is observed as during the pre-monsoon the water is found at 5–10-m depth but during the post monsoon it is available at 2–5-m depth. Groundwater is extensively used for irrigation; however, it has not affected the development of groundwater aquifers as a positive development of groundwater resources has been reported and any long-term decline in groundwater table is not observed (CGWB 2013).
Collection of groundwater samples and field analysis
A set of 65 groundwater samples were collected from 15 to 18 March 2019 (Fig. 1a). To eliminate the impacts of iron cast pipes on collected well water samples, wells were purged for 5–6 min before the collection of samples. Physical water quality parameters such as pH and electrical conductivity (EC) were tested onsite using pH tester HANNA (HI98107P) and DiST waterproof EC tester HANNA (HI98303) probe. The pH probes were calibrated every day before use with standard solution of 4.0, 7.0, and 10.0 for pH; however, the EC probe was calibrated with 84 µS/cm, 1413 µS/cm, and 12.8 mS/cm solutions. No any systematic or temporal drift was observed in the reading; hence, the probe was not corrected. In addition, the information about the depth of the wells, age, and person depending on the well water for daily need was gathered from the well owner.
Two different sets of samples were collected in prewashed and dried high-density polyethylene (HDPE) bottles each for cations and anions. One set of sample was acidified with analytical grade 6 N HNO3 (Ultrapure Merck) and it was used for cation analysis in lab; however, the other set was not acidified and it was used for anion analysis. To avoid any headspace all the samples were filled up to top, and to avoid direct sunlight, it was kept in the ice box. After collection samples were brought to the laboratory and preserved in the laboratory freeze. The samples were analyzed within 10 days of sampling.
Lab analysis of groundwater
The pre-acidified groundwater samples were tested with inductively coupled plasma, optical Emission Spectroscopy, Agilent (OES 700 series) to detect the cations including Na+, Ca2+, Mg2+, K+, As, Fe, and Mn concentration in groundwater. The reproducibility of the analysis was in order of 5% for all the cations. However, un-acidified set of sample was used to determine the concentration of anions. The bicarbonate (HCO3−) and chloride (Cl−) concentration was analyzed using the standard titrimetric method as prescribed in American Public Health Association (APHA 2008); however, the other anions were analyzed on UV–Visible Spectrophotometer (Labman) followed by the prescribed method in APHA 2008. The concentration of NO3− was measured using the Screening Method within 3 days of the collection of samples. The sample was treated with 1 N HCl which prevents interference from hydroxide/carbonates. To get the correct NO3− reading the absorbance was recorded at 220 nm along with 275 nm as organic matters also interfere the 220-nm absorbance along with the NO3− (APHA 2008). Fifty-milliliter aliquot has been mixed with 1 mL of 1 N HCl and diluted to prepare 0, 1, 1.75, and 3.5 mg/L of intermediate nitrate concentration (APHA 2008). The SO42− concentration was analyzed using the turbidimetric method and the absorbance was recorded at 420-nm wavelength using spectrophotometer (APHA 2008). The concentration of F in groundwater samples was determined using the SPADNS methods as prescribed in APHA 2008. F− in sample reacts with the dye within 1 min and the discoloration of a red zirconium dye solution containing sodium 2-(p-sulfophenylazo)-1,8-dihydroxynaphthalene-3,6-disulfonate (SPADNS) takes place. The absorbance was recorded at 570-nm wavelength (APHA 2008). As the amount of F− in the solution increases the color of the solution became progressively lighter. For quality assurance all the chemical used during the analysis was of Merck.
The normalized charged balance index (NCBI) of the groundwater samples was calculated using the following equation:
where Tz− is the total sum of anions (in epm) and Tz+ is the total sum of cations (in epm).
Geochemical modeling
Geochemical modeling of groundwater quality parameters is a mass balance approach which describes the variation in chemical composition of groundwater due to dissolution/precipitation of minerals or gases along the flow path. The fate of minerals, i.e., either dissolution or precipitation, can be inferred using the saturation index (SI) as it evaluates the degree of equilibrium between water and respective minerals. Geochemical models work on certain assumption, i.e., (1) the flow path of the groundwater samples is same, (2) there is no major role of dispersion and dispersion is not much significant on hydro-geochemistry, (3) aquifer is in chemical steady state, and (4) the considered mineral phases are present in the study area (Zhu and Anderson 2002). As the study area is the part of alluvial floodplain of river and there is no evidence of alteration in flow path, we assume that all these assumption are filled in this region.
Geochemical modeling was performed using Aquachem 4.0 database and SI along with dominant water types were calculated. The formula used for SI calculation is as below:
where IAP is the ionic activity product and Ktsp(T) represents the equilibrium solubility product of an individual mineral.
Further the values of SI are used to infer the major hydro-chemical processes governing the groundwater composition. The negative values of SI suggest under-saturation and the positive value suggests oversaturation of groundwater with respective minerals (Singh et al. 2017).
Geo-statistical tools
The spatial location of wells were recorded with Global Positioning System (GPS) during the collection of groundwater samples, and it was used to generate the spatial interpolation map using inverse distance weighted (IDW) technique. IDW is extensively used to generate surface maps in groundwater studies as it relies on the local factors which have substantial influence (Singh et al., 2017; Kumar and Singh 2020; Mallick et al., 2021). As the local influence decreases with distance, it calculates moving average of the variable (Singh et al. 2017; Kumar and Singh 2020). The spatial analyst module of ArcGIS 10.3.1 is used to generate the interpolation map using IDW techniques for each of the groundwater quality parameters. However the geological map of the study area was procured from Geological Survey of India (GSI) and digitized using ArcGIS.
Risk assessment
Potential health risk of the exposed population was calculated using the model proposed by US Environment Protection Agency which is based on the daily intake and the standard threshold (USEPA 1989). This mathematical model is extensively used globally as it is considered most expedient and accurate technique to calculate the probability of health risk due to contaminated groundwater (Peluso et al. 2013; Magesh et al. 2018; Adimalla 2019; Rao et al. 2019; Kumar and Singh 2020; Maurya et al. 2020). The value of F−, Mn, Fe, and As was considered to assess the health risk in residing population. The health risk indices assess the exposure through ingestion and dermal pathway; however, the dermal exposure is not much significant. Exposure through ingestion for two age groups, i.e., children (6 years) and adults (70 years), was estimated using Eqs. (3) and (4).
where CDI is the chronic daily intake, EC is the elemental concentration (mg/L), IngR is the ingestion rate (L/day), EF is the exposure frequency (days/year), ED is the exposure duration (year), AT = average time (ED × 365), and BW is the body weight (kg).
In Eq. (4), HQ is the hazardous quotient which is calculated using CDI and reference dose (RfD). The RfD values used for F−, Mn, Fe, and As are 0.06, 0.02, 0.3, and 0.0003, mg/kg/day, respectively, taken from ICMR 2009 and USEPA 2010. However, it has extensively used to study health hazards through contaminated groundwater (Magesh et al. 2018; Adimalla 2019; Rao et al. 2019; Maurya et al. 2020; Kumar et al. 2020). The cumulative health risk due to these contaminants on both age groups in the study area was calculated using Eq. (5).
where i is the HQ of each contaminant considered in this study.
As per the USEPA recommendation, the values > 1 were considered as not suitable due to their non-carcinogenic health risk. The details of the data, variables used, and the conversion factors are provided in Table 1.
Results and discussion
Hydrochemistry
Based on the pH values groundwater of the study area is found alkaline as the pH varies from 6.86 to 8.29 with an average of 7.6. All the samples have pH > 7 except 1, suggesting dominance of alkaline condition. When rain water passes through soil during the process of groundwater development, its interaction with soil imparts alkalinity into groundwater (Subramanian and Saxena 1983). The concentration of EC ranges from 198 to 2370 µS/cm with the average of 809.8 µS/cm (Supplementary Table 1). High EC in groundwater suggests high ionic strength of groundwater which may be imparted due to variation in soil types and agriculture activities in study region (Kumar et al., 2015). According to Davis and Dewiest classification, approximately 17% of the collected samples belongs to fresh water type, 77% under marginal water type, and rest 6%, i.e., 4 out of 65 samples, falls under the brackish water type (Davis and Dewiest 1967). High EC might also be attributed due to mineralization of soil and it indicates increase in ionic activities of groundwater (Singh et al. 2014).
Based on the average concentration cations are found as Ca2+ > Na+ > Mg2+ > K+. Ca2+ is found as the most dominant cation as it ranges from 20 to 160 mg/L with an average of 74.4 mg/L followed by Na+ which varies from 14 to 250 mg/L with an average of 60.5 mg/L. The concentration of cations in groundwater is mostly governed by the natural processes including weathering of minerals, exchange of ions, and the precipitation and dissolution of ions (Kumar and Singh 2020). As the study area is under extensive agriculture and the alluvial soil in this region has high organic matter, root respiration/oxidation of organic matter might also contribute Ca2+ in groundwater (Diwakar et al., 2015). Mg2+ concentration in groundwater varies from 4.8 to 102.1 mg/L and the value of K+ varies from 1 to 65 mg/L; however, the average concentrations of Mg2+ and K+ ions in groundwater are found as 28 mg/L and 11.3 mg/L, respectively. Weathering of silicate minerals is considered as the major contributor of Mg2+ ions; however, the K+ in groundwater might be attributed due to weathering of minerals or leaching of agricultural runoff. The wide variation in groundwater quality parameters indicates that the water quality in the study area is not homogenous; it indicates that the groundwater quality in the study area is not only influenced by the natural activities but also significantly affected by change in land use land cover and other anthropogenic activities.
Based on the average values, HCO3− is found as the most dominant anion followed by Cl−, SO42−, and NO3−. The HCO3− concentration varies from 80 to 584 mg/L with an average of 284.6 mg/L. In natural condition weathering of silicate/carbonate minerals is the major source of HCO3− in groundwater. Study area is the alluvial floodplain with high organic matter and extensive agriculture degradation of organic matter and root respiration are also considered as the major contributor of HCO3− in groundwater (Singh et al. 2017). High concentration of cations, i.e., Ca2+, Na+, and HCO3−, determines the hardness of groundwater. The concentration of Cl− ions ranges from 3.8 to 289.3 mg/L with an average of 55.5 mg/L. In absence of any major geogenic source of Cl−, sewage effluents coming from the villages might attribute Cl− in groundwater. Concentration of SO42− and NO3− ions ranges from 1.65 to 394 mg/L and 0.07 to 18.7 mg/L with an average of 27.9 mg/L and 3.7 mg/L, respectively. Leaching of the sewage, municipal waste, septic tank, and other anthropogenic activities including agriculture excessive use of fertilizers can contribute SO42− and NO3− in groundwater (Elisante and Muzuka 2015; Singh et al. 2018). The spatial variability of major ions in the study area is represented in Fig. 2a, which indicates that the high concentrations of SO42−, HCO3−, Ca2+, and Na+ are found in the central part of the study area, which is mostly covered by the newer alluvial sediments deposited by the rivers.
Distribution of major contaminants
High concentration of F− in groundwater is found as a public health concentration along with Fe and Mn. The F− concentration ranges from BDL to 2.1 mg/L with an average of 0.8 mg/L. Of the collected groundwater, 15.4% has F− above the WHO guideline of 1.5 mg/L. Groundwater samples along with the older alluvium have elevated F− as 7 out of 10 high F samples are from older alluvium (Fig. 2b). Despite being very close to the high As region, the concentration of As was found within the WHO guidelines and it varies from BDL to 9.0 µg/L. High As in groundwater is reported from the other bank of river Sone from Bhojpur district in reducing environment (Saha et al. 2010; Donselar et al. 2016). The distribution of As is not homogenous along the river banks and similar contrasting behavior of As across the banks has been reported from the Rapti river basin (Singh et al. 2018) and lower reaches of River Ganges (Mukherjee et al. 2018). The concentration of Fe and Mn varies from BDL to 4.6 mg/L with an average of 0.57 mg/L and 0.01 to 0.2 mg/L with the mean concentration of 0.05 mg/L, respectively. Out of 65 collected samples 52.3% has high Fe and 24.6% has high Mn above WHO guideline (Supplementary Table 1). Six out of 10 high F− samples also have high Fe above WHO guidelines; it indicates co-existence of these contaminants in the study region.
Hydro-geochemical process
The relationship between the ions present in the groundwater reveals the processes responsible for groundwater quality in the study area. The scatter plots and ionic ratio are often used to determine the geochemical process that develops the chemical signature of groundwater. Na+ normalized plot between Ca2+ and HCO3− indicates the influence of silicate or carbonate weathering along with the evaporate dissolution on groundwater quality (Singh et al. 2017; Kumar and Singh 2020). The graphical plot infers that the groundwater quality of the study area is largely controlled by silicate weathering and dissolution of carbonate minerals (Fig. 3a). Similarly, the Na+ normalized plot between Ca2+ and Mg2+ suggests that groundwater quality is mostly govern by the silicate weathering (Fig. 3b). The major source of Ca2+ and Mg2+ in groundwater is dolomite or calcite to understand the source of these ions Ca/Mg ratio used.
The Ca/Mg ratio below 1 indicates dissolution of dolomite; however, greater values suggest calcite dissolution as a major governing factor. Out of 65, 90%, i.e., 59 samples, that has Ca/Mg values > 1 indicates dissolution of calcite minerals as a major contributor of Ca2+ in groundwater (Supplementary Fig. 1). As the study area is the part of alluvial floodplain weathering of silicate minerals and ion exchange are found to be responsible for high Na in groundwater (Bhatt et al., 2021). As Cl− is persistent in groundwater hence in scatter plot between Ca2+ + Mg2+ and Cl− is used to understand the evolution of these cations in groundwater. High concentration of Cl− in case of evaporation dominance will tend to keep the points below the equiline; however, due to ion exchange, points will shift above the equiline (Singh et al. 2017). In present study most of the samples are found above the equiline, which confirms the ion exchange as major process (Fig. 3c). Apart from these major processes, the graphical plot between Na/Cl and EC is used as an indicator of influence of evaporation on groundwater. The value of Na/Cl will be close to 1 in case of halite dissolution; however, the greater value, i.e., > 1, indicates excess of Na+ ions contributed due to weathering of silicate minerals (Kumar and Singh 2020). Approximately, 87% of the samples, i.e., 57 out of 65, that has high Na/Cl values > 1 indicates dominance of silicate weathering (Supplementary Fig. 2). Scatter plot between Na + K and Cl shows that 26% samples is below equiline; however, remaining 76% that is above the equiline indicates silicate weathering as a source of cations (Fig. 3d). Interestingly, high Na+ in groundwater is observed compared to the K+ although K+ is resistant to chemical weathering; however, apart from ion exchange anthropogenic activities might also contribute high Na+ in groundwater (Mallick et al. 2018).
Geochemical control of groundwater
Water type
The contribution of geochemical attributes on groundwater quality is often summarized by Piper diagram (Piper 1944). In this study, the piper plot suggests dominance of HCO3−; however, among cations, Ca2+ is most dominant (Fig. 4). The dominance of ionic species in ground water depends on the aquifer minerals, residence time, and rock-water interaction. The result of geochemical modeling also confirms Ca-HCO3 as the dominant water type with 70% of the groundwater samples; however, 23% of the samples have Na-HCO3 water type. Out of 65 collected samples, around 3%, i.e., 2 samples, have Mg-HCO3 water type; however, 1.5%, i.e., 1 sample, have Mg-SO4 water type (Supplementary Table 2). Weathering and dissolution of silicates, calcite, and gypsum along with ion-exchange contribute Mg2+, Ca2+, Na+, and HCO3− ions in groundwater (Singh et al. 2017). The study area is the active floodplain with dominance of organically rich sediment; the oxidation of these organic matter might also contribute HCO3− in groundwater. The dissolution of gases during precipitation and infiltration, particularly CO2 either from atmosphere or in the unsaturated zones, initiates weathering of aquifer minerals and resulting HCO3− type signature in water facies (Salama et al. 1999; Singh and Kumar 2020).
Saturation indices and major ions
The saturation indices are used as an effective indicator to infer the fate of minerals in groundwater. The positive SI value indicates precipitation of the mineral; however, negative values suggest dissolution of minerals in groundwater. In this study, SI of calcite (CaCO3) and dolomite (CaMg(CO3)2) indicates that, respectively, 88% (57 out of 65) and 65% (54 out of 65) samples are oversaturated with respect to these minerals and hence tend to precipitate. In contrast, the SI values of fluorite (CaF2), anhydrite (CaSO4), and gypsum (CaSO4.2H2O) are found negative and suggest dissolution of these minerals in groundwater. Along with alkaline aquifer environment undersaturation of groundwater with respect to calcite also favors the dissolution of fluorite minerals, which results to excess of F− in groundwater (Mrazovaca et al. 2013).
To understand the geochemical control, the dominant ionic species present as a major chemical constituent of the minerals are plotted against the SI values. SI of calcite and dolomite is found positive in most of the groundwater samples, and hence, it does not show any consistent relation with Ca + HCO3 and Ca + Mg ion concentrations in groundwater (Fig. 5a, b). Apart from calcite and dolomite gypsum can also contribute to the Ca2+and SO4− ion concentrations in groundwater. Samples from the study area are found under saturated with gypsum, and it shows consistent relation with Ca + SO4 indication dissolution of gypsum as major source of these ions in groundwater (Fig. 5d). Similarly, halite and fluorite also show positive relation with their major constituent; i.e., Na + Cl and F− is indicative of dissolution of minerals as major contributor of these ions in study area (Fig. 5c). Oversaturation of these minerals suggests that the groundwater has enough residence time to reach up to equilibrium. F− shows positive relation with fluorite (Fig. 5e). As discussed the F− ions that are found in dominant pH condition with high HCO3 might have significant influence on the F−mobilization in groundwater.
Geochemical control of high F − in groundwater
The concentration of F− in groundwater is controlled by both natural and anthropogenic activities (Edmunds and Smedley 2013; Singh et al. 2017; Maurya et al. 2020). In present study, the association between groundwater F− and NO3− is not found significant enough to infer anthropogenic input. In alluvial floodplain clay mineral plays vital role as it absorbs F− in acidic pH (Su et al. 2019); however, the study area has alkaline pH, with an average of 7.39. Alkaline condition of groundwater favors the dissolution of F− along with high HCO3− and Na+ ions (Singh et al. 2013, 2017). In alkaline condition substitution of F− by OH− acts as a major factor controlling F− enrichment in groundwater. An insignificant but positive association between F− and HCO3− is observed (Fig. 6). High HCO3− in groundwater favors the release of F− as it acts as a major competitor and restricts desorption of F−, resulting to increase of F− in groundwater (Su et al. 2019).
The scattered plot between Ca2+ + Mg2+ and SO42− + HCO3− suggests that ion exchange is a major process; however, the exchange of cations., i.e., Na+ from clay minerals with Ca2+ and Mg2+, might increase F− concentration in groundwater. The scatter plot between (HCO3−-SO42−, Ca2+-Mg2+) against (Cl−-Na+-K+) also confirms the dominance of ion exchange in the study area (Fig. 6). In Fig. 6, the significant negative correlation (R = 0.67) indicates that high Na+ in groundwater is attributed to cation exchange and it plays important role in dissolution of F−-bearing minerals. Seven out of 10 high F− samples in this study lie in older alluvium, and most of these samples with high F− are found under saturated with respect to fluorite, however oversaturated with calcite (Fig. 5e). High Ca2+ restricts the dissolution of F− due to common ion effects, resulting to negative correlation between these ions (Handa 1975). In this study, although not much significant but negative correlation between Ca2+ and F− is observed and groundwater is mostly found oversaturated with calcite and undersaturated with fluorite suggests dissolution of fluorite mineral responsible for high F− in groundwater (Fig. 5f).
Human health risk
Among the multiple pathways of exposure to the inorganic contaminants, exposure through drinking is a major concern due to excessive use and health implications. Groundwater is considered safe in terms of microbial contaminants and used widely to fulfill the drinking and domestic need; however, it is vulnerable to both geogenic and anthropogenic activities. The severity and health implications of exposed population depend on various factors including the type of contaminants, dose, nourishment, and other factors. However, these contaminants may pose both acute and chronic health implications. In this study the population is divided in two groups, i.e., children (up to 6 years) and adults (up to 70 years), and to understand the health risk due to ingestion of contaminated groundwater, CDI, HQ, and HI are calculated. Contaminants including F−, As, Fe, and Mn are considered to calculate the health risk on the residing population. The detailed statistical summary of the CDI, HQ, and HI for both age groups is provided in supplementary Tables 3 and 4. HQ > 1 indicates that the water is unsafe for drinking in terms of that particular contaminant; however, the HI values are used to infer the cumulative impacts and health risk due to all contaminants on human health. F− has major contribution on the HI as 10.7% of the samples has HQ F− > 1 for adults; however, 18.4% of samples has high HQ > 1 for children. Fluoride is essential for bone formation but the high concentration of fluoride in drinking water may cause skeletal and dental fluorosis, stiffness, spine, and major joints, osteosclerosis, intellectual impairment, and mental retardation in children, however loss of fertility and miscarriage in adults (Ayoob and Gupta 2006; Zhang et al. 2017). Despite being above the WHO guidelines, the individual health risk of Mn and Fe is less as all the samples have HQ < 1 for both the age groups. The cumulative impact of all these contaminants is assessed by calculating HI and indicates that 26.15% of samples has high HI > for adults; however, 50.76%, i.e., 33 out of 65 samples, has high HI > 1 for children. The population residing along the older alluvial floodplain on the eastern part of the study area is under high risk as distribution of HI for children and adults is high along older floodplain (Fig. 7). The higher values of HI are found for children, which suggest that the degree of health risk in children is much higher compared to adults. This cloud can be due to low body weight and the reference dosage of contaminants.
Conclusion
Groundwater in alluvial aquifers is extensively used for agriculture and domestic purpose; however, in recent past quality assurance before its use has become a major public health concern. To understand the groundwater quality and process controlling evolution of groundwater in the study area conventional graphical plots and geochemical modeling are used. Groundwater is found alkaline with high HCO3− ions that suggests interaction between CO2 present in soil with water during infiltration. Based on the SI values dissolution of anhydrite, gypsum, and fluorite along with the precipitation of calcite and dolomite minerals are found as the major hydro-geochemical process in the study region. Results suggest that silicate weathering, ion exchange, and dissolution and precipitation of aquifer minerals are the controlling factors, responsible for ionic evolution of groundwater in the study area. Ca-HCO3 is the major water type followed by Na-HCO3 and Mg-HCO3 water type. Dissolution of fluorite minerals in alkaline condition seems the major factor controlling F− enrichment in groundwater. Exceeding concentration of F− along with Fe and Mn above the WHO guidelines are found as the major public health concern. Groundwater in the older alluvial plain is found much contaminated and the health of the community residing in this area is under threat. High HQ value of F− indicates high F− hazard for children and adults; however, HI suggests that long-term use of this for drinking might have significant health impacts. The evaluation of groundwater quality is required in the study region at household level to ascertain the groundwater quality and its suitability for human consumption. Testing of wells in the alluvial floodplain to identify the safe and unsafe well along with alternative source of drinking water is recommended to reduce exposure through contaminated groundwater.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Acharya SK (2005) Arsenic levels in groundwater from Quaternary alluvium in the Ganga Plain and the Bengal Basin, Indian subcontinent: insights into influence of stratigraphy. Gondwana Res 8(1):55–66. https://doi.org/10.1016/S1342-937X(05)70262-8
Adimalla N, Li P, Qian H (2018). Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: a special emphasis on human health risk assessment (HHRA). Hum Ecol Risk Assess Int J https://doi.org/10.1080/10807039.2018.1460579
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11(2):109–123. https://doi.org/10.1007/s12403-018-0288-8
Association APH, (APHA, (2008) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington DC
Avigliano E, Schenone NF (2015) Human health risk assessment and environmental distribution of trace elements, glyphosate, fecal coliform and total coliform in Atlantic Rainforest mountain rivers (South America). Microchem J 122:149–158. https://doi.org/10.1016/j.microc.2015.05.004
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487. https://doi.org/10.1080/10643380600678112
Barbieri M, Boschetti T, Petitta M, Tallini M (2005) Stable isotopes (2H, 18O and 87Sr/86Sr) and hydrochemistry monitoring for groundwater hydrodynamics analysis in a karst aquifer (Gran Sasso, Central Italy). Appl Geochem 20:2063–2081. https://doi.org/10.1016/j.apgeochem.2005.07.008
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—a case study: Ain Azel plain (Algeria). Geoderma 159:390–398. https://doi.org/10.1016/j.geoderma.2010.08.016
Belkhiri L, Narany TS (2015) Using multivariate statistical analysis, geostatistical techniques and structural equation modeling to identify spatial variability of groundwater quality. Water Resour Manag 29:2073–2089. https://doi.org/10.1007/s11269-015-0929-7
Bhatt AG, Kumar A, Trivedi PR (2021) Integration of multivariate statistics and water quality indices to evaluate groundwater quality and its suitability in middle Gangetic floodplain. Bihar SN Appl Sci 3:426. https://doi.org/10.1007/s42452-021-04394-x
Central Groundwater Board, Ground Water Information Booklet Jehanabad District (2013) Bihar State. http://cgwb.gov.in/District_Profile/Bihar/Jehanabad.pdf
Crandall C, Katz BG, Berndt MP (2013) Estimating nitrate concentrations in groundwater at selected wells and springs in the surficial aquifer system and Upper Floridan aquifer, Dougherty Plain and Marianna Lowlands, Georgia, Florida, and Alabama, 2002–50: U.S. Geol. Surv. Investig. Rep. 2013–5150, 65, http://pubs.usgs.gov/sir/2013/5150/
Davis SN, Dewiest RJM (1967) Hydrogeology. Wiley, New York
Diwakar J, Johnston SG, Burton ED, Shrestha SD (2015) Arsenic mobilization in an alluvial aquifer of the Terai region. Nepal J Hydrol Reg Stud 4:59–79. https://doi.org/10.1016/j.ejrh.2014.10.001
Donselaar ME, Bhatt AG, Ghosh AK (2017) On the relation between fluvio-deltaic flood basin geomorphology and the wide-spread occurrence of arsenic pollution in shallow aquifers. Sci Total Environ 574:901–913. https://doi.org/10.1016/j.scitotenv.2016.09.074
Drozd VM, Branovan I, Shiglik N, Lushchyk ML, Platonova TY, Pashkevich VI, Kudelsky AV, Shimanskaya I, Danilova LI, Biko J, Reiners C (2016) Effect of nitrates in drinking water on the prevalence of thyroid cancer and other thyroid diseases: a literature review and post-Chernobyl research experience in Belarus. Cytol Genet 50(6):372–376. https://doi.org/10.3103/S0095452716060074
Edmunds WM, Smedley PL (2013) Fluoride in natural waters. In: Essentials of Medical Geology. Revised Edition. 311–336. https://doi.org/10.1007/978-94-007-4375-5_13
El Alfy M, Lashin A, Abdalla F, Al-Bassam A (2017) Assessing the hydrogeochemical processes affecting groundwater pollution in arid areas using an integration of geochemical equilibrium and multivariate statistical techniques. Environ Pollut 229:760–770. https://doi.org/10.1016/j.envpol.2017.05.052
Elisante E, Muzuka ANN (2015) Occurrence of nitrate in Tanzanian groundwater aquifers: a review. Appl Water Sci 7(1):71–87. https://doi.org/10.1007/s13201-015-0269-z
Fleming RE, Ponka P (2012) Iron overload in human disease. New Engl J Med 366(4):348–359. https://doi.org/10.1056/NEJMra1004967
Ganguli SS, Singh S (2016) Delineation of Holocene-Pleistocene aquifer system in parts of Middle Ganga Plain, Bihar, Eastern India through DC resistivity survey. Appl Water Sci 6(4):359–370. https://doi.org/10.1007/s13201-014-0220-8
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Groundwater 13:275–281
He J, An Y, Zhang F (2013) Geochemical characteristics and fluoride distribution in the groundwater of the Zhangye Basin in Northwestern China. J Geochem Explor 135:22–30. https://doi.org/10.1016/j.gexplo.2012.12.012
Huang D, Yang J, Wei X, Qin J, Ou S, Zhang Z, Zou Y (2017) Probabilistic risk assessment of Chinese residents’ exposure to fluoride in improved drinking water in endemic fluorosis areas. Environ Poll 222:118–125. https://doi.org/10.1016/j.envpol.2016.12.074
Kim YS, Park HS, Kim JY, Park SK, Cho BW, Sung IH, Shin DC (2004) Health risk assessment for uranium in Korean groundwater. J Environ Radioact 77(1):77–85. https://doi.org/10.1016/j.jenvrad.2004.03.001
Kumar A, Roy SS, Singh CK (2020) Geochemistry and associated human health risk through potential harmful elements (PHEs) in groundwater of the Indus basin. India Environ Earth Sci 79(4):86. https://doi.org/10.1007/s12665-020-8818-7
Kumar A, Singh CK (2015) Characterization of hydrogeochemical processes and fluoride enrichment in groundwater of South-Western Punjab. Water Qual Expo Health 7(3):373–387. https://doi.org/10.1007/s12403-015-0157
Kumar A, Singh CK (2020) Arsenic enrichment in groundwater and associated health risk in Bari doab region of Indus basin, Punjab. India Environ Poll 256:113324. https://doi.org/10.1016/j.envpol.2019.113324
Lafthouhi N-E, Vanclooster M, Jalal M, Witam O, Aboufirassi M, Bahir M, Persoons E (2003) Groundwater nitrate pollution in the Essaouira Basin (Morroco). Compt Rendus Geosci 335:307–317. https://doi.org/10.1016/S1631-0713(03)00025-7
Lapworth DJ, Krishan G, MacDonald AM, Rao MS (2017) Groundwater quality in the alluvial aquifer system of northwest India: new evidence of the extent of anthropogenic and geogenic contamination. Sci Total Environ 599:1433–1444. https://doi.org/10.1016/j.scitotenv.2017.04.223
Ledesma-Ruiz R, Pastén-Zapata E, Parra R, Harter T, Mahlknecht J (2015) Investigation of the geochemical evolution of groundwater under agricultural land: a case study in north-eastern Mexico. J Hydrol 521:410–423. https://doi.org/10.1016/j.jhydrol.2014.12.026
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165. https://doi.org/10.1016/j.scitotenv.2014.11.045
P Liu N Hoth C Drebenstedt SY Xu Z 2017 Hydro-geochemical paths of multi-layer groundwater system in coal mining regions—using multivariate statistics and geochemical modeling approaches Sci Total Environ 601 1 14 https://doi.org/10.1016/j.scitotenv.2017.05.146
Magesh NS, Chandrasekar N, Elango L (2018) Trace element concentrations in the groundwater of the Tamiraparani river basin, South India: insights from human health risk and multivariate statistical techniques. Chemosphere 185:468–479. https://doi.org/10.1016/j.chemosphere.2017.07.044
Mallick J, Singh CK, Almesfer MK, Kumar A, Khan RA, Islam S, Rahman A (2018) Hydro-geochemical assessment of groundwater quality in Aseer Region, Saudi Arabia. Water 10(12): 1847. https://www.mdpi.com/2073-4441/10/12/1847
Mallick J, Kumar A, Almesfer MK, Alsubih M, Singh CK, Ahmed M, Khan RA (2021) An index-based approach to assess groundwater quality for drinking and irrigation in Asir region of Saudi Arabia. Arab J Geosci 14(3):1–17. https://doi.org/10.1007/s12517-021-06506-8
Maurya J, Pradhan SN, Seema GAK (2020) Evaluation of ground water quality and health risk assessment due to nitrate and fluoride in the Middle Indo-Gangetic plains of India. Hum Ecol Risk Assess Int J 11:1–7. https://doi.org/10.1080/10807039.2020.1844559
Mrazovaca S, Miloradov MV, Matic I, Maric N (2013) Multivariate statistical analyzing of chemical parameters of groundwater in Vojvodina. Chem Erde 73:217–225. https://doi.org/10.1016/j.chemer.2012.11.002
Mukherjee A, Fryar AE, Eastridge EM, Nally RS, Chakraborty M, Scanlon BR (2018) Controls on high and low groundwater arsenic on the opposite banks of the lower reaches of River Ganges, Bengal basin, India. Sci Total Environ 645:1371–1387. https://doi.org/10.1016/j.scitotenv.2018.06.376
Pant N, Rai SP, Singh R, Kumar S, Saini RK, Purushothaman P, Pratap K (2021) Impact of geology and anthropogenic activities over the water quality with emphasis on fluoride in water scarce Lalitpur district of Bundelkhand region. India Chemosphere 279:130496. https://doi.org/10.1016/j.chemosphere.2021.130496
Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan KM, Kline J, Ahsan H, Graziano JH (2011) Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect 119:1665–1670. https://doi.org/10.1289/ehp.1103548
Pietrangelo A (2010) Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 139(2):393–408. https://doi.org/10.1053/j.gastro.2010.06.013
Piper AM (1944) A graphic procedure in the chemical interpretation of water analysis. Am Geophys Union Trans 25:914–923
Podgorski JE, Labhasetwar P, Saha D, Berg M (2018) Prediction modeling and mapping of groundwater fluoride contamination throughout India. Environ Sci Tech 52(17):9889–9898. https://doi.org/10.1021/acs.est.8b01679
Rahman MM, Ng JC, Naidu R (2009) Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health 31(1):189–200. https://doi.org/10.1007/s10653-008-9235-0
Rahman SM, Kippler M, Tofail F, Bölte S, Derakhshani Hamadani J, Vahter M (2017) Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ Health Perspect 125(5):057003. https://doi.org/10.1289/EHP631
NS Rao BLS Sunitha BD Spandana M Chaudhary 2019 Mechanisms controlling groundwater chemistry and assessment of potential health risk: a case study from South India Geochem 125568 https://doi.org/10.1016/j.chemer.2019.125568
Sahu S, Raju NJ, Saha D. (2010) Active tectonics and geomorphology in the Sone-Ganga alluvial tract in mid-Ganga Basin, India. Quatern Int 15;227(2):116–26 https://doi.org/10.1016/j.quaint.2010.05.023
Saini K, Singh P, Bajwa BS (2016) Comparative statistical analysis of carcinogenic and non-carcinogenic effects of uranium in groundwater samples from different regions of Punjab. India Appl Radiat Isot 118:196–202. https://doi.org/10.1016/j.apradiso.2016.09.014
Salama RB, Otto CJ, Fitzpatrick RW (1999) Contributions of groundwater conditions to soil and water salinization. Hydrogeol J 7(1):46–64. https://doi.org/10.1007/s100400050179
Singh CK, Kumar A, Shashtri S, Kumar A, Mallick J, Singh A, Ranjan S (2021) Geochemical modeling to infer genetic origin of groundwater and associated health risks in desertic aquifers. Groundw Sustain Dev 13:100569. https://doi.org/10.1016/j.gsd.2021.100569
Singh CK, Kumar A, Bindal S (2018) Arsenic contamination in Rapti River basin, Terai region of India. J Geochem Explor 192:120–131. https://doi.org/10.1016/j.gexplo.2018.06.010
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J Geochem Explor 175:59–71. https://doi.org/10.1016/j.gexplo.2017.01.001
Singh CK, Rina K, Singh RP, Mukherjee S (2014) Geochemical characterization and heavy metal contamination of groundwater in Satluj River Basin. Environ Earth Sci 71(1):201–216. https://doi.org/10.1007/s12665-013-2424-x
Singh UK, Kumar B (2017) Pathways of heavy metals contamination and associated human health risk in Ajay River Basin, India. Chemosphere 174:183–199. https://doi.org/10.1016/j.chemosphere.2017.01.103
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568. https://doi.org/10.1016/S0883-2927(02)00018-5
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavihar M, Peter J, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Metturtaluk, Salem district, Tamil Nadu. J Earth Syst Sci 117:49–58. https://doi.org/10.1007/s12040-008-0012-3
Su H, Wang J, Liu J (2019) Geochemical factors controlling the occurrence of high-fluoride groundwater in the western region of the Ordos basin, north-western China. Environ Pollut 252:1154–1162. https://doi.org/10.1016/j.envpol.2019.06.046
Subramanian V, Saxena K (1983) Hydro-geochemistry of groundwater in the Delhi region of India, relation of water quality and quantity. Proc Hamberg Symp IAHS 146:307–316
USEPA (1989) United States Environmental Protection Agency, risk assessment guidance for Superfund, Volume I: human health evaluation manual (Part A), interim fnal, ofce of emergency and remedial response. EPA/540/1‐89/002 https://www.osti.gov/biblio/7037757 Accessed 30 Oct 2019.
USEPA (2002) United States Environmental Protection Agency, Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites, Appendix D-Dispersion Factors Calculations., Washington, DC, USA, OSWER93552002 (2002) 4–24.
USEPA (2014) Human health evaluation manual, supplemental guidance: update of standard default exposure factors, OSWER Directive 9200.1–120. United States Environmental Protection Agency, Washington, DC. https://nepis.epa.gov/Exe/ZyNET.exe/P100NQNA.TXT Accessed 20 Oct 2019.
van Geen A, Farooqi A, Kumar A, Khattak JA, Mushtaq N, Hussain I, Ellis T, Singh CK (2019) Field testing of over 30,000 wells for arsenic across 400 villages of the Punjab plains of Pakistan and India: implications for prioritizing mitigation. Sci Total Environ 654:1358–1363. https://doi.org/10.1016/j.scitotenv.2018.11.201
GA Wasserman X Liu F Parvez H Ahsan P Factor-LitvakA Geen van V Slavkovich NJ Lolacono Z Cheng I Hussain H Momotaj 2004 Water arsenic exposure and children’s intellectual function in Araihazar Bangladesh Environ Health Perspect 1329–1333 https://doi.org/10.1289/ehp.6964
Xiao J, Jin Z, Zhang F (2015) Geochemical controls on fluoride concentrations in natural waters from the middle loess plateau, China. J Geochem Explor 159:252–261. https://doi.org/10.1016/j.gexplo.2015.09.018
Yadav KK, Kumar S, Pham QB, Gupta N, Rezania S, Kamyab H, Talaiekhozani A (2019) Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol Environ Saf 182:109362. https://doi.org/10.1016/j.ecoenv.2019.06.045
Yidana SM, Yidana A (2010) Assessing water quality using water quality index and multivariate analysis. Environ Earth Sci 59(7):1461–1473. https://doi.org/10.1007/s12665-009-0132-3
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Zhu C, Anderson G (2002) Environmental application of geochemical modelling. Cambridge University Press, Cambridge
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhatt, A.G., Kumar, A. & Singh, S.K. Hydro-geochemical evolution of groundwater and associated human health risk in River Sone subbasin of Middle-Gangetic floodplain, Bihar, India. Arab J Geosci 15, 405 (2022). https://doi.org/10.1007/s12517-021-09269-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-09269-4