Abstract
The aim of the present work is to evaluate the suitability of Churni River water for irrigation based on major physical and chemical parameters of 83 water samples collected during February 2011–December 2017 (one in every month) at two stations: Majdia and Ranaghat. The physical parameters measured are electrical conductivity (EC) (204–697 μS/cm for Majdia and 182–731 μS/cm for Ranaghat) and total dissolved solids (TDS) (40–526 mg/L for Majdia and 84–496 mg/L for Ranaghat). Besides, ion chemistry of four cations (concentration order: Ca2+ > Mg2+ > Na+ > K+) and four anions (HCO3− > CO32− > Cl− > SO42−) depicts good ionic combination (ion balance error within 10%) and suitability of water for irrigation as indicated by the lower value of sodicity hazard (sodium absorption ratio (SAR) of 0.07–0.52 for Majdia and 0.05–0.52 for Ranaghat), alkalinity hazard (residual sodium carbonate of 2–10 for Majdia and 2–13 for Ranaghat) and permeability hazard (permeability index of 39–74 for Majdia and 40–139 for Ranaghat). Similarly, the compound ranking method locates the water samples of both the stations at 1.57 on a 1–3 scale when 1, 2 and 3 indicate good, permissible and unsuitable, respectively. Finally, ANOVA shows no significant difference in water quality except SAR between the upstream (Majdia) and downstream (Ranaghat) areas of the river.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Physicochemical analysis of water is essential to determine the quality of water in every sector. Analyses are conducted on water sampled from aquiferous layers (Kumar et al. 2014; Ali Moasheri et al. 2012; Singh and Kumar 2015), surface water (Kumarasamy et al. 2013; Disli 2017; Shakir et al. 2017) and wastewater (Baig Asadullah et al. 2018) because water from different sources is used for multiple purposes. The river is an important source of surface water used for potable water, irrigation, manufacturing industries, etc. Sustainable agricultural return largely depends on improved performance of an irrigation system which is evaluated by analysing the quality of water delivered. However, sustainability in agricultural return depends not only on improved irrigation systems but also on crop yields and market prices of inputs and outputs. Many scholastic works have identified the suitability of river water for irrigation (Kumarasamy et al. 2013; Disli 2017; Costa and Aparicio 2015; Huong et al. 2008; Fulazzaky 2010; Khan et al. 2005; Rao et al. 2015; Shakir et al. 2017). As the river gets polluted from different sources, identification of that sources and their proper management is a major challenge. Besides, compositional proportions of different physicochemical properties may induce water pollution. Therefore, measuring the concentration of each parameter and finding out the principal factors of water pollution are essential to evaluate its suitability for irrigation. For this purpose, some indices such as sodicity hazard [sodium absorption ratio (SAR) and percent sodium or (% Na)], alkalinity hazard (residual sodium carbonate (RSC)) and permeability hazard (permeability index (PI)) have widely been used (Piper 1944; Eaton 1950; Richards 1954; Todd 1959; Gibbs 1970; Ravikumar et al. 2015; Reddy 2013; Sadick et al. 2017; Satyanarayana et al. 2016; Smith et al. 2014; Wilcox 1955). A good share of global agricultural products comes from irrigated lands. In India, ~ 63.25 million ha is the net area under irrigated land where principal sources of irrigation are tube wells and other wells (62%), canals (26%), tanks (3%) and other sources (9%) (Directorate of Economics and Statistics 2018). Regarding canal irrigation, most of the canals are taken off from perennial rivers. The alluvial plains in India having perennial rivers and excessive groundwater storage are the areas of the highest percentage of irrigation. One of the high-intensity irrigation areas of India is the Gangetic Plain where, apart from groundwater, river water plays a significant role to boost up the agricultural production. But, water, both the groundwater and surface water, is of concern because in the Gangetic Plain, rivers and groundwater are getting polluted. So, in this regard, it is important to monitor the quality of the river to receive pollution-free water. In the context of Bengal Delta, rivers are deteriorating at a faster rate since the sixteenth century (Majumdar 1941; Parua 2010; Rudra 2010, 2011, 2014). Like the decay of Bhagirathi-Hooghly River (Guchhait et al. 2016; Islam and Guchhait 2018), many other rivers in the Bengal Delta such as Bhirab-Jalangi and Mathabhanga-Churni are still facing heavy siltation at their source and are separated from the Ganga River throughout the year except monsoon period (Chapman and Rudra 2015). Consequently, velocity has become too feeble to carry suspended load and therefore has allowed a greater part of sediment to deposit on its bed. Thus, the channels have become chocked and finally turned into decayed rivers. This phenomenon is natural and caused by continuous south-eastward swing of the Ganga-Padma River as a result of neo-tectonic movements (Allison 1998; Allison and Kepple 2001; Goodberd 2003; Kuehl et al. 1997; Islam and Guchhait 2017a). Besides, some scholars have addressed few anthropogenic activities like the release of urban industrial wastewater, runoff from agriculture, urbanisation and rapid land use and land cover change as potential factors affecting the chemical composition of river water (Das 2015). Similarly, some have paid attention to water pollution and its effects on aquatic ecology and fish community structure (Das and Chakrabarty 2007; Das 2015) of the Churni River. Based on the available literature, it can be mentioned that assessing the suitability of river water for irrigation is explored by a few scholars in general and for river Churni in particular. Keeping this gap in mind, this paper would address the following objectives:
- i.
To analyse the physicochemical properties of water for assessing its suitability for irrigation, and
- ii.
To find out the variations in the suitability of water between the upstream and downstream segments.
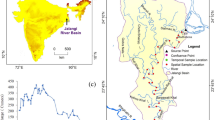
Study area
Three rivers, the Bhagirathi, the Jalangi and the Mathabhanga in Nadia District of West Bengal playing an important role in influencing local geomorphology, geohydrology, climate, soil, land use and socioeconomic spectrum, are known as ‘Nadia Rivers’ (Majumder 1978). River Churni has been selected for study which is the extended part of the Mathabhanga River. The Mathabhanga originates from the Padma River in Bangladesh (24° 03′ 19″ N and 88° 24′ 21″ E) and is bifurcated into two channels: the Ichhamati and the Churni near Majdia (23° 40′ N, 88° 70′ E) in Nadia District. The Churni runs through Shibnivas, Hanskhali, Birnagar, Aranghata and Ranaghat and finally falls into the Hooghly River near Chakdah (23° 13′ N, 88° 50′ E). It flows for ~ 56 km. Two stations, Majdia (upstream) and Ranaghat (downstream), have been selected as water sample locations for analysing the suitability of water quality for irrigation (Fig. 1). Geographically, the area is a featureless monotonous deltaic plain with an imperceptible gentle slope (Islam and Guchhait 2017b). The climate of this area is characterised by tropical monsoon recording an average annual temperature of about 27 °C and average precipitation of about 1353 mm. The precipitation regime in this climate portrays that rainfall is concentrated only in 3 months (July–September), triggering a significant change in upland discharge from agricultural fields increasing total dissolved solids (TDS) and electrical conductivity (EC). The area (four community development blocks of Nadia District: Krishnaganj, Hanskhali, Ranaghat-I and Ranaghat-II) is densely populated with the rural (80%) and urban (20%) people (Directorate of Census Operation 2011). The economy of the area is dependent mostly on agriculture. Most of the agricultural tracts are irrigated by the water lifted from the Churni River. Thus, the government has installed many irrigation pumps on the Churni River (Fig. 2).
Database and methods
Database
We observed 83 water samples (one in every month) during February 2011–December 2017 at two stations: Majdia and Ranaghat. The tests on water quality were done by Kankinara Laboratory under West Bengal Pollution Control Board. For assessing the suitability of water for irrigation, we have simultaneously taken two physical parameters (EC and TDS) though they are similar in measuring the salinity level of water. However, TDS analysis measures salinity intrusion more effectively than EC (Khaki et al. 2015). Thus, in our present study, in order to segregate the level of salinity intrusion in the tidal stretch from the non-tidal stretch of the Churni River, we have applied TDS analysis. Besides, TDS and EC are not linearly correlated and hence the derivative measures may not represent the reality because, with a slight change in temperature, the constant in the TDS-EC equation (TDS = k EC (in 25 °C)) often changes (Rusydi 2018). In the present context, the EC data used are taken as temperature compensating. Thus, to detect the minute difference in water quality for irrigation, simultaneous use of EC and TDS is justified. Besides, ion chemistry has been measured with reference to four cations [calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+)] and four anions [bicarbonate (HCO3−), carbonate (CO32−), chloride (Cl−) and sulphate (SO42−)].
Methods
Chemical classification of water
Using the AquaChem 2014.2 software, hydrochemical classification of the water samples of the Churni River has precisely been done through developing a piper trilinear diagram. Testing of ionic balance of the water sample is vital while analysing the physicochemical quality of water. In principle of electroneutrality, the concentration or sum of the positive ions (cations) must be equal to the sum of the negative ions (anions) (Nag and Das 2014). So, ion balancing is one of the most important validation tests for surface water and groundwater quality analysis. For calculation of ion balance, the concentration of each ion in water samples is measured in mEq/l. The most commonly used formula for ion balance is as follows (Nag and Das 2014):
In good water quality, both the groundwater and surface water, % of ion balance should be within the range of + 10% to − 10%. If the value of a particular sample exceeds the range, the analysis does not pass the validation check.
Measurement of the suitability of water for irrigation
The suitability of the Churni River for irrigation has been assessed using three types of hazards: sodicity, alkaline and permeability hazard. Different types of indices have been employed for measuring each hazard as follows:
- i.
Sodicity hazard: Sodicity implies the amount of dissolved sodium present in water. Irrigation water having a high concentration of sodium brings about hazard deforming soil physical structure and making plant growth difficult. The physical processes directed by the high concentration of sodium are soil dispersion and clay platelet and aggregate swelling (Sarah 2004). The degree of force that binds clay particles together becomes feeble at the presence of the large amount of sodium ions in the pore space. Therefore, clay particles swell which leads to soil dispersion. Consequently, soil pores are plugged by clay particles resulting in poor soil permeability. More particularly, the three main types of clay (montmorillonite, illite and kaolinite) have their different sodium ion holding capacity. Montmorillonite clay having high sodium holding capacity is considerably more prone to swelling and dispersion than other clays while kaolinite is least prone to swelling and dispersion (DeCarlo and Shokri 2014; Grim 1939). Therefore, sodium hazard is a good measure for assessing the suitability of irrigation water and widely evaluated through SAR and % Na.
- a.
SAR: It is a ratio of sodium ion to calcium and magnesium ions which helps in predicting the degree to which irrigation water tends to enter into cation-exchange reaction in soil, and it is expressed as follows (Asadollahfardi et al. 2013):
The significant relationship between SAR values and the extent to which sodium is absorbed by soil has been proved and accepted.
- b.
% Na: Sodium in water is generally expressed in two ways: percent sodium and soluble sodium percentage. Apart from creating obstacles for plants’ growth directly, it changes the physical and chemical properties of soil. Percent sodium is calculated using the following formula (Singh and Kumar 2015):
-
ii.
Alkaline hazard measured by RSC decides the degree of alkaline hazard for soil (Richards 1954). Alkalinity in the most natural surface water is derived from dissolution of carbonate and CO2 present in the atmosphere. Normally, alkalinity in water is determined by three carbonate species (H2CO3, HCO3− and CO32−) and their proportion is dependent on pH and temperature. When the concentration of carbonate exceeds the total amount of calcium and magnesium, the quality of water for irrigation deteriorates. In water, if the concentration of carbonate and bicarbonate is too high, there is a tendency for calcium and magnesium to form solid materials (scale) and precipitates as calcium carbonate (CaCO3) and magnesium carbonate (MgCO3) as a result of a chemical reaction (Dinka 2016; Silva 2004). Consequently, the relative proportion of sodium in water has a tendency to be increased as sodium bicarbonate. This excess (amount of sodium increased due to precipitation of calcium and magnesium) of sodium is known as residual sodium carbonate and calculated using the following equation (Richards 1954):
-
iii.
Permeability hazard measured by PI defines the potentiality of water to hinder soil permeability. Permeability of soil is greatly influenced by continuous irrigation with the water having a high concentration of sodium, calcium, magnesium and carbonates assembled in soil (Doneen 1964). It was Doneen who evaluated the suitability of water for irrigation based on permeability index using the following equation (Doneen 1964):
Results
EC and TDS
Electrical conductivity determines the quality of water for both drinking and irrigation purposes because salinity hazard and suitability of water for irrigation are, to a great extent, determined by the concentration of EC. It is the capacity of water to conduct electrical current, and this capacity is directly proportional to the ions and solid particles present in water. The mean EC for both Majdia and Ranaghat is about 510 μS/cm (Table 1) while slightly higher coefficient of variation (CV) of about 12% has been recorded by Ranaghat compared to Majdia (CV = 9%). Total dissolved solids refer to all organic and inorganic substances present in water. In other words, it is a measure of any minerals, salts, cation and anion dissolved in water. The acceptable range of TDS for irrigation is from 200 to 500 mg/L. If the concentration of TDS is higher than 1500, attention should be paid on the selection of crops and the duration and frequency of irrigation and soil drainage (Table 2). The mean TDS for Majdia and Ranaghat is about 337 mg/L and 329 mg/L (Table 1), respectively, and CV for both the stations is about 8%.
Ionic chemistry of water
From the chemical analysis, it has been found that the concentration of all cations is in the order of Ca2+ > Mg2+ > Na+ > K+ and that of anions is of HCO3− > CO32- > Cl− > SO42− at Majdia and Ranaghat (Table 3). Water samples from both the stations reveal neutral to the alkaline type of water (pH ranging from 7.2 to 8.4 at Majdia and from 7 to 8.3 at Ranaghat). The piper diagram depicts that the dominant water facies is Ca2+–CO32-–HCO3−. The cationic triangle indicates Ca2+-dominated water while anionic triangle indicates carbonate and bicarbonate–dominated water. Moreover, the diagram shows that all the samples fall in the zones of 1, 3 and 5 (Fig. 3a–c), indicating that alkaline earth (Ca2+ + Mg2+) exceeds alkalies (Na+ + K+), weak acids (CO32- + HCO3−) exceed strong acids (SO42− + Cl−) and carbonate hardness (secondary alkalinity) exceeds 50%. The average concentration of the cations and anions is similar in both the measuring stations except relatively higher values for Na+, Cl− and SO42− at Ranaghat (Table 3). The similar results have been observed regarding other descriptive statistics—minimum, maximum and CV (Table 3). To comprehend the association between the anions and cations, a correlation matrix has been derived (Table 4) which depicts that calcium, magnesium, sodium, CO32-, HCO3− and Cl− are strongly correlated with the other ions and most correlation is significant at the 0.01 level (2-tailed) at both the stations. But, potassium and sulphate are poorly correlated with the other anions and cations. Except for some minor irregularity, the overall strong and significant relation between the ions may ensure the quality water for irrigation at both the stations. Besides, the ion balancing test also reveals that all the values of water samples are within 10% ion balance errors except the average value − 16 found in 2011 at Ranaghat (Fig. 4). The average (based on mod values) and median of ion balance error during 2011–2017 are 2.5 and 1.8 at Majdia and 4.1 and 1.8 at Ranaghat, respectively, which signify good ionic combination which also ensures the quality of water for irrigation.
Piper trilinear diagram (a Majdia; b Ranaghat) representing hydrochemical classification of the water samples of the Churni River c. Hydro-chemical zones -in zone 1, alkaline earth (Ca2+ + Mg2+) exceeds alkalies (Na+ + K+); in zone 2, alkalies exceed alkaline earth; in zone 3, alkalies exceed alkaline earth; in zone 4, strong acids exceed weak acid; in zone 5, carbonate hardness (secondary alkalinity) exceeds 50%; in zone 6, non-carbonate hardness (secondary alkalinity) exceeds 50%; in zone 7, non-carbonate alkali (primary salinity alkalinity) exceeds 50%; in zone 8, carbonate alkali (primary alkalinity) exceeds 50%; in zone 9, no cation-anion pair exceeds 50%
Irrigation hazards
Sodicity hazard
The high concentration of sodium in water is not suitable for irrigation because it creates sodium hazard in soil destroying soil aggregates and decreasing soil permeability which jointly triggers hindrance for good agricultural production. Sodium hazard has been quantitatively analysed by SAR and % Na.
- a.
SAR: Higher sodium absorption ratio in water badly affects the soil in such a way that sodium ion tends to be absorbed by clay particles, displacing Ca2+ and Mg2+, resulting in its low permeability and poor soil aeration and soil with poor drainage (Kelly 1951; Ishaku et al. 2011). For measuring the salinity and sodium hazard of irrigation water, Richards (1954) classified the concentration of soluble salt into four classes based on EC and concentration of SAR. Different classes of salinity hazard are low or C1 (EC < 250 μS/cm), medium or C2 (EC 250–750 μS/cm), high or C3 (EC 750–2250 μS/cm) and very high or C4 (EC > 2250 μS/cm). Similarly, classes of sodium hazard are low or S1 (SAR < 10), medium or S2 (SAR 10–18), high or S3 (SAR 18–26) and very high (SAR > 26). After plotting all the values of SAR against EC in a U.S. Salinity Laboratory Staff (USSL) diagram, it has been found that 100% of the samples fall in S1 and C2 classes, indicating that the water is free from sodium hazard but has medium salinity hazard (Fig. 5a, b). In Majdia, the value of EC ranges from 204 to 697 μS/cm with the mean value of 517.89 μS/cm, and in Ranaghat, it ranges from 182 to 731 μS/cm with the mean value of 516 μS/cm.
- b.
% Na: Percentage of sodium is commonly used for assessing the suitability of water for irrigation. When the concentration of sodium in irrigation water is high, sodium has a tendency to be absorbed by clay particles, displacing calcium and magnesium ions. This process reduces the soil permeability. The values of percent sodium are plotted against the values of electrical conductivity using the Wilcox diagram (Wilcox 1955) which categorises water for irrigation into five classes: excellent (< 20% Na), good (20–30% Na), permissible (40–60% Na), doubtful (60–80% Na) and unsuitable (> 80% Na). From the tested value of the water sample, both the stations come under excellent to good category (Figs. 3b and 6a).
Alkalinity hazard
The water with high residual sodium carbonate has high pH value and, when irrigated with such type of water, can cause infertile soil owing to the deposition of sodium carbonate. As per the USSL classification, the value of residual sodium carbonate less than 1.25 signifies good quality of water, 1.25–2.50 signifies medium quality of water and more than 2.50 signifies bad quality of water for irrigation. Moreover, the constant use of water which contains the RSC value of more than 2.5 may hinder the movement of air and water in the soil by clogging the soil pores. In Majdia, the value of RSC ranges from 4.5 in 2013 to 5.6 in 2011 with the mean value of 5, and in Ranaghat, it ranges from 4.5 to 7.5 with the mean value of 5.1 (Table 5). Based on the RSC classification, all the tested samples of Majdia and Ranaghat exceed the range of 2.5 which signifies that the water containing an excess amount of bicarbonate is not suitable for irrigation.
Permeability hazard
Doneen (1964) categorised the values of PI into three classes: values less than 80 (class 1) as good (75% permeability), from 80 to 100 (class 2) as moderate (25–75% permeability) and more than 100 (class 3) as poor (25% of maximum permeability) and as bad quality of water for irrigation. The result shows that at Majdia, the PI value ranges from 39 to 74 with an average value of 52 and, in Ranaghat, the PI value ranges from 40 to 139 with an average value of 55 (Fig. 7a, b; Table 5). Based on the PI values, all the tested samples of Majdia and Ranaghat fall in class 1 which signify that the water is suitable for irrigation.
Suitability of water for irrigation
To assess the suitability of water for irrigation, a compound-ranking method was applied. Ranks have been assigned to seven parameters based on their degree of suitability for irrigation (Table 6). Ranks 1, 2 and 3 represent good, permissible and unsuitable, respectively. The average composite rank scores for both Majdia and Ranaghat have been found to be 1.57 (Table 7), indicating good to the permissible category of water. But if a sophisticated look is embraced into absolute values of seven hazard parameters (Table 7), it is found that Majdia has registered a higher score regarding three parameters (EC, TDS and PI) showing that the lesser hazard score for Ranaghat makes this station more suitable for irrigation.
Upstream-downstream variation in irrigation water quality
To find out any statistically significant difference between two stations in terms of seven selected variables (EC, TDS, ion balance, SAR, % Na, RAC and PI), separate ANOVA for each variable has been run. The ‘F critical’ for 162 degrees of freedom at the 0.05 significance level is 3.89 while each computed F value remains well below that range except SAR (Table 8) which portrays that null hypothesis is accepted for every case except SAR. Similarly, the significance value, i.e. P value (Table 8) in case of every parameter except the SAR, exceeds 0.05, signifying that the null hypothesis is accepted. It proves that there is no significant difference between the upstream (Majdia) and downstream (Ranaghat) segments of the river in water quality for irrigation.
Discussions
The ANOVA findings indicate that there is upstream-downstream variation in water quality only in terms of SAR. And, the rest of the parameters have strongly nullified this trend. However, a slight difference though not statistically significant exists in overall water quality between the stations. Therefore, this work intends to address these variations. First, the value of SAR is more for Ranaghat than Majdia because downstream of the Churni River is a two-way semi-tidal channel having a comparatively more concentration of soluble salts by the saline intrusion. And, it is established that the SAR is linearly correlated with the tidal water intrusion (Shammi et al. 2016). Thus, Ranaghat is revealing more salinity hazard than Majdia. Second, the slight difference vis-à-vis overall water pollution is due to the discharge of effluents from wastewater released by Carew and Company located at Darshana in Bangladesh while manufacturing sugar, wine and chemicals (Das and Chakrabarty 2007). Throughout the year, this company releases its stored wastewater into the river Mathabangha which ultimately finds its way into the river Churni, thereby increasing carbonate hardness and alkalinity. More particularly, the tests depict that in normal situation, the concentrations of carbonate hardness and alkalinity in the river are 321 mg/L and 216 mg/L, respectively, which, during the discharge of industrial effluents from Carew and Company, increase up to 324 mg/L for carbonate hardness and 328 mg/L for alkalinity. As the level of pollution decreases with increasing distance, the effect of wastewater of this industry is found to be minimum for Ranaghat located downstream. Moreover, another potential source of water pollution in the downstream segment is the urban effluents especially from the built-up area of Ranaghat municipality and the agricultural runoff from the on-bed and off-bed intensive agricultural practice. Ozaki et al. (2014) pointed out that the pollution of water is largely controlled by the balance between the volume of toxic effluents and the magnitude of dilution effects by the tidal action. Similarly, many scholastic works established that the overall quality of water is relatively poorer in low tidal stretches of the river and the better quality in high tidal one. However, few scholars strongly established that seawater intrusion is the principal factor for the stagnation of river water by lowering the discharge and scaling up the pollution loads at a significant rate (Hosoi et al. 1996; Akther and Ahmed 2019). The Churni River, although receiving little toxic loads from urban effluents because of the less spatial urbanisation (only one municipality, i.e. Ranaghat, having an area of about 8 km2), gets substantial agricultural runoff. However, the tidal flux from the Hooghly River into the downstream part of the Churni triggers a proactive dilution process especially during the high tide and thereby maintaining the overall water pollution lesser at the lower stretch of the river.
Conclusion
The present study reveals that all the samples fall under low sodium hazard (S1) and medium salinity hazard (C2) in the USSL diagram while all samples come under ‘excellent to good category’ for irrigation to sensitive crops on the Wilcox diagram. As per the RSC, water is not suitable for irrigation due to the high concentration of carbonate ions whereas Doneen’s PI values indicate that the river water is free from permeability hazard. River Churni has been facing multiple pressures since long back, and now, the river has become a subject of an international issue as Dasrana sugar mill situated in Bangladesh (a neighbouring country of India) is releasing industrial wastewater into the Churni River. Besides, the lower reach of the river is receiving industrial effluents and urban sewage. Consequently, the water of the entire river has been affected by pollutants, leaving an unhealthy environment for aquatic lives and scaling down the level of well-being of the people depending upon the river. Though no serious issues have been faced using water for irrigation (good composite rank score of 1.57), the water may be harmful if the pollution level continues to be raised. Although there is very slight difference between the hazard scores for irrigation, the ANOVA reveals that there is no significant difference of the physicochemical parameters between the upper (Majdia) and the lower (Ranaghat) stretches of the river.
References
Akther S, Ahmed KR (2019) Water chemistry and water quality of a tidal river system in relation with riverbank land use pattern and regional climate in the southwest Bengal Delta of Bangladesh. Sustain Water Resour Manag 5:1259–1279. https://doi.org/10.1007/s40899-019-00308-3
Ali Moasheri, A., Tabatabai, S. M., Sarani, N., & Alai, Y. (2012). Estimation spatial distribution of sodium adsorption ratio (SAR) in groundwater’s using ANN and geostatistics methods, the case of Birjand Plain, Iran. International Conference on Chemical, Ecology and Environmental Sciences (ICEES’ 2012), (pp. 123–129).
Allison MA (1998) Historical changes in the Ganges-Brahmaputra Delta Front. Journal of Coastal Research 14(4):1269–1275
Allison M, Kepple E (2001) Modern sediment supply to the lower delta plain of the Ganges-Brahmaputra River in Bangladesh. Geo-Mar Lett 21:66–74. https://doi.org/10.1007/s003670100069
Asadollahfardi G, Hemati A, Moradinejad S, Asadollahfardi R (2013) Sodium adsorption ratio (SAR) prediction of the Chalghazi River using artificial neural network (ANN) Iran. Curr World Environ 8(2):169–178. https://doi.org/10.12944/CWE.8.2.02
Baig Asadullah MW, Wanjuleb RV, Shindec HH (2018) Assessment of wastewater quality of Kham River for irrigation. Mater Today: Proceedings 5:113–119. https://doi.org/10.1016/j.matpr.2017.11.061
Chapman, G., & Rudra, K. (2015). Time streams: history and rivers in Bengal. Centre for Archaeological Studies & Training, Eastern India.
Costa JL, Aparicio VC (2015) Quality assessment of irrigation water under a combination of rain and irrigation. Agric Water Manag 159:299–306. https://doi.org/10.1016/j.agwat.2015.06.017
Das BC (2015) Socio-economic impact of a decaying river on fishermen: a case study of Taranipur Village, West Bengal. International Journal of Research in Management, Science & Technology 3(4):141-149
Das SK, Chakrabarty D (2007) The use of fish community structure as a measure of ecological degradation: a case study in two tropical rivers of India. BioSystems 90:188–196. https://doi.org/10.1016/j.biosystems.2006.08.003
DeCarlo KF, Shokri N (2014) Salinity effects on cracking morphology and dynamics in 3-D desiccating clays. Water Resour Res 50(4):3052–3072
Dinka MO (2016) Quality composition and irrigation suitability of various surface water and groundwater sources at Matahara Plain. Water Res 43(4):677–689. https://doi.org/10.1134/S0097807816040114
Directorate of Census Operation (2011). Village and town wise primary census abstract, District census handbook, Nadia, Series 20 Part XII-B, Census of India.
Directorate of Economics and Statistics (2018). Agricultural statistics at a glance, 2017, Ministry of Agriculture & Farmers Welfare, Department of Agriculture, Cooperation & Farmers Welfare, Government of India. https://eands.dacnet.nic.in/PDF/Agricultural%20Statistics%20at%20a%20Glance%202017.pdf. Accessed 4 July 2018
Disli E (2017) Hydrochemical characteristics of surface and groundwater and suitability for drinking and agricultural use in the Upper Tigris River Basin, Diyarbakır–Batman, Turkey. Environ Earth Sci 76:1–23. https://doi.org/10.1007/s12665-017-6820-5
Doneen LD (1964) Water quality for agriculture. Department of Irrigation. University of California, Davis
Eaton EM (1950) Significance of carbonate in irrigation water. Soil Sci 69:12–133. https://doi.org/10.1097/00010694-195002000-00004
Fulazzaky MA (2010) Water quality evaluation system to assess the status and the suitability of the Citarum River water to different uses. Environ Monit Assess 168:669–684. https://doi.org/10.1007/s10661-009-1142-z
Gibbs RJ (1970) Mechanisms Controlling World Water Chemistry. Science 170 (3962):1088-1090
Goodberd S (2003) Response of the Ganges dispersal system to climate change: a source-to sink-view since the last interstade. Sediment Geol 162:83–104. https://doi.org/10.1016/S0037-0738(03)00217-3
Grim RE (1939) Relation of the composition to the properties of clays. J Am Ceram Soc 22(1–12):141–151. https://doi.org/10.1111/j.1151-2916.1939.tb19440.x
Guchhait SK, Islam A, Ghosh S, Das BC, Maji NK (2016) Role of hydrological regime and flood plain sediments in channel instability of the Bhagirathi River, Ganga Brahmaputra Delta, India. Phys Geogr 37(6):1–35. https://doi.org/10.1080/02723646.2016.1230986
Hosoi Y, Kido Y, Nagira H, Yoshida H, Bouda Y (1996) Analysis of water pollution and evaluation of purification measures in an urban river basin. Waf Sci Tec 34(12):33–40
Huong NT, Li MO, Higashi T, Kanayama M (2008) Assessment of the water quality of two rivers in Hanoi City and its suitability for irrigation water. Paddy Water Environ 6:257–262
Ishaku JM, Ahmed AS, Abubaka MA (2011) Assessment of groundwater quality using chemical indices and GIS mapping in Jada area, Northeastern Nigeria. J Earth Sci Geotech Eng 1(1):35–60
Islam A, Guchhait SK (2017a) Analysing the influence of Farakka Barrage Project on channel dynamics and meander geometry of Bhagirathi River of West Bengal, India. Arab J Geosci 10(11):245
Islam A, Guchhait SK (2017b) Search for social justice for the victims of erosion hazard along the banks of river Bhagirathi by hydraulic control: a case study of West Bengal, India. Environ Dev Sustain 19(2):459. https://doi.org/10.1007/s10668-015-9739-6
Islam A, Guchhait SK (2018) Analysis of social and psychological terrain of bank erosion victims: a study along the Bhagirathi River, West Bengal, India. Chin Geogr Sci 28:1009–1026. https://doi.org/10.1007/s11769-018-0937-7
Kelly WP (1951) Alkali soils—their formation, properties and reclamation. Reinhold, New York
Khaki M, Yusoff I, Ismalami N (2015) Application of the artificial neural network and neuro fuzzy system for assessment of groundwater quality. Clean - Soil, Air, Water 43(4):551–560
Khan R, Israili SH, Ahmad H, Mohan A (2005) Heavy metal pollution assessment in surface water bodies and its suitability for irrigation around the Neyveli lignite mines and associated industrial complex, Tamil Nadu, India. Mine Water Environ 24:155–161. https://doi.org/10.1007/s10230-005-0087-x
Kuehl S, Levy ML, Moore WS, Allison M (1997) Subaqueous delta of the Ganges-Brahmaputra River system. Mar Geol 144:81–96. https://doi.org/10.1016/S0025-3227(97)00075-3
Kumar SK, Bharani R, Magesh S, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4:341–350. https://doi.org/10.1007/s13201-013-0148-4
Kumarasamy P, Dahms H, Jeon H, Rajendran A, James RA (2013) Irrigation water quality assessment—an example from the Tamiraparani River, Southern India. Arab J Geosci 7:5209–5220. https://doi.org/10.1007/s12517-013-1146-4
Majumdar SC (1941) Rivers of Bengal Delta. Bengal Government Press, Kolkata
Majumder, D. (1978). West Bengal District Gazetters Nadia. Govt. of W. B.
Nag SK, Das S (2014) Quality assessment of groundwater with special emphasis on irrigation and domestic suitability in Suri, I & II Blocks, Birbhum District, West Bengal, India. Am J Water Resour 2(4):81–98
Ozaki H, Co TH, Le AK, Pham VN, Nguyen VB, Tarao M et al (2014) Human factors and tidal influences on water quality of an urban river in Can Tho, a major city of the Mekong Delta, Vietnam. Environ Monit Assess 186:845–858. https://doi.org/10.1007/s10661-013-3421-y
Parua P (2010) The Ganga: water use in the Indian subcontinent. Springer
Piper AM (1944) A geographic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Rao PV, Rao SA, Rao NS (2015) Suitability of groundwater quality for drinking, irrigation and industrial purposes in the Western Delta Region of the river Godavari, Andhra Pradesh. J Geol Soc India 86:181–190. https://doi.org/10.1007/s12594-015-0297-1
Ravikumar P, Somashekar RK, Prakash K (2015) A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Sci 80:31073–31077
Reddy KS (2013) Assessment of groundwater quality for irrigation of Bhaskar Rao Kunta watershed, Nalgonda District, India. Int J Water Resour Environ Eng 5(7):418–425. https://doi.org/10.5897/IJWREE2012.0375
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. USDA hand book.
Rudra K (2010) Dynamics of the Ganga in West Bengal, India (1764–2007)—implications for science-policy interaction. Quat Int 227:161–169. https://doi.org/10.1016/j.quaint.2009.10.043
Rudra K (2011). The encroaching Ganga and social conflict: the case of West Bengal, India. Kolkata.
Rudra K (2014) Changing river courses in the western part of the Ganga Brahmaputra Delta. Geomorphology 227:87–100. https://doi.org/10.1016/j.geomorph.2014.05.013
Rusydi AF (2018) Correlation between conductivity and total dissolved solid in various type of water: a review. IOP Conf. Series: Earth and Environmental Science. IOP. 118:012019. https://doi.org/10.1088/1755-1315/118/1/012019
Sadick A, Prince Charles Asante PC, Dugan E, Asaana J (2017) Correlation analysis of irrigation water quality parameters from Lake Bosomtwe in the Ashanti Region of Ghana. SCIREA J Agric 2:2
Sarah P (2004) Soil sodium and potassium adsorption ratio along a Mediterranean–arid transect. J Arid Environ 59:731–741. https://doi.org/10.1016/j.jaridenv.2004.02.007
Satyanarayana E, Ratnakar D, Muralidhar M (2016) Major ion chemistry of groundwater and surface water in parts of Mulugu-Venkatapur Mandal, Warangal District, Telangana State, India. Hydrol Current Res 7(3):253. https://doi.org/10.4172/2157-7587.1000253
Shakir E, Zahraw Z, Al-Obaidy AH (2017) Environmental and health risks associated with reuse of wastewater for irrigation. Egypt J Pet 26:95–102. https://doi.org/10.1016/j.ejpe.2016.01.003
Shammi M, Rahman R, Rahman MM, Moniruzzaman M, Bodrud-Doza M, Karmakar B, Uddin M (2016) Assessment of salinity hazard in existing water resources for irrigation and potentiality of conjunctive uses: a case report from Gopalganj District, Bangladesh. Sustain Water Resour Manag 2:369–378. https://doi.org/10.1007/s40899-016-0064-5
Silva EL (2004) Quality of irrigation water in Sri Lanka—status and trends. Asian J Wat Environ Pollution 1(1–2):5–12
Singh AK, Kumar SR (2015) Quality assessment of groundwater for drinking and irrigation use in semi-urban area of Tripura, India. Eco Env & Cons 21(1):97–108
Smith CJ, Oster JD, Sposito G (2014) Potassium and magnesium in irrigation water quality assessment. Agric Water Manag 157:59–64. https://doi.org/10.1016/j.agwat.2014.09.003
Todd DK (1959) Groundwater hydrology. Wiley, New York
Wilcox LV (1955). Classification and use of irrigation water. USDA.
Acknowledgements
We are thankful to the anonymous reviewers and responsible editor for their valuable and constructive suggestions.
Funding
This study was financially supported by the Indian Council of Social Science Research (ICSSR) given to the first author of this paper (vide File number RFD/2017-18/ENVI/GEN/324) to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Broder J. Merkel
Rights and permissions
About this article
Cite this article
Sarkar, B., Islam, A. Assessing the suitability of water for irrigation using major physical parameters and ion chemistry: a study of the Churni River, India. Arab J Geosci 12, 637 (2019). https://doi.org/10.1007/s12517-019-4827-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-019-4827-9