Abstract
The Al-Kharrar Lagoon (KL) and Salman Bay (SB) are located on the eastern Red Sea coast, Saudi Arabia. The concentrations of heavy metals such as Fe, Mn, Zn, Cu, Ni, Pb, Co, and Cd in their bottom sediments were measured and correlated with the surface water temperature, salinity, pH and dissolved oxygen (DO), organic carbon (OC), carbonates, and sediment grain size. The highest concentration of metals in the KL is mostly attributed to influx of siliciclastics from wadies to the center and south-eastern parts of the lagoon where metals were directly correlated with salinity, pH, and mud. At the inlet stations, the metals were the lowest indicating that the KL acts as a buffer zone, preventing metals from dispersion into the Red Sea waters. But also, the enrichment factor (EF), there, for Mn, Zn, Cu, Co, and Ni, was the highest due likely to the effectiveness of biosorption by mucus algae that proliferate on coral debris and sand grains. The metals had no relationships with the OC, but with mud and Fe-Mn oxides due possibly to the presence of high DO. The brine waters of SB showed inverse relationships with the metals that ascribed probably to the occurrence of many soluble salts preventing precipitation of heavy metals into the sediments. Normalizing the heavy metals with Fe and the world average shale indicated that the bottom sediments of the KL and SB were uncontaminated and mainly influenced by natural sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (commonly of Fe, Mn, Al, Cu, Zn, Cd, Ni, Cr, Co, Pb, Ag, and As) in the shallow water sediments of the eastern Red Sea coast have been studied by many authors in order to evaluate their natural background levels against the effect of anthropogenic activities (El Sayed and Basaham 2004; Turki 2007; Badr et al. 2009; Pan et al. 2011; Abu-Zied et al. 2013; Ghandour et al. 2014; Basaham et al. 2015; Abu-Zied et al. 2016; Youssef and El-Sorogy 2016; Abu-Zied and Hariri 2016; Al-Mur et al. 2017). Some of these studies have showed that the shallow water sediments of the Red Sea that are far away from cities are not polluted and have natural heavy metal accumulations except, for example, those of the coastal area of Jeddah City that has been highly affected by human and industrial pollutions leading to occurrence of high concentration of heavy metals in their sediments (Abu-Zied and Hariri 2016; Al-Mur et al. 2017). In the un-polluted areas, the nearshore sediments of the Red Sea were mainly composed of biogenic sands with very low metal concentrations which primarily originated from the influx of wadies that are numerous in the eastern coastal plain of the Red Sea (Abu-Zied and Hariri 2016). These wadies are active for a short period during winter and originate from the escarpment of the Al-Hejaz and Asir mountains; they end with mouths (such as bays, lagoons and sharms) or disappear in the alluvium of the eastern coastal plain of the Red Sea.
Few studies have dealt with the factors that control the concentrations of heavy metals in nearshore sediments of the Red Sea. Among of these studies, Abu-Zied et al. (2013, 2016) and Abu-Zied and Hariri (2016) have showed that the distribution and accumulations of heavy metals (e.g., Fe, Mn, Cu, Zn, Cd, Ni, Cr, Co, and Pb) in the surficial bottom sediments of the eastern coast of the Red Sea have been affected by factors such as temperature, salinity, pH, dissolved oxygen, organic carbon, carbonates, sediment grain size, closeness to natural or pollution point-source, and redox potential. Also, the degree of bioavailability, mobility, and toxicity of heavy metals in the environment depend strongly on their specific chemical forms (Yu et al. 2010; Tamunobereton-ari et al. 2011). Pan et al. (2011) studied the speciation of heavy metals (Cd, Zn, Ag, Cu, Pb, As, and Hg) in the sediments of eastern coast of the Red Sea and concluded that the sequential extraction indicated that the anthropogenic input of metals was not significant in the studied areas except in the local fish market of Jeddah City. They also mentioned that the metals of the exchangeable phase which is an indication of the anthropogenic origin and of high potential bioavailability were low in all studied sites, whereas those of Fe-Mn oxides and organic phases were high in the sediments of the local fish market of Jeddah City. This is because of Fe-Mn oxides and organic matters are important metal scavengers in sediments through various mechanisms including coprecipitation, adsorption, surface complex formation, and ion exchange.
In addition, bacteria play a role in metal distributions, relationships, and Fe-Al complexation in metal-polluted lake sediment due to having mineral coatings of varying composition and texture formed by adsorption of detrital clay and precipitation of authigenic oxides and clay (Jackson et al. 2011). They also reported that some bacteria mobilize metals by releasing water-soluble chelating agents, while others immobilize them by producing H2S. Also, algae, specially the brown seaweed Sargassum sp., can mediate and change the concentration of heavy metals in seawaters and hence in sediments due to having high potentials for the accumulation of heavy metals, as compared to other algal genera (Da Costa et al. 2001; Bina et al. 2006). They also mentioned that this seaweed is mainly constituted by the polysaccharide alginate (usually calcium, magnesium, sodium, and potassium salts) produced due to the interaction between alginic acid and alkaline and alkaline-earth elements from seawater, then acting as efficient ion-exchangers (biosorbents) for heavy metals present in solution.
The heavy metals in seawaters and sediments have a twofold of importance. They are essential nutrients for marine organisms and become very toxic if its concentrations exceed the requirements of metabolic processes of these organisms which are being used as good food sources for human (Du Laing et al. 2009; Tamunobereton-ari et al. 2011; Oves et al. 2016). Also, Tchounwou et al. (2012) reported that metals such as Co, Cu, Cr, Fe, Mg, Mn, Mo, Ni, Se, and Zn are essential nutrients that are required for various biochemical and physiological functions, but inadequate supply of these micronutrients results in a variety of deficiency diseases or syndromes.
The Saudi Red Sea lagoons and bays and its natural resources that are close to coastal cities are at risk due to pollution from domestic and industrial wastes and their use as fish farms (Abu-Zied et al. 2013; Al-Mur et al. 2017). Such anthropogenic activities are, at present, growing rapidly and could lead to increase of heavy metal concentrations above the permissible limits and change the water quality of these aquatic environments (Badr et al. 2009), especially the ones (Al-Kharrar and Salman lagoons) that are under the concern of this study.
Therefore, this study aims to determine the concentrations of the heavy metals such as iron (Fe), manganese (Mn), nickel (Ni), zinc (Zn), copper (Cu), cobalt (Co), cadmium (Cd), and lead (Pb) in the surficial bottom sediments of Al-Kharrar (hypersaline) and Salman (brine pool) lagoons in order to compare them with the natural background level and make distinction between the heavy metal inventory of these lagoons. Also, the heavy metal concentrations in the studied sediments will be correlated with the hydrogeochemical parameters (temperature, salinity, pH, dissolved oxygen, and organic carbon and carbonates) and sediment grain size to shed light on the factors affecting their concentrations and distribution.
The study area
Al-Kharrar Lagoon
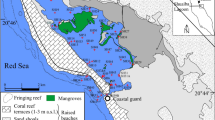
Al-Kharrar Lagoon (KL) is located 9 and 140 km north of Rabigh and Jeddah cities, respectively. It occurs in the center of the eastern coast of Red Sea, Saudi Arabia, between latitudes 22.84° and 23.00° N and longitudes 38.83° and 38.98° E (Fig. 1). The KL is a normal to hypersaline water body with a salinity ranging from 38.8 to 41.5‰, during winter (this study). It has an elongated shape parallel to the Red Sea with a total area of 82 km2 and elongated small islets at its northern and center of its southern parts (Fig. 1). Mangrove shrubs grow abundantly around the islets and at the northern and southern shorelines (Fig. 1). It is only connected with the Red Sea water via a narrow (120 m wide) and deep (14 m) inlet located at its northwestern corner (Al-Dubai et al. 2017). The eastern side of the KL is connected with several dry wadies (such as: Rabigh, Rahab, Murayyikh and Al-Khariq) originating from the escarpment of the Al-Hejaz mountains (Fig. 1). These wadies feed the KL with an irregular influx of freshwaters only during winter for a short period. The influx of freshwaters via the Wadi Rabigh is no longer reaching the KL since 2009 due to the construction of Rabigh Dam about 35 km east of Rabigh City.
Salman Bay
Salman bay (SB) is located north of Jeddah City, center of the eastern coast of Red Sea, Saudi Arabia (Fig. 1). It occurs between latitudes 21.84° and 21.95° N and longitudes 38.96° and 39.04° E (Fig. 1). The SB has an elongated shape parallel to the Red Sea with a total area of 32 km2 (Fig. 1). It is only connected with the Red Sea water via a very narrow, shallow inlet located at its northwestern corner. It is a brine water body with a salinity ranging from 70 to 80‰, during winter (this study). Owing to shallowness of SB and restriction of water exchange between it and Red Sea, its water quality is deteriorated. Field work indicated that the SB is not connected either to wadies of the catchment area or to industrial effluents, but layer of black sludge was noticed on its eastern shoreline. The brine waters of the SB are used for salt extraction by the SASA salt company located at the eastern coast of the bay (Fig. 1).
Materials and methods
Field work
Twenty five and Thirteen surficial (upper 2 cm) bottom sediments were collected from the Al-Kharrar Lagoon (KL) and Salman Bay (SB) during December 2016, respectively. The KL’ samples were collected on board using the Van Veen grab sampler and those of the SB were collected from close to shoreline using a stainless steel knife. Immediately after collection, the substrate types were described, and the upper 2 cm was cut off and placed in plastic bags, then transported to laboratory for geochemical and heavy metal analyses. At each station of the sediment samples, the hydrochemical parameters such as water depth (measured by Eco-sounder UWTEC), temperature, salinity, pH, and dissolved oxygen (DO) were, in situ, measured by YSI 556 MPS Meter. At some stations of the KL (KH3, KH8, and KH10), vertical profiles for water column temperature, salinity, and DO were undertaken. The coordinates of sediment samples at each station were determined by Garmin II GPS and listed with substrate types and hydrochemical parameters in Table 1.
Granulometry
Sediment grain size variations were determined by wet sieving method; this is because the collected sediment samples are small and consist mainly of bioclastics grains that when dried, they get solidified. So, it is better to disintegrate them by wet sieving. A known dry weight of the sediment sample was passed through > 2 and 0.063 mm sieve openings using a shower of tape water until all mud grains passed through the 0.063 mm sieve opening. The fractions that were retained in the sieves were dried in an oven at 60 °C for 72 h. After that, the dried fractions in each sieve were weighed to determine the percentages of the gravel (> 2 mm) and sand (0.063–2 mm) fractions. Mud fraction was determined by subtracting the total gravel and sand fraction from the total dry weight of sediments.
Geochemical analysis
It was performed on bulk sediment dry weight, because it is a good representation for all metals since some samples contain insignificant amounts of fine-grained sediments and simplifies sample processing and potential artifacts that might change the original composition (Herut and Sandler 2006). Sediment samples were dried on room temperature. Total organic carbon (OC) was determined by the loss on ignition (LOI) technique (Heiri et al. 2001; Abu-Zied et al. 2011). Total carbonate (CaCO3) was determined by etching 0.5 g of the dry weight sediment sample in 50 ml of 1 M HCl solution at room temperature for 24 h. After that, the residue was filtered, oven dried, and weighed. Then, the CaCO3 in the sediment sample was determined by the loss in weight.
Concentration of heavy metals (Fe, Mn, Zn, Cu, Ni, Pb, Co, and Cd) in bulk, dry weight sediment samples was measured by the atomic absorption spectrophotometer (AAS), GBC-93, Australian Model at the National Institute of Oceanography and Fisheries, Hurghada, Egypt. Approximately, 0.5 g of each pre-powdered samples was digested with a mixture of concentrated HF, HNO3, and HClO3 acids (5:3:1). The digested solution was evaporated to near dryness. The digested samples were filtered and diluted with de-ionized water (Chester et al. 1994). After that, measurement of heavy metal concentrations was undertaken by the AAS, GBC-932. To insure that maximum accuracies have been obtained, the AAS was adjusted to provide mean value of triplicate measurements of each metal with precision of < 3% and detection limit of 0.01 mg/kg; the obtained results were expressed in mg/kg (Table 2). The zero level of the AAS was adjusted by blanks and the quality control tools were adopted to avoid possible contamination. Meanwhile, the chemical reagents are of high analytical grade and all of glassware were washed with diluted acid and later rinsed with double-distilled water before use.
Enrichment and pollution factors were applied in this study to normalize and compare the metal concentrations of the studied sediments between different parts of the lagoons and between lagoons itself and to see the degree of metal enrichment in the studied sediment samples relating this enrichment either to natural or anthropogenic sources. Armstrong-Altrin and Machain-Castillo (2016) reported that the enrichment factor (EF) is a useful tool to evaluate the heavy metal contamination in sediments against their natural background. In this study, the enrichment factor (EF) of the recorded metal concentrations was applied using the Fe as normalizer to correct for difference in sediment grain size (Loring 1991; Acevedo-Figueroa et al. 2006). This is also because Fe showed a perfect correlation (r values were higher than 0.9) with all metals. The EF was calculated using this equation: EF = (M/Fe)sediment/(M/Fe)background (Abrahim and Parker 2008; Ghandour et al. 2014; Nowrouzi and Pourkhabbaz 2014; Youssef and El-Sorogy 2016). The (M/Fe)sediment represents the metal concentrations in the studied sediments and the (M/Fe)background represents the metal concentrations in the average shale background (Turekian and Wedepohl 1961). The EF < 1.5 indicates that the metals are originated from natural sources, whereas the EF > 1.5 means that the metals are relatively higher than the natural background due to contribution from natural and/or anthropogenic sources (Zhang and Liu 2002; Xu et al. 2015; Al-Mur et al. 2017).
Contamination factor (CF) was applied to determine the degree of anthropogenic contamination to the studied sediment samples. It was calculated using this equation: CF = Msediment/Mbackground. When CF < 1, this indicates that the heavy metal concentrations in sediments are uncontaminated, whereas CF > 1 means that the sediments are contaminated (Hakanson 1980; Tomlinson et al. 1980).
Pollution load index (PLI) was calculated using this equation: PLI = (CF1 × CF2 × CF3...CFn)(1/n). When PLI < 1 indicates there is no pollution and PLI > 1 indicates there is a pollution (Tomlinson et al. 1980; Al-Mur et al. 2017).
Index of geoaccumulation (Igeo) was calculated using this equation: Igeo = log2 (Cn/(1.5 × Bn)) following Muller (1969). The Cn is the measured concentration of metal (n), and Bn the metal’s concentration in the average shale (Turekian and Wedepohl 1961). The factor 1.5 is introduced to include possible variations of the background values due to lithogenic effects. Value < 0.0 indicates the sediments uncontaminated, and 0–1 indicates that the sediments are uncontaminated to moderately contaminated.
Statistical analysis
The relationships and structures that may exist between the recorded heavy metals, hydrogeochemical parameters, and sediment grain size were best obtained by the correlation matrix of the principal component analysis (PCA) and by the correlation similarity index of the R-mode hierarchical clustering (UPGMA algorithm), using the PAST software (Hammer et al. 2001). Also, the Bray-Curtis similarity index of the Q-mode hierarchical clustering (UPGMA algorithm) was performed for the data of Salman Bay.
Results
Al-Kharrar Lagoon
Hydrogeochemical parameters
Bathymetry of the Al-Kharrar Lagoon (KL) shows a gradual increase in the depth towards its opening (inlet) with the Red Sea, reaching a maximum water depth of 15 m at station 1 (Table 1). In the center of the lagoon water depth stabilizes around 8 m. In the upper lagoon (southern part), water depth averages 4 m which rapidly decreases to 0.5–1 m near-shore of the southern stations (Table 1).
Surface water temperature of the KL averaged 27.3 ± 0.8 °C during December 2016 (Fig. 2). It showed maximum values up to 29 °C at the inner inlet stations KH1–4, but at the rest of the studied stations, it decreased to an average value of 26.5 °C (Fig. 2). The bottom water temperature showed the same pattern as those of the surface water temperature, but with a subtle decrease in its mean value of 27 ± 0.8 °C (Table 1).
Surface water salinity of the KL averaged 40.8 ± 1‰ during December 2016 (Fig. 2). It showed the lowest values 38.8‰ at the inner inlet stations KH1–4. It increased to 41.5‰ at the stations of the southern part, the upper lagoon (Fig. 2). Bottom water salinity of the KL showed the same pattern as those of the surface water salinity, displaying an average of 41.1 ± 0.8‰ (Table 1). At the stations KH1–10, the bottom water salinity increased from those of the surface water by about 0.5‰, whereas at the rest of the stations (southern stations), the surface and bottom salinities are more or less similar, but with a subtle surface salinity increase of about 0.1‰ (Fig. 3, Table 1).
The surface and bottom water pH were very similar in all stations, displaying average values 8.87 and 8.86, respectively (Fig. 2, Table 1). The surface and bottom water dissolved oxygen (DO) were also the same in all stations, displaying a mean value of 6.4 mg/l (Fig. 2, Table 1). In all the studied stations (except stations KH7–10), the surface DO was a slightly higher than those of the bottom DO with a mean difference of 0.1 mg/l (Table 1). At the stations KH7–10 (center of the lagoon, 8 m water depth), the bottom water DO decreased from the overlying surface DO by a value of 0.5 mg/l (Fig. 3).
The vertical profiles of temperature, salinity, and dissolved oxygen in the water column of the KL showed that the water column consists of surface and bottom layers (Fig. 3) (Al-Dubai et al. 2017). The surface layer was less saline, warm with high DO, whereas the bottom layer was more saline, colder with slightly low DO (Fig. 3).
The total LOI organic carbon (OC) in the surficial bottom sediments of Al-Kharrar Lagoon (KL) averaged 8.4 ± 4%. It was the lowest near the inner inlet stations (KH1–7) and at the south-eastern stations of the lagoon, displaying value of 5% (Fig. 4). In the center of the lagoon, the OC was the highest displaying a value of 12% (Fig. 4). The CaCO3 in the surficial bottom sediments of KL averaged 59.24% ± 28. It was the highest near the inner inlet stations (KH1–4), displaying value of 95% (Fig. 4). In the center of the lagoon, it decreased to 50%. At the south-eastern stations of the lagoon, the CaCO3 decreased to the lowest value of 25% (Fig. 4).
Sediment grain size
The gravel fraction (> 2 mm) represents about 5 to 10% of the total bottom sediments (dry wt) at the inlet stations of the KL (Fig. 4). At these stations, it consists of rounded, yellow to brown-coated coral debris, small bivalves, and gastropods. In the rest of the lagoon, it is very low or absent, consisting only of small bivalves and gastropods, echinoid fragments, and the larger benthic foraminifera Sorites.
The sand fraction (0.063–2 mm) is the highest at the inlet stations of the lagoon, representing about 80% the total bottom sediments (Fig. 4). It is composed of the same bio-clastics of the gravel fraction, but with a plenty of benthic foraminifera. In the rest of the lagoon, it is very low (15%), enriched with small bivalves and benthic foraminifera and with small components of small gastropods, echinoid fragments/spines, and ostracod shells. At the south-eastern stations, the above components are dominated by the mica flakes.
Mud fraction (> 0.063 mm) is very low (14%) at the inlet stations of the KL (Fig. 4), whereas, in the rest of the stations, it is the highest reaching 90% of the total bottom sediments (dry wt) of the KL.
Heavy metals
Fe concentration in the surficial bottom sediments of Al-Kharrar Lagoon (KL) averaged 18,500 ± 11,200 mg/kg (Fig. 4). It was the lowest at the northern stations, near the inlet stations, displaying values around 1500 mg/kg. After that, Fe concentration increased gradually as it moves through the lagoon’s center and towards the south, reaching concentrations up to 30,000 mg/kg (Fig. 4). At the south-western stations, it showed low values around 10,000 mg/kg, whereas, at the south-eastern stations of the lagoon, highest concentrations of Fe were recorded with values up to 30,000 mg/kg (Fig. 4).
The spatial concentration distribution patterns of the rest of metals (Mn, Zn, Ni, Cu, and Co) in the surficial bottom sediments of KL are very similar to those of the Fe. The concentration of Mn averaged 330 ± 140 mg/kg (Fig. 4). It was the lowest at the northern stations, near the inlet stations, displaying values around 100 mg/kg. After that, Mn concentration increased gradually as it moves through the lagoon’s center and towards the south, reaching concentrations up to 400 mg/kg (Fig. 4). At the south-western stations, it showed low values around 200 mg/kg, whereas, at the south-eastern stations of the lagoon, highest concentrations of Mn were recorded with values up to 500 mg/kg (Fig. 4).
Zn concentration in the surficial bottom sediments of KL averaged 23 ± 15 mg/kg (Fig. 4). It was undetectable at the northern stations, near the inlet stations. After that, Zn concentration increased gradually as it moves towards the lagoon’s center, reaching concentrations up to 30 mg/kg (Fig. 4). At the south-western stations, it showed low values around 15 mg/kg, whereas, at the south-eastern stations of the lagoon, highest concentrations of Zn were recorded with values up to 45 mg/kg (Fig. 4).
Ni, Cu, and Co concentrations in the surficial bottom sediments of KL averaged 26 ± 17, 22 ± 11 and 9 ± 4 mg/kg, respectively (Fig. 4). They were the lowest at the northern stations, near the inlet stations, displaying values around 5 mg/kg. After that, their concentrations increased gradually as it moves through the lagoon’s center and towards the south, showing concentrations up to 30, 30, and 14 mg/kg, respectively (Fig. 4). At the south-western stations, they showed low values around 20, 20, and 6 mg/kg, respectively, whereas, at the south-eastern stations of the lagoon, highest concentrations of Ni, Cu, and Co were recorded with values up to 45, 35, and 14 mg/kg, respectively (Fig. 4).
Pb and Cd were undetected in the surficial sediments of the KL except at station KH15 of the south-western corner of the lagoon; they showed values of 1.3 and 7.8 mg/kg, respectively (Table 2).
Correlation of heavy metals with the hydrogeochemical parameters and sediment grain size
In the ordination plot of the PCA, the components 1 and 2 explain 47 and 16% of the variance, respectively (Fig. 5a). There is a close similarity correlation between the heavy metals Co, Ni, Zn, Mn, Fe, and Cu, and the sediment samples of the south-eastern stations of the KL (Fig. 5a). These heavy metals showed also direct relationships with the hydrochemical parameters such as salinity, dissolved oxygen, and pH. On the other hand, they were inversely correlated with the water temperature and carbonate (CaCO3), and they showed no correlation with the OC (Fig. 5a).
The heavy metals Pb and Cd showed no relationships with the other heavy metals and hydrogeochemical parameters (Fig. 5a).
The water temperature and carbonates (CaCO3) showed a close kinship correlation with the sediment samples of the near inner inlet stations KH1–6 (Fig. 5a). This correlation was also indicated by the R-mode hierarchical cluster analysis (Fig. 5b) and by the matrix of Pearson correlation coefficients (r) (Table 3).
Salman Bay
Hydrogeochemical parameters
The studied stations of Salman Bay were collected from close to the shoreline at water depths not more than 0.5 m (Table 1). The water temperature averaged 26.4 ± 2 °C during December 2016 (Fig. 2). It was the lowest (24 °C) in southern part of the bay, stations SA1–5, but at the rest of the stations, it was the highest, up to 28 °C.
The water salinity was averaged 71.8 ± 4.2 ‰. Its highest values up to 80 ‰ were recorded in southern part of the bay, stations SA1–4, but at the rest of the stations, the salinity was around 70‰ (Fig. 2).
The water pH of the Salman Bay during December 2016 averaged 8.6 ± 0.2 (Fig. 2). It was the highest up to 8.8 at southern stations SA2–5, but at the rest of the stations, it was low displaying a value of 8.55 (Fig. 2).
The dissolved oxygen was low in the Salman Bay showing a mean value of 4.5 ± 0.8 mg/l (Fig. 2). It was, more or less, the lowest at the southern stations, showing values of 3.5 mg/l, but at northern stations, it increased to 5 mg/l (Fig. 2).
The total LOI organic carbon (OC) and CaCO3 in the surficial bottom sediments of Salman Bay averaged 10.2 ± 5.3 and 70.7 ± 13%, respectively (Fig. 4). They did not show significant changes between the southern and northern part of the bay (Fig. 4).
Sediment grain size
The gravel fraction (> 2 mm) represents about 10% of the total bottom sediments (dry wt) at the studied stations of the Salman Bay (Fig. 4). It consists of blackish to white, solidified sediments, with a plenty of small gastropods, mainly of Cerithium sp., but the small bivalves are low and restricted to the Mytilus sp. At the southern stations, the above components are dominated by evaporitic crystals of gypsum.
The sand fraction (0.063–2 mm) represents about 60% of the total dry wt sediments of the studied stations of the SB (Fig. 4). It is composed of the same materials of the gravel fraction, but with a plenty of reworked benthic foraminifera.
Mud fraction (> 0.063 mm) shows 30% of the total dry wt sediments of the studied stations of the SB (Fig. 4).
Heavy metals
The distribution of heavy metals in the Salman Bay (SB) divided the bay into southern and northern parts. Fe concentration in the surficial bottom sediments of Salman Bay (SB) averaged 6150 ± 2400 mg/kg (Fig. 4). It was the lowest at the southern stations (stations SA1–5), displaying values around 4000 mg/kg. In the southern part of the SB, Fe concentration increased to 8000 mg/kg, except at the stations 12–13 that showed a value of 2500 mg/kg (Fig. 4).
The concentration distribution patterns of the rest of metals (Mn, Zn, Ni, Cu, and Co) in the surficial bottom sediments of SB are very similar to those of Fe. The concentration of Mn averaged 94 ± 40 mg/kg (Fig. 4). It was the lowest (60 mg/kg) in the surficial sediments of the southern part of the SB, displaying values around 60 mg/kg. In the northern part of the SB, Mn concentration increased to 140 mg/kg, except at the stations SA12–13 that showed value of 40 mg/kg (Fig. 4).
Zn concentration distribution in the surficial bottom sediments of SB averaged 9 ± 3 mg/kg (Fig. 4). It was, more or less, low (5 mg/kg) in surficial sediments of the southern part of the bay, except at the stations SA1–2, the highest concentration 14 mg/kg was recorded. In the northern part of the SB, Zn concentration increased to 10 mg/kg, except at the station SA13 a value of 2 mg/kg was recorded (Fig. 4).
Cu concentration distribution in the surficial bottom sediments of SB averaged 7 ± 4 mg/kg (Fig. 4). It was the lowest at the southern part of the bay, displaying values around 4 mg/kg. In the northern part of the SB, Cu concentration increased to 8 mg/kg, except at the station SA13 a value of 2 mg/kg was recorded (Fig. 4).
Ni and Co concentrations in the surficial bottom sediments of the southern part of SB were undetectable (Fig. 4). In the northern part of the SB, they increased to the highest values of 8 and 3 mg/kg, respectively (Fig. 4).
Pb and Cd were undetected in the surficial sediments of the SB except at station SA4 of the southern part of the bay, they showed values of 1.8 and 3 mg/kg, respectively (Table 2).
Correlation of heavy metals with the hydrogeochemical parameters and sediment grain size
In the ordination plot of the PCA, the components 1 and 2 explain 29 and 19% of the variance, respectively (Fig. 6a). It shows that the Fe, Mn, Ni, Cu, and Zn have direct relationships with the water temperature, but their relationships with the OC are weak. On the other hand, they are inversely correlated with the water salinity, pH, and CaCO3 (Fig. 6a). The concentrations of Pb, Cd, and Co were very low or undetectable in the surficial sediments of Salman Bay, but they showed direct relationships with pH, CaCO3, and DO. On the other hand, they are inversely correlated (or no correlation) with the water salinity, temperature, and the other heavy metals (Fig. 6a). The abovementioned relationships were also indicated by the correlation similarity index of the R-mode hierarchical cluster (Fig. 6b) and by the matrix of Pearson correlation coefficients (r) (Table 3).
Comparison of Al-Kharrar and Salman Lagoons
The Salman Bay (SB) is very shallow water body, whereas the KL has a maximum water depth of 9 m. The water temperature of the SB was lower than of the KL, although its salinity was 72‰. The water salinity of the KL was 41‰. The pH of both of the SB and KL was similar, whereas the DO of the SB was lower than of the KL. The total OC and CaCO3 of the SB were higher than those of the KL.
The concentrations of Fe, Mn, Zn, and Cu in the surficial sediments of the KL are threefold higher than those of the SB, whereas Ni and Co of KL were 10 times higher than those of the SB. The concentrations of Pb and Cd in the sediments of the SB and KL were very low or undetectable (Table 4).
In Table 4, the heavy metal concentrations (Fe, Mn, Zn, Cu, Ni, Pb, Co, and Cd) in the bottom sediments of the KL are more or less similar to those of lagoonal conditions such as Al-Arbaeen and Al-Shabab inlets (Abu-Zied et al. 2013), Shuaiba and Al-Mejarma lagoons (Basaham et al. 2015), and Sharm Obhur (Ghandour et al. 2014). They showed also a similarity to those of Gulf of Aqaba (Abu-Hilal et al. 1988) and Jizan Shelf (Basaham 2009). In other hand, these concentrations are lower than those recorded for the same area by Basaham et al. (2015) and the world average shale (Turekian and Wedepohl 1961).
The heavy metal concentrations in the shoreline sediments of SB showed, more or less, a similarity to those of the Red Sea coast (Badr et al. 2009; Abu-Zied et al. 2013; Abu-Zied and Hariri 2016; Al-Mur et al. 2017), but they are very low in comparison with the lagoons of the Red Sea and the world average shale (Table 4).
Heavy metal enrichment/pollution factors
The enrichment factors (EFs) of heavy metals (Mn, Cu, Co, and Ni) in the studied bottom sediment samples of the KL were slightly below 1.5 except at the near-inlet samples (located in the channel of bottom water outflow), they exhibited higher values than the threshold 1.5 value (Fig. 7). In the near-inlet sediment samples, their EFs were the highest showing 3.5, 3.5, > 4, and 6, respectively (Fig. 7). The EF of Zn was around 0.5 in all stations except at the inlet, it increased to 1. The average EFs indicated that the concentration behavior of heavy metals relative to the world average shale in the KL follows this order: Co > Cu > Ni > Mn > Zn (Table 5).
The PLI of the studied bottom sediment samples of the KL was around 0.5, except, at the near-inlet samples, it decreased to 0.0 (Fig. 7).
In the Salman Bay (SB), the EFs of the heavy metals (Mn, Cu, and Ni) were also slightly below 1.5, whereas those of the Zn and Co were around 0.5 (Fig. 7). The PLI of the studied sediment samples of the SB was around 0.0 (Table 5). The average EF of heavy metals in the SB follows this order: Cu > Mn > Zn > Co > Ni.
In both of the KL and SB, the average CF was < 1 and average Igeo was lower than 0.0 indicating uncontaminated sediment samples (Table 5).
Discussion
Heavy metals of the Al-Kharrar Lagoon
The Al-Kharrar Lagoon (KL) is very virgin and natural, and it is, up to date, not connected to any source of anthropogenic pollution. Thus, its heavy metal inventory could be originated from the siliciclastic influx through several wadies originated from the upland Al-Hejaz catchments such as: Rabigh (south), Rahab and Murayyikh (east), and Al-Khariq (North) and probably from in situ conditions such as separation from seawaters, authigenic or diagenetic processes. The siliciclastic materials of Wadi Rabigh are no longer reaching the KL since 2009 due to the construction of Rabigh Dam about 35 km east of Rabigh City. This conclusion is testified by comparing the recorded concentrations of heavy metals with the world average shale background. The enrichment/pollution factors (EF, CF, PLI, and Igeo) applied for the concentrations of heavy metals were lower than the threshold values of these factors, indicating unpolluted, natural conditions for the KL. The average EFs indicated that the concentration behavior (relative to the world average shale) of heavy metals in the surficial bottom sediments of KL follows this order: Co > Cu > Ni > Mn > Zn.
The heavy metals of the surficial bottom sediments of KL could be also influenced by the eolian deposition in the catchment area and by the ability of sediments to bind and release metals that mainly controlled by salinity, pH, cation exchange capacity and organic matter content, redox conditions (Du Laing et al. 2008). Moreover, bacteria and algae play rule in the accumulation of heavy metals into the sediments (Da Costa et al. 2001; Bina et al. 2006; Jackson 2008; Jackson et al. 2011). Some sea-weeds are very efficient in scavenging the heavy metals, and after death, they decompose contributing to the sediment budget of heavy metals; this is because they are mainly constituted of polysaccharide alginate, usually calcium and sodium alginates which are very efficient in scavenging the heavy metals (Da Costa et al. 2001).
The concentration distribution of the heavy metals Fe, Mn, Zn, Ni, Cu, and Co in the KL showed the same pattern. These metals were the lowest in the sediments of the near-inlet stations and at the south-western stations. On the other hand, they were the highest in the sediments of the lagoon’s center and at the southeastern stations, testifying the effect of wadies on the geochemical inventory of the KL. At the near-inlet stations, the concentration of metals was the lowest, indicating that the siliciclastic influx from the surrounding wadies, at the present, was not strong enough to pass through the lagoon’s inlet to the open waters of the Red Sea. Another explanation for the low concentration of heavy metals at the near-inlet station sediments could be attributed to the winnowing of bottom sediments which are mainly of coral debris (95% carbonates) by currents so that one important scavenger for heavy metals such as organic matters was the lowest in this channel (the inlet passage). However, the EF of the bottom sediments at the inlet stations showed a striking feature that is the EF values of the Mn, Zn, Cu, Co, and Ni, relative to the world average shale (Turekian and Wedepohl 1961) were the highest, but in the rest of samples, they were the lowest. These high EF values for metals at the inlet stations of the KL could not be anthropogenic but rather a natural biosorption by the polysaccharide alginates of the mucus algae that predominate, covering the coral debris and sand grains in this area. Xu et al. (2015) mentioned that EF value larger than 1.5 could indicate an important proportion of non-crustal materials delivered from either natural processes such as biota and/or anthropogenic influences. Also, a higher level of Zn in the deep-sea sediments of Gulf of Mexico was attributed to long residence time of organic matter in the water column (Armstrong-Altrin et al. 2015).
The statistical analysis (PCA, cluster analysis, and Pearson correlation coefficients) indicated that the metals of the KL sediments have no relationships with the organic carbon of the studied sediments. This may be related to the great influence of terrestrial influx on the concentrations of heavy metals in the lagoon, and most of organic carbon (OC) is short-lived in the lagoon’s water column and freshly derived from marine sources such as the decay of algae and sea-grasses (Abu-Zied and Hariri 2016). Ramos-Vázquez et al. (2017) concluded that the river-influenced coastal areas are considered as active interfaces between the continental and oceanic environments, which have huge dispersal of detrital, fine-grained sediments enriched in heavy metals.
Also, these organic matters may not be partially oxidized to form complexes attractive to heavy metals due to occurrence of highly oxygenated water. Inside the Al-Arbaeen and Al-Shabab inlets (Jeddah’s coast), the concentrations of Fe and Mn showed an inverse relationship with the OC due to the occurrence of reducing conditions so the bottom sediments exporting Fe-Mn to the waters, whereas outside the inlets, the OC showed a direct relationship with Fe-Mn due to the occurrence of oxidizing conditions leading to fixation of Fe and Mn into the sediments (Abu-Zied et al. 2013). The OC was inversely correlated with gravel and sand sediments, but with a direct correlation with the mud fraction which showed positive relationships with heavy metals such as Fe, Mn, Zn, Cu, and Ni. However, with a plenty of dissolved oxygen in the KL, the organic matters are a favorable place for aerobic bacteria to play an important role in metal fixation. Jackson et al. (2011) discussed that most bacteria play a rule in the variations of element distribution and relationships controlling diagenesis, precipitating oxides, degradation of adsorbed clay, and synthesis of intracellular and extracellular clay. They also reported that Fe oxides precipitated abiotically and indiscriminately on bacteria and minerals, but Mn oxides were purely biogenic, precipitating only on certain bacteria. Thus, it is possible that the occurrence of plenty of Fe-Mn oxides and high oxygenated waters in the KL may be deleterious on the role of organic matter for fixation or precipitation of heavy metals in the bottom sediments of the KL. On the other hand, the heavy metals showed an opposite relationships with the water temperature and CaCO3.
The heavy metals (Fe, Mn, Zn, Ni, Cu, and Co) and the hydrochemical parameters (salinity, dissolved oxygen, and pH) are positively correlated. Taking in our mind that the mean source of these metals is via wadies influx, this means that these metals come to the Al-Kharrar Lagoon (KL) as detrital, colloidal, or soluble substances (as reduced forms with lower oxidation state) in a freshwater medium. Once these substances, specially the soluble substances, reach high saline, alkaline waters of the lagoon, they get oxidized and precipitated immediately near the lagoon’s shoreline or spread far away from it depending on the magnitude of flood events. Takáč et al. (2009) reported that the decreased availability of metals is affected by higher adsorption and precipitation in alkaline and neutral environments. Also, the dissolution of heavy metals in sediment pore-waters from minerals and their availability in a solution decreased with increasing pH (Buykx et al. 2002).
The direct relationship of DO with the heavy metals is apparent, giving information about the redox potential in the KL that may need a further study due to its control on the bio-availability of these metals in the lagoon’s water. Violante et al. (2010) reported that redox reactions, both biotic and abiotic, are of great importance in controlling the oxidation state and thus, the mobility and the toxicity of many heavy elements depending on the particular metal species and microenvironments. During this study (winter), the bottom water of KL was well ventilated with DO not less than 5 mg/l; thus, any humic substances carrying heavy metals get readily oxidized and precipitated on the lagoon’s bed. The surficial bottom sediments of the KL have high load of heavy metals, for example; the concentrations of Fe, Mn, Ni, Zn, Cu, and Co were 18,734 ± 11,202, 328.94 ± 143, 26.85 ± 17, 23.61 ± 16, 22.42 ± 12, 9.18 ± 4.5 mg/kg, respectively. These concentrations are higher than those of the eastern Red Sea coast sediments (Badr et al. 2009; Abu-Zied and Hariri 2016; Abu-Zied et al. 2016; Al-Mur et al. 2017), so under reducing conditions, they may be more mobile, exporting bio-reactive metals into the lagoon’s water. However, the highest concentrations of Fe and Mn may be advantageous for the fixation of extra heavy metals into the lagoon sediments, since they have very high adsorptive capacity (Violante et al. 2008; Marchand et al. 2011; Abu-Zied et al. 2013). But, if the precipitates formed during oxidative precipitation are nano-particulate and colloidal, they may greatly enhance the mobility of associated trace metals in aquatic and subsurface environments (Violante et al. 2010). Therefore, the KL acts as a buffer zone, preventing heavy metals from dispersion into the Red Sea waters. Kuriata-Potasznik et al. (2016) mentioned that natural lakes, connected to rivers, can counteract stress by temporarily removing toxic metals form circulation and depositing them mostly around the delta, where heavy metals undergo an array of biogeochemical processes on natural reactive surfaces such as surfaces of clay minerals, metal oxides and oxyhydroxides, humic substances, plant roots, and microbes.
The mean concentration of heavy metals in the surficial bottom sediments of the KL is twofold lower than those recorded by Basaham et al. (2015) and higher than those recorded by Youssef and El-Sorogy (2016) in the same lagoon (Table 4). This is because authors did not use the surficial (upper 2 cm) bottom sediments as this study did or may be as a result of using different analytical techniques. They are, more or less, similar to those of the lagoonal systems such as Sharm Obhur (Ghandour et al. 2014), Al-Mejarma and Shuaiba (Basaham et al. 2015). On the other hand, the heavy metals of the KL (this study) are threefold higher (especially Fe and Mn) than those of the open shoreline sediments of the eastern Red Sea coast (Abu-Zied and Hariri 2016; Abu-Zied et al. 2016; Al-Mur et al. 2017) and those of the surficial sediments of the Salman Bay (this study). This testifies that the lagoons of the Red Sea act as a filter inhibiting the dispersion of a high quantity of heavy metals into the open waters of the Red Sea. Vodyanitskii (2010) concluded that iron compounds are used as ameliorating agents and geochemical barriers for fixing heavy elements. The concentrations of Ni and Co in the KL were 10 times higher than those of the Salman Bay, whereas the concentrations of Pb and Cd in the sediments of the both of SB and KL were very low or undetectable, indicating that no industrial effluents enter these water bodies and their pristine natural conditions are still unaffected.
Heavy metals of the Salman Bay
The Salman Bay (SB) is nearly closed, very shallow water body with very high salinity up to 76‰. It is not directly connected to the siliciclastic materials of the catchment area. Therefore, its heavy metals may originate from marine, atmospheric sources and water runoff (winter rainfalls) from adjoining alluvium. The concentration of heavy metals in SB shoreline sediments is threefold lower than those of the Al-Kharrar Lagoon, but they are, more or less, similar to those of the open shoreline sediments of the eastern Red Sea coast (Abu-Zied and Hariri 2016; Abu-Zied et al. 2016; Al-Mur et al. 2017), in spite of the spread of thick layer of black sludge emitting bad odors at the studied sites (Fig. 8a). This means that this black layer of sludge could be mainly derived from the decay of algal mats that after death, they get drift by wind and current to accumulate on the eastern shoreline of the bay; they do not accumulate on the western side of the bay. In fact, there is one or couple houses at the western coast of the bay, but field observations could not attribute this black sludge to wastewaters of these houses. To confirm that this black sludge is attributed whether to natural or anthropogenic conditions, stable isotopes of nitrogen (δ15N) in the organic matters should be carried out in a further study. The EF, CF, PLI, and Igeo of the heavy metals in the shoreline sediments of SB showed also very low values, suggesting that these sediments may occur in uncontaminated conditions. The average EF indicated that the concentration behavior (relative to the world average shale) of heavy metals in the surficial bottom sediments of SB follows this order: Cu > Mn > Zn > Co > Ni. The heavy metal concentrations in the shoreline sediments of SB was much lower than those recorded by Youssef (2015) in the same area, due probably to his extraction of heavy metals only from the shells of calcareous benthic foraminifera.
The spatial distribution of hydrogeochemical parameters and heavy metals in the shoreline sediments of SB have divided the bay into southern and northern parts. This division is also indicated by the Q-mode hierarchical cluster (Fig. 9). The concentrations of Fe, Mn, Zn, Cu, and Ni were the highest in the sediments of the northern part of the bay and decreased to lower values in the southern part, following the trend of water temperature that increased towards the northern part. These distribution concentrations of heavy metals were in opposite correlations with water salinity and pH which were the highest in the southern part; also, the CaCO3% showed an inverse relationship with the heavy metals. Moreover, the OC and DO showed no relationships with the heavy metals, except Zn and Cu showed a weak correlation with the OC.
First, the inverse correlation between heavy metals and the shoreline sediment carbonates (CaCO3) of Salman Bay could be normal due to its dilutive effect where the carbonates in the bulk sediments were around 71% and contain a considerable amount of biogenic components such as small gastropods that tolerate the high salinity (76‰) of the bay (Fig. 8b). Second, the inverse correlation between heavy metals and water parameters of SB such as salinity and pH is elusive. It is possible that the high salinity (brine) of the SB leads to formation of many soluble salts preventing precipitation of heavy metals into the sediments, and hence, they get disposed into the sea via the inlet. Also, the precipitation of a high quantity of mineral salts may act a dilution factor as carbonates do. However, some results pointed out that the availability of reactive heavy metals increased in the pore water of the sediments with increasing salinity (McLaughlin et al. 1994; Kadkhodaie et al. 2012). Du Laing et al. (2008) mentioned that increasing organic matter and clay contents and sulfide contents were found to enhance metal accumulation in the intertidal sediments, whereas higher salinities resulted in reduced metal accumulation. Also, increasing metal mobilization with increasing salinities was demonstrated by both of the laboratory (Paalman et al. 1994) and field experiments (Hatje et al. 2003). So, the SB brine ions could prevent heavy metals to precipitate into the sediments, leading to the occurrence of inverse relationships between metals in sediments and water salinity of the Salman Bay.
The water temperature in both of the Al-Kharrar Lagoon and Salman Bay behaved with the heavy metals in two opposite ways, showing an inverse correlation in the KL and direct correlation in the SB. This indicates that each environment has its own geological, physical, and biological characters interacting with the chemical species to give, at the ultimate, the geochemical inventory of the environment. For example, the accumulation of heavy metals in the sediments of each environment is determined by an input amount from rivers, atmosphere or industrial and domestic waste, and the ability of sediment to bind and release metals (Du Laing et al. 2008). All of these processes are governed by certain biogeochemical processes on natural reactive surfaces such as surfaces of clay minerals, metal oxides and oxyhydroxides, humic substances, plant roots, microbes (Jackson 2008; Kuriata-Potasznik et al. 2016), sediment characteristics such as pH, cation exchange capacity and organic matter content, redox conditions, and chloride content (Du Laing et al. 2008).
The southern part of Salman Bay of the highest salinity showed a lower temperature than that recoded in the northern part by about 3 °C. The explanation for this could be related to the effect of brine water (salinity > 50‰) on temperature which decreases when salinity of the brine increases.
Conclusions
The concentrations of heavy metals Fe, Mn, Ni, Zn, Cu, Co, Cd, and Pb were studied in the bottom sediments of the Al-Kharrar Lagoon (KL) and Salman Bay (SB) and correlated with the hydrogeochemical parameters and sediment grain sizes. In the KL, the concentrations of metals were the highest in the center and southeastern stations where the influx of heavy metal-rich siliciclastics from wadies dominated, but they were low in the sediments of the near-inlet stations.
Also, the occurrence of hypersaline, high pH, and dissolved oxygen conditions in the lagoon could favor the oxidation and precipitation of soluble heavy metals near the lagoon’s shoreline or could spread far away depending on the magnitude of the flood events.
In the KL, the metals were also directly correlated with Fe and Mn, but with no relationships with the organic carbon due probably to the occurrence of a plenty of Fe-Mn oxides and highly oxygenated waters that could affect the role of organic matters to form complexes readily for fixation and precipitation of heavy metals into the lagoon’ sediments. Where, all metals showed positive relationships with the dissolved oxygen and muddy sediments.
The EF of metals in the bottom sediments of the KL were the highest at the inlet sediments, due probably to a natural biosorption by the polysaccharide alginates of the mucus algae that cover the coral debris and sand grains in this area. In the rest of sediment samples, the EF were the lowest far below the threshold level, indicating that the KL was influenced mainly by natural processes. Also, the CF, PLI, and Igeo showed very low values, categorizing the KL under uncontaminated conditions.
The concentrations of Fe-Mn and Ni-Co in the sediments of KL were threefold and 10 times higher than those of the Salman Bay, respectively, whereas the concentrations of Pb and Cd in the sediments of the SB were undetectable, indicating that no industrial effluents enter this water body. The occurrence of black sludge on the eastern shoreline of SB may be related to the decay of algal mats, a natural process, since the concentrations of heavy metals in this sludge and their CF, PLI, and Igeo values were very low. The metals of the SB showed a positive relationship with temperature and no relationships with DO, OC, carbonate, and sediment grain size, but with an inverse relationship with salinity. The brine water of the SB showed an adverse effect on the concentrations of heavy metals in the sediments of the bay due to the occurrence of many soluble salts that could preclude precipitation of heavy metals into the sediments.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Abu-Hilal A, Badran M, de Vaugelas J (1988) Distribution of trace elements in Callichirus laurae burrows and nearby sediments in the Gulf of Aqaba, Jordan (Red Sea). Mar Environ Res 25:233–248
Abu-Zied RH, Bantan RA (2015) Palaeoenvironment, palaeoclimate and sea-level changes in the Shuaiba Lagoon during the late Holocene (last 3.6 ka), eastern Red Sea coast, Saudi Arabia. Holocene 25:1301–1312
Abu-Zied RH, Hariri MS (2016) Geochemistry and benthic foraminifera of the nearshore sediments from Yanbu to Al-Lith, eastern Red Sea coast. Saudi Arabia. Arab J Geosci 9:245. https://doi.org/10.1007/s12517-015-2274-9
Abu-Zied RH, Bantan RA, Basaham AS, El Mamoney MH, Al-Washmi HA (2011) Composition, distribution, and taphonomy of nearshore benthic foraminifera of the Farasan Islands, southern Red Sea, Saudi Arabia. J Foraminifer Res 41:349–362
Abu-Zied RH, Basaham AS, El Sayed MA (2013) Effect of municipal wastewaters on bottom sediment geochemistry and benthic foraminifera of two Red Sea coastal inlets, Jeddah, Saudi Arabia. Environ Earth Sci 68:451–469
Abu-Zied RH, Al-Dubai TAM, Bantan RA (2016) Environmental conditions of shallow waters alongside the southern corniche of Jeddah based on benthic foraminifera, physico-chemical parameters and heavy metals. J Foraminifer Res 46:149–170
Acevedo-Figueroa D, Jiménez BD, Rodríguez-Sierra CJ (2006) Trace Metals in Sediment of Two Estuarine Lagoons from Puerto Rico. Environ Pollut 141:336–342
Al-Dubai TA, Abu-Zied RH, Basaham AS (2017) Present environmental status of Al-Kharrar lagoon, central of the eastern Red Sea coast, Saudi Arabia. Arab J Geosci 10(305). https://doi.org/10.1007/s12517-017-3083-0
Al-Mur BA, Quicksall AN, Al-Ansari AMA (2017) Spatial and temporal distribution of heavy metals in coastal core sediments from the Red Sea, Saudi Arabia. Oceanologia 59:262–270
Armstrong-Altrin JS, Machain-Castillo ML (2016) Mineralogy, geochemistry, and radiocarbon ages of deep sea sediments from the Gulf of Mexico, Mexico. J S Am Earth Sci 71:182–200
Armstrong-Altrin JS, Machain-Castillo ML, Rosales-Hoz L, Carranza-Edwards A, Sanchez-Cabeza JA, Ruíz-Fernández AC (2015) Provenance and depositional history of continental slope sediments in the southwestern Gulf of Mexico unraveled by geochemical analysis. Cont Shelf Res 95:15–26
Badr NBE, El-Fiky AA, Mostafa AR, Al-Mur BA (2009) Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environ Monit Assess 155:509–526
Bantan RA, Abu-Zied RH (2014) Sediment characteristics and molluscan fossils of the Farasan Islands shorelines, southern Red Sea, Saudi Arabia. Arab J Geosci 7:773–787
Basaham AS (2009) Geochemistry of Jizan shelf sediments, southern Red Sea coast of Saudi Arabia. Arab J Geosci 2:301–310
Basaham AS, El Sayed MA, Ghandour IM, Masuda H (2015) Geochemical background for the Saudi Red Sea coastal systems and its implication for future environmental monitoring and assessment. Environ Earth Sci 74:4561–4570
Bina B, Kermani M, Movahedian H, Khazaei Z (2006) Biosorption and recovery of copper and zinc from aqueous solutions by nonliving biomass of marine Brown algae of Sargassum sp. Pak J Biol Sci 9:1525–1530
Buykx SEJ, van den Hoop MAGT, Loch JPG (2002) Dissolution kinetics of heavy metals in Dutch carbonate- and sulfide-rich freshwater sediments. J Environ Qual 31:573–580
Chester R, Bradshaw GF, Corcoran PA (1994) Trace metal chemistry of the North Sea particulate aerosol; concentrations, sources and sea water fates. Atmos Environ 28:2,873–2,883
Da Costa ACA, Tavares APM, de França FP (2001) The release of light metals from a brown seaweed (Sargassum sp.) during zinc biosorption in a continuous system. Electron J Biotechnol 4:125–129
Du Laing G, De Vos R, Vandecasteele B, Lesage E, Tack FMG, Verloo MG (2008) Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuar Coast Shelf Sci 77:589–602
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
El Sayed MA, Basaham AS (2004) Speciation and mobility of same heavy metals in the coastal sediments of Jeddah, eastern Red Sea. Mans J Environ Sci 27:57–92
Ghandour IM, Basaham S, Al-Washmi A, Masuda H (2014) Natural and anthropogenic controls on sediment composition of an arid coastal environment: Sharm Obhur, Red Sea, Saudi Arabia. Environ Monit Asses 186:1465–1484
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hassan MAH, Nadia AA (2000) Trace metals in fish, mussels, shrimp and sediment from Red Sea coast of Yemen. Bull Inst Oceanogr Fish 26:339–353
Hatje V, Payne TE, Hill DM, McOrist G, Birch GF, Szymczak R (2003) Kinetics of trace element uptake and release by particles in estuarine waters: effects of pH, salinity, and particle loading. Environ Int 29:619–629
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Herut B, Sandler A (2006) Normalization methods for pollutants in marine sediments: review and recommendations for the Mediterranean Israel oceanographic and Limnological research. IOLR Report pp 1–23
Jackson TA (2008) Interactions between heavy metals, colloidal minerals, and microorganisms–their environmental significance. In: The roles of the geosphere in environmental changes (proc. 35th international symposium, National Academy of Sciences, Republic of Korea (17th October, 2008; Seoul, Korea), pp 25–132
Jackson TA, West MM, Leppard GG (2011) Accumulation and partitioning of heavy metals by bacterial cells and associated colloidal minerals, with alteration, neoformation, and selective adsorption of minerals by bacteria, in metal-polluted lake sediment. Geomicrobiol J 28:23–55
Kadkhodaie A, Kelich S, Baghbani A (2012) Effects of salinity levels on heavy metals (cd, Pb and Ni) absorption by sunflower and sudangrass plants. Bull Environ Pharmacol Life Sci 1:47–53
Kuriata-Potasznik A, Szymczyk S, Skwierawski A, Glińska-Lewczuk K, Cymes I (2016) Heavy metal contamination in the surface layer of bottom sediments in a flow-through Lake: a case study of Lake Symsar in northern Poland. Water 8:358. https://doi.org/10.3390/w8080358
Loring DH (1991) Normalization of heavy-metal data from estuarine and coastal sediments. ICES J Mar Sci 48:101–115
Marchand C, Allenbach M, Lallier-Vergès E (2011) Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (Conception Bay, new-Caledonia). Geoderma 160:444–456
McLaughlin MJ, Tiller KG, Beech TA, Smart MK (1994) Soil salinity causes elevated cadmium concentrations in field growth potato tubers. J Environ Qual 23:1013–1018
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2:108–118
Nowrouzi M, Pourkhabbaz A (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara biosphere reserve, Iran. Chem Speciat Bioavailab 26:99–105
Oves M, Khan MS, Qari AH, Felemban MN, Almeelbi T (2016) Heavy metals: biological importance and detoxification strategies. J Bioremediat Biodegrad 7:334. https://doi.org/10.4172/2155-6199.1000334
Paalman MAA, van der Weijden CH, Loch JPG (1994) Sorption of cadmium on suspended matter under estuarine conditions: competition and complexation with major seawater ions. Water Air Soil Pollut 73:49–60
Pan K, Lee OO, Qian PY, Wang WX (2011) Sponges and sediments as monitoring tools of metal contamination in the eastern coast of the Red Sea, Saudi Arabia. Mar Pollut Bull 62:1140–1146
Ramos-Vázquez MA, Armstrong-Altrin JS, Rosales-Hoz L, Machain-Castillo ML, Carranza-Edwards A (2017) Geochemistry of deep-sea sediments in two cores retrieved at the mouth of the Coatzacoalcos River delta, western Gulf of Mexico, Mexico. Arab J Geosci 10(148). https://doi.org/10.1007/s12517-017-2934-z
Saleh SMK (2006) Environmental assessment of heavy metals pollution in bottom sediments from the Gulf of Aden. PhD thesis, 235 p, Alexandria University, Egypt
Takáč P, Szabová T, Kozáková Ľ, Benková M (2009) Heavy metals and their bioavailability from soils in the long-term polluted central Spiš region of SR. Plant Soil Environ 55:167–172
Tamunobereton-ari I, Omubo-Pepple VB, Tamunobereton-ari NA (2011) Speciation of heavy metals (cu, Pb, Ni) pollutants and the vulnerability of groundwater resource in Okrika of rivers state, Nigeria. Am J Sci Ind Res 2:69–77
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology. Experientia Supplementum, vol 101. Springer, Basel
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33:566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth crust. Geol Soc Am Bull 72:175–192
Turki AJ (2007) Metal speciation (cd, cu, Pb and Zn) in sediments from Al Shabab lagoon, Jeddah, Saudi Arabia. J KAU Mar Sci 18:191–210
Violante A, Krishnamurti GSR, Pigna M (2008) Mobility of trace elements in soil environments. In: Violante A, Huang PM, Gadd GM (eds) Biophysico-chemical processes of metals and metalloids in soil environments. Wiley, Hoboken, pp 169–213
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and biovailability of heavy metals and metalloids in the soil environments. J Soil Sci Plant Nutr 10:268–292
Vodyanitskii YN (2010) The role of Iron in the fixation of heavy metals and metalloids in soils: a review of publications. Eurasian Soil Sci 43:519–532
Xu G, Liu J, Pei S, Hu G, Kong X (2015) Geochemical background and ecological risk of heavy metals in surface sediments from the West Zhoushan fishing ground of East China Sea. Environ Sci Pollut Res 22:20283–20294
Youssef M (2015) Heavy metals contamination and distribution of benthic foraminifera from the Red Sea coastal area, Jeddah, Saudi Arabia. Oceanologia 57:236–250
Youssef M, El-Sorogy A (2016) Environmental assessment of heavy metal contamination in bottom sediments of Al-Kharrar lagoon, Rabigh, Red Sea, Saudi Arabia. Arab J Geosci 9(474). https://doi.org/10.1007/s12517-016-2498-3
Yu XJ, Yan Y, Wang WX (2010) The distribution and speciation of trace metals in surface sediments from the Pearl River estuary and the Daya bay, southern China. Mar Pollut Bull 60:1364–1371
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54:1051–1070
Acknowledgments
The authors acknowledge with thanks DSR technical and financial support. Nizam Farooqui and Talha Al-Dubai thanked for their help and assistance during the field work of this research. Also, authors are grateful for Dr. Hussein Touliabah for his help during the field work and preparing samples for heavy metal measurements.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. 219-662-1437.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hariri, M.S.B., Abu-Zied, R.H. Factors influencing heavy metal concentrations in the bottom sediments of the Al-Kharrar Lagoon and Salman Bay, eastern Red Sea coast, Saudi Arabia. Arab J Geosci 11, 495 (2018). https://doi.org/10.1007/s12517-018-3838-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3838-2