Abstract
This work presents results of the hydrogeological and hydrochemical studies on groundwater samples from the alluvial aquifer of Merdja in Tébessa, located in the Western part of this town. Its groundwater resources are used mainly for crop irrigation in an agriculture dominated area. Hydrochemical and water quality data obtained through a sampling period (December 2008) and analysis program indicate that nitrate pollution can be a serious problem affecting groundwater due to the use of nitrogen (N) fertilizers in agriculture. The concentration of nitrate in groundwater ranged from 19 to 281 mg/l. Considerable seasonal fluctuations in groundwater quality were observed as a consequence of agricultural practices and other factors such as annual rainfall distribution and the wadi El Kebir flow regime. The chemical composition of the water is not only influenced by agricultural practices, but also by interaction with the alluvial sediments. The dissolution of evaporites accounts for part of the Na+, K+, Cl−, SO 2−4 , Mg2+, and Ca2+, but other processes, such as calcite precipitation and dedolomitization, also contribute to groundwater chemistry.
ملخص
يستعرض هذا العمل نتائج الدراسات الهيدروجيولوجية و الهيدروكيميائية لعينات من المياه الجوفية للطبقة الغرينية لسهل المرجة المحاذي لمدينة تبسة من الجهة الغربية, أين تستخدم المياه الجوفية بشكل رئيسي لسقي المساحات الزراعية.
نوعية المياه و معطيات التحليل الهيدروكيميائي المتحصل عليها خلال شهر ديسمبر 2008 أظهرت قابلية للتلوث بالنترات التي قد تشكل مشكلة خطيرة لهذه المياه الجوفية بسبب الاستخدام المفرط للأسمدة الكيميائية في الزراعة.
يتراوح تركيز النترات في المياه الجوفية بين 19 إلى 281 ملغم/لتر, وترتبط بطبيعة الموسم الزراعي و كمية التساقط الفصلي إلى جانب المياه المستعملة التي تجري خلال وادي الكبير الذي يقطع طوليا سهل المرجة. يرتبط التركيب الكيميائي للمياه أيضا بتفاعلها مع ترسبات الطمي و انحلال التراكيب الملحية الجبسية التي ترفع تركيز العناصر الكيميائية المعدنية و تساهم بذلك و بشكل ملحوظ في التركيبة الكيميائية للمياه الجوفية بسهل المرجة.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture can have a significant impact on the quality of groundwater. Nitrate and chloride pollution of surface water is of high concern as it may have negative impacts on water supply and ecosystems (Postma et al. 1991; McNeely et al. 1979; Rouabhia et al. 2004 and 2008a, 2008b, Baali et al. 2007). High nitrate concentrations in water serving as a source for drinking water is a serious health problem, being known for many years as the cause of blue baby syndrome and related to increased levels of diarrhea of children (Fehdi et al. 2008, Hamilton and Helsel 1995).
Return flows from irrigated agriculture may increase the salt, nitrate, and chloride concentrations of the receiving water bodies, limiting their agricultural, industrial, urban, and ecological uses. Irrigated agriculture in semi-arid areas significantly contributes to crops productivity, stability, and diversification. However, the return flows from irrigated agriculture are considered a major diffuse contributor to the contamination of surface and groundwater bodies (Rouabhia et al. 2004).
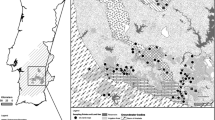
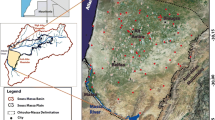
The area of study is near the city of Tebessa in eastern Algeria (Fig. 1a, b). This area lies in the semi-arid region of Algeria and is susceptible to the various threats common in both growing urban areas and developing agricultural areas. The city of Tebessa and the surrounding villages (Bekkaria and Youkous les bains: Hammamet) have seen a great deal of growth in the past decade, with the establishment of new industries and farms. The most important economic activity of the area is agriculture. Large amounts of synthetic nitrogen fertilizers, such as urea 46%, ammonium nitrate 33.5%, liquid fertilizer N-32%, or commercial complex fertilizers with different proportions (%) of nitrogen, phosphorous, and potassium [(N = 15, P2O5 = 15, K2O = 15), (N = 8, P2O5 = 15, K2O = 15), and (N = 9, P2O5 = 18, K2O = 28)] are applied during farming season (summer and autumn). The amount of nitrogen fertilizers used varies significantly within the study area depending on the type of crop, the amount of nitrogen in the irrigation water and soil, and also on each farmer’s practices. The consequent paucity of information makes it difficult to establish a nitrogen balance in the aquifer. In addition to agricultural practices, other non-point sources of nitrogen include precipitation and irrigation water containing nitrogen. Point sources of nitrogen such as septic tanks and dairy lagoons are not significant in this area.
The main aims of this study are: (1) to identify the spatial and temporal trends of nitrate in groundwater and (2) to evaluate the characteristics, spatial distribution, and variations in groundwater chemistry as a result of agricultural practices and water–sediment interactions within the aquifer.
The Tebessa area contains two aquifers: the limestone aquifer and the alluvial aquifer. In general, the shallow groundwater in Merdja area is found in the alluvial fan deposits from plio-quaternary age (Fig. 2). This aquifer overlies geologic formations consisting of cenomanian marly layers. The recharge of this alluvial aquifer is combined and is done from: (1) highlands of Dyr Bouroumane on the East and Doukkane on the West (precipitation infiltrating through alluvial fans where the mountain range meets the plain); (2) from losing streams; and (3) through cross-formational flow.
Annual precipitation in the studied area ranges from 200 to 320 mm (Rouabhia et al. 2008a, 2008b); thus, this is considered to be a semi-desert area. Summer temperatures can reach 45°C. This situation of dryness accentuates the drawdown of water resource especially during the last decade because the renewal of this resource is very weak. Dryness generates sometimes very unfavorable effects on groundwaters. (Aquifer refill decreases considerably whereas the exploitation increases). The dry climate, atmospheric dust, and low precipitation can also affect the water quality generally causing increased salt content (Fehdi et al. 2008).
Geological and hydrological setting
The geology of the studied area was investigated by several authors (Blés and Fleury 1970; Vila 1980). The micropaleontological and biostratigraphical analyses have showed that, from the stratigraphical point of view, the studied area includes the plio-quaternary tectonic depression of Tebessa; this depression separates the highlands of Dyr situated in the North from the Doukkane and Mestrie highlands located in the South. Most of the area is of Cretaceous age (Fig. 2), and forms a series of anticlines and synclines. The stratigraphic consists of alternating sequence alternation carbonate formations of limestones, marly-limestones, and argillaceous marls. The plio-quaternary and quaternary terrains occupy the central part; they are consisted by actual and recent alluvial deposits, conglomerates, gravels, sandstones, etc. Analysis of the hydrostratigraphic column of the studied area suggests the presence of two aquifers including the formation of plio-quaternary age (Djabri 1987). This large alluvial aquifer occupies the major part of the tectonic depression, limited at the North and at the South by two great faults of W–E orientation (Fig. 2). It is composed of diverse deposits such as alluvial fans, silts, calcareous crust, conglomerates, and gravels. This aquifer plays an important role in the drinking water supply for the local population, where it undergoes a strong solicitation, which generates an anthropogenic pollution.
Different wells have been drilled into the alluvial aquifer, supplying water mainly for irrigation, show groundwater levels from approximately 20 to 30 m in depth. Average water level fluctuations are not greater than 1.5 m between dry and wet seasons. The general direction of groundwater flow is from east to west. Average permeability in the sandy levels is around 10−3 m/day (Djabri 1987). The aquifer is mainly recharged by precipitation, irrigation water, and El Kebir Wadi. At some places the wadi may recharge or drain the aquifer depending on several factors such as river flow, water table, and the permeability of the river bed materials.
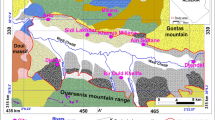
Hydrogeological cross-section (Fig. 3) shows that the depth to the top of the water table vary between 25 and 30 m, this groundwater level near to surface, causes a degradation of the alluvial aquifer waters by the various external agents (anthropogenic pollution, evaporation, etc.).
Hydrogeological cross-section through the Merdja plain. 1 Permeable zone (marly-limestones, alluvial fans, silts, calcareous crust, conglomerates, and gravels). 2 Impermeable zone (clay and marl). 3 Marly bedrock. 4 Screened interval. 5 Piezometric head, 6 well name. * Elevation above sea level. E East, W West
Materials and methods
The hydrochemical properties of groundwater samples collected from the quaternary aquifer system are showed in Table 1. The sites which samples were taken are shown in Fig. 1b. The experimental data, corresponding to December 2008, were obtained from field surveys and from chemical analyses performed in the laboratory. Portable equipment was used to obtain in situ readings of temperature, pH, and electrical conductivity (EC). At the same time, samples of non-acidified water in 500 ml polyethylene bottles were taken. The measurement of HCO3 and Ca2+ was carried out in the shortest time practicable, although the field campaigns normally took 3 or 4 days; during this period, the samples were kept at a low temperature in a portable refrigerator. The HCO3 content was determined as the total alkalinity, by titration with HCl 0.05 N and methyl orange as indicator. The cations were analyzed by atomic absorption spectrometry (Ca and Mg) and by emission spectrometry (Na and K). A visible light spectrophotometer was used to analyze SO4 by turbidimetry and the SiO2, by colorimetry. The concentrations of Cl ions were determined by argentometric titration, using AgNO3 0.01 N and 5% K2CrO4 as indicator.
The hydrochemical calculations were performed using the AQUACHEM program (Calmbach 1997), which makes it possible, in a straightforward way, to use of PHREEQC (Parkhurst and Apello 1999). The computer processing of these hydrochemical data had a main objective: determination the saturation indices of calcite, dolomite, and gypsum, and the partial equilibrium pressure of CO2.
Results and discussion
The physico-chemical and chemical data of all the investigated groundwater are summarized in Table 1. According to the analytical data, parameters values range widely except for the pH and dissolved oxygen (DO) and nitrate concentrations. DO and pH values increase slightly eastwards up to a maximum value of 8.6 mg/l and 7.92 respectively, whereas nitrate concentration increases westwards, with a maximum of 281 mg/l in December 2008. Significant changes in water quality occur along the river course due to the influence of its tributaries, wastewater disposal, agricultural activities, and large seasonal variations in the discharge rate of the Merdja plain. Variations in selected water quality parameters (EC, NO −3 , Cl−, SO 2−4 , and Na2+) along the Merdja plain are illustrated in Fig. 4. In general, water quality becomes considerably worse downstream of the Merdja plain due to a significant increase in Cl− (>200 mg/l) and SO 2−4 (>250 mg/l) concentrations. This is attributed to the Triassic unit rich in evaporites.
Hydrochemistry
Results of analyzed parameters in groundwater samples are given in Table 1. Temperature ranged from 11.9°C to 21.2°C and pH values were near neutral to slightly basic, with an apparent increase in pH towards the eastern part of the aquifer, where the maximum value of 7.92 was reached. The other quality parameters have wider ranges due to spatial changes in the lithology of the alluvial sediments that constitute the aquifer and seasonal changes in fertilizer application and irrigation practices.
Two hydrochemical facies identified are Ca–Mg–SO4–HCO3–Cl and Na–Ca–Mg–Cl–SO4 (Fig. 5). Ca–Mg–SO4–HCO3–Cl type was found upstream (Bekkaria sector). Downstream, groundwater evolves to Na–Ca–Mg–Cl–SO4 types along the flow path. That change of facies is accompanied by a gradual increase in the groundwater total dissolved solids (TDS) and is mainly controlled by evaporites dissolution present in the Triassic unit.
The spatial distribution of the values of EC, NO −3 ,Cl−, SO 2−4 , Ca2+, and Na+ in groundwater in December 2008 shown in Figs. 6 and 4. These maps provide a basis for making area-wide generalizations concerning the distribution of water quality parameters. Cl− and SO 2−4 spatial distribution patterns are very similar, and show that the dissolution of evaporites significantly affect groundwater chemistry. Mention should be made of the high concentrations of Cl− (>250 mg/l), Na+ (>200 mg/l), SO 2−4 (>350 mg/l), and Ca2+ (>200 mg/l) in wells located between Tébessa and Chabro wadi and downstream of the Ain Chabro.
A high correlation is also observed between Sr2+ and SO 2−4 (n = 25; r = 0.855; p < 0.01; Fig. 7), which suggests the dissolution of strontium sulfate (celestite; often associated with gypsum, anhydrite, and halite) as the main source of strontium.
Celestite deposits occur in Triasic unit of Djebissa Diapir. The main mineralization consists of granular celestite nodules. Dolomite, calcite, quartz, and gypsum are associated to celestite in secondary mineralizations (Djabri, 1987).
Other sources of sulfate in groundwater may be agricultural practices, although fertilizers containing sulfate are not commonly used in the study area.
The presence of carbonate rocks such as calcite and dolomite in the sediments determines the high HCO −3 concentrations throughout the aquifer, which range between 60.8 and 523 mg/l. The spatial distribution of Ca2+ and Mg2+ concentrations in groundwater is determined by the dissolution/precipitation of calcite and dedolomitization processes, as discussed below. Potassium concentrations are low (mean value of 5.72 mg/l) and show no significant changes across the basin. The source of potassium in groundwater is likely to be the weathering of K-feldspar present in the sediments and the application of synthetic fertilizers.
Impact of agriculture on groundwater
Within the study area, agricultural activities that involve the application of urea, ammonium nitrate, liquid fertilizer N-32%, and other commercial complex fertilizers are the main source of elevated nitrate concentrations in groundwater (up to 281 mg/l). About 56% of the samples have nitrate concentrations that exceed the drinking water limit of 50 mg/l (WHO 2006), with an average nitrate concentration in the groundwater of 82 mg/l. As illustrated in Fig. 4, anomalously high NO −3 concentrations (>100 mg/l) are found in irrigation wells located between Aeroport and Chabro wadi, where there is heavy agricultural activity above the aquifer and considerable amounts of synthetic fertilizers (300–600 kg/ha/year) are applied during the farming season. Nitrite usually occurs in small quantities in groundwater, is unstable in the presence of oxygen, and occurs as an intermediate form between ammonia and nitrate or nitrate and nitrogen gas (McNeely et al. 1979).
NO −2 concentrations vary between <0.1 (detection limit) and 3.66 mg/l, and NH +4 concentrations vary between <0.1 (detection limit) and 6.12 mg/l. Phosphorous is also a component of complex synthetic fertilizers widely applied to farming land. The proportion of P2O5 in the applied fertilizers varies between 15% and 18% (150–180 g of P2O5 in each kilogram of fertilizer).
However, phosphate was not detected (concentrations below the detection limit: 0.1 mg/l) in groundwater. When phosphate is added to a soil, its concentration in solution initially declines very rapidly. This is followed by a more gradual decrease in solution concentration that can continue for weeks. Under aerobic conditions, adsorption on the surface of iron and aluminum hydroxides coating clay-sized particles controls the amount of phosphate in solution (Patrick and Khalid 1974; Elrashidi and Larsen 1978).
The concentration of dissolved oxygen in groundwater (mean value of 6.69 mg/l) indicates the presence of aerobic conditions in the aquifer and, therefore, the aforementioned mechanism might be the main factor leading to the low phosphate concentrations in the groundwater (Mcdonald et al. 2001).
The application of liquid fertilizer N-32% (N32) through fertilization is a common practice during the growing season (June–July) in the area, which contains 16% urea, 8% ammonium, and 8% nitrate. Several studies have reported that a reduction in the proportion of NO −3 in applied fertilizers can lead to a considerable decrease in the potential for NO −3 leaching (Baali et al. 2007). The amount of N32 applied varies between 30 and 50 m3/ha/year depending on the crop and on each farmer’s practices (Rouabhia et al., 2008a, Rouabhia et al. 2008b).
A highly permeable, alluvial aquifer provides favorable conditions for the vertical transport of oxygen to deeper parts of the aquifer (Hamilton and Helsel 1995; Kraft et al. 1999; Nash and McCall 1995). Consequently, the alluvial aquifer of the Merdja plain is aerobic throughout its depth, with a mean DO concentration in groundwater of 6.69 mg/l. Under these conditions, urea and ammonium are readily transformed into nitrate. The nitrification rate of ammonium is very fast under aerobic conditions (15–20 days at 20°C; Kpomblekou and Killorn 1996). Therefore, infiltration of nitrogen-rich waters into the aerobic groundwater system seems to be the major cause of high nitrate concentrations in groundwater.
The spreading of the nitrate front
Nitrate concentration in groundwater depends on the hydrogeological conditions and the availability of electrons donors for denitrification (Postma et al. 1991). In the unconfined shallow aquifer (a few meters deep), the decrease in nitrate with depth can be due to the process of the reduction of nitrate by oxidation of organic matter within the sediments (Gillham and Cherry 1978). In deeper, unconfined aquifers (water table depth more than 10 m) small available amounts of labile organic carbon are not sufficient to support the denitrification process (Starr and Gillham 1993). If the later is the case of Merdja aquifer, denitrification does not take place, and NO −3 behaves as a conservative chemical factor (see Fig. 4 ). The use of second Fick’s low (Appelo and Postma 2005; Appelo et al. 1990; Appelo and Postma 1993; Appelo 1994; Appelo and Postma 1999), under specific boundary conditions could provide the appropriate model to estimate the transport of NO −3 and furthermore to estimate the propagation of groundwater contamination. In Merdja plain the distance of the first 500 m from the continuous “source” a main pollution spread of 0.201 m/day if v (groundwater velocity) = 4,6.10−6 m/s, Df (diffusion coefficient) = 0, D L (coefficient of the longitudinal dispersion) = 10−6 m2/s (calculated using PHREEQC version 2, Parkhurst and Apello 1999).
Water–rock interaction process
Interaction between groundwater and surrounding host rocks are believed to be the main process responsible for the observed chemical characteristics of groundwater in the Merdja plain. Evaluation of such process requires the description of the mineral assemblage of the rocks in which water is found, and the identification of chemical reaction responsible for the geochemical evolution of groundwater. From available studies in the literature, such reactions generally include chemical weathering of rock-forming minerals, dissolution–precipitation of secondary carbonates, and ion exchange between water and clay minerals.
Two approaches, mathematical and graphical, are generally used to investigate hydrogeochemical evolution. The mathematical approach is often used for the calculation of saturation indices of groundwater with respect to mineral phases, thus providing some indication upon the equilibrium state between groundwater and the surrounding mineral rock assemblages. Several geochemical programs (Plummer et al. 1976; Plummer et al. 1991) have been developed for such calculations. The graphical approach describes the mineral stability fields of minerals in equilibrium with groundwater, in terms of activity ratio (on a log scale) of ions in groundwater (Plummer et al. 1976, 1991).
In the present study, saturation indices (SI) with respect to carbonate (calcite, dolomite, and aragonite) and evaporite (gypsum and anhydrite) minerals, as well as activities of soluble species (Table 2), were calculated by using the computer chemical program PHREEQC. Because all the investigated groundwaters have very low total dissolved solids, the expression of Debye and Hückel (1923) was used for the computation of activity coefficient.
Figure 8 shows the poles of SI against total dissolved solids for all investigated groundwater. In the following discussion, we may assume that SI values falling within the range of ±0.5 U from zero indicate the equilibrium state. All of considered groundwaters are in the state of equilibrium with respect to calcite and most of them are undersaturated with respect to dolomite; however, all samples are undersaturated with respect to aragonite (Fig. 8a–e) indicating phases undergoing dissolution especially for dolomite (log Ks = −17.09 in WATEQ)
On the other hand, groundwater samples are found to be undersaturated with respect to evaporates minerals (gypsum and anhydrite), suggesting that these evaporite mineral phases are absent in the corresponding host rock.
Conclusions
Groundwater is unique for agriculture in the study area. However, elevated nitrate concentrations are of great concern in the groundwater of the alluvial aquifer of the Merdja plain. Agricultural practices involving synthetic fertilizers have been identified as the main source of nitrate contamination in groundwater. Concentrations of NO –3 higher than 50 mg/l were observed in irrigation wells located in center part of the plain, where there is heavy agricultural activity above the aquifer and crops are still irrigated using surface irrigation systems. In addition, it has been observed that denitrification is not a significant process in this shallow aquifer due to relatively high dissolved oxygen concentrations in groundwater.
Not only agricultural activities but also the mineralogical composition of the sediments in contact with groundwater determines the spatial distribution of the major ions and groundwater chemistry. Two hydrochemical facies have been identified. The change of facies is accompanied by a gradual increase in the groundwater TDS and is mainly controlled by dissolution of evaporites present in the Triassic unit. The analysis of saturation indices for calcite, dolomite, and gypsum showed that evaporites dissolution and dedolomitization (calcite precipitation and dolomite dissolution driven by gypsum dissolution) are the main hydrogeochemical processes that control groundwater chemistry.
References
Appelo CAJ (1994) Cation and proton exchange, pH variations, and carbonate reactions in a freshening aquifer. Water Resources Res 30:2793–2805
Appelo CAJ, Postma D (1993) Groundwater, geochemistry and pollution. Balkema, Rotterdam
Appelo CAJ, Postma D (1999) Variable dispersivity in a column experiment containing MnO and FeOOH-coated sand. J Contam Hydrol 40(1999):95–106
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Balkema, Rotterdam, p 649p
Appelo CAJ, Willemsen A, Ikekman HE, Griffioen J (1990) Geochemical calculations and observations on salt water intrusions. II. Validation of a geochemical model with column experiments. J Hydrol 120:225–250
Baali F, Rouabhia A, Kherici N, Djabri L, Bouchaou L, Hani A (2007) Underground water quality and contamination risk. The case of the basin of Chéria. N.E. Algeria. Estudios Geol 63(1):127–133 ISSN: 0367–0449
Blés JL, Fleury JJ (1970) Carte géologique de l’Algérie au 1/50000: feuille n°178, Morsott, avec notice explicative détaillée. Service de cartes Géologique et Sonatrach, Division d’hydrocarbure. Direction des explorations, Alger, Algérie
Calmbach L (1997) AquaChem Computer Code Version 3.7.42, Waterloo, Ontario, Canada. N2L 3L3
Debye P, Hückel E (1923) The theory of electrolytes. I. Lowering of freezing point and related phenomena. Physikalische Zeitschrift 24:185–206
Djabri L (1987) Contribution to the hydrogeological study of the subsidence plain of Tebessa NE Algerie. Attempt of modelling. Doctorate Thesis, University of Franche Comté. France
Elrashidi MA, Larsen S (1978) The effect of phosphate addition on the solubility of phosphate in soil. Plant Soil 50:585–594
Fehdi Ch, Rouabhia A, Baali F, Boudoukha A (2008) The hydrogeochemical characterization of Morsott-El Aouinet aquifer, Northeastern Algeria. Environ Geol. doi:10.1007/s00254-008-1667-4.
Gillham RW, Cherry AJ (1978) Field evidence of denitrification in shallow groundwater flow system. Water Pollut Res Can 13:53–71
Hamilton PA, Helsel DR (1995) Effects of agriculture on groundwater quality in five regions of the United States. Ground Water 33:217–226
Kpomblekou AK, Killorn R (1996) Nitrification of ammonium nitrogen in soils treated with XDE-474. Soil Sci Soc Am J 60:1482–1489
Kraft GJ, Sities W, Mechenich DJ (1999) Impacts of irrigated vegetable agriculture on a humid north-central U.S. sand plain aquifer. Ground Water 37:572–580
Mcdonald AE, Grant BR, Plaxton WC (2001) Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. J Plant Nutr 24:1505–1519
McNeely RN, Neimanis VP, Dwyer L (1979) Water quality sourcebook a guide to water quality parameters: inland waters directorate. Water Quality Branch, Ottawa 88p
Nash H, McCall GJH (1995) Groundwater quality, 17th Special Report. Chapman & Hall, London, pp 109–122
Parkhurst DL, Apello CAJ (1999) User guide to PHREEQC (version 2)-a computer program for speciation, batch reaction, one dimensional transport, and inverse geochemical Calculations: U.S. Geological Survey Water Resources Investigations Report: 99–4259, 312 p
Patrick WH Jr, Khalid RA (1974) Phosphate release and sorption by soils and sediments: effect of aerobic and anaerobic conditions. Science 186:53–55
Plummer LN, Jones BF, Trusedall AH (1976) WATEQ-a Fortran IV version of WATEQA computer program for calculating chemical equilibrium of natural waters. U.S Geol-Surv. Water Res, Washington DC, vol 76, pp 13–61 (Revised 1978, 1984)
Plummer L, Presemon E, Parkhurst D (1991) An interactive code (NETPATH) for modelling Net geochimical reactions along a flow Path. USGS Water Investigation Rep no 91-4078
Postma D, Boesen C, Kristiansen H, Larsen F (1991) Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resour Res 27:2027–2045
Rouabhia A, Baali F, Kherici N, Djabri L (2004) Vulnérabilité et risque de pollution des eaux souterraines de la nappe des sables miocènes de la plaine d’El MA EL Abiod (Algérie). Rev Sécheresse 15:347–352
Rouabhia A, Fehdi Ch, Baali F, Djabri L, Rouabhi R (2008) Impact of human activities on quality and geochemistry of groundwater in the Merdja area, Tebessa, Alegria. Environ Geol. doi:10.1007/s00254-008-1225-0.
Rouabhia A, Baali F, Fehdi Ch, Kherici N, Djabri L (2008) Hydrochemical and isotopic investigation of a sandstone aquifer groundwater in a semi arid region, El Ma El Abiod, Algeria. Environ Geol doi:10.1007/s00254-008-1451-5.
Starr RC, Gillham RW (1993) Dinitrification and organic carbon availability in two aquifers. Ground Water 6:934–947
Vila JM (1980) Algerian mountain chain and the Algerian–Tunisian confines [La chaine alpine de l’Algérie orientale et des confins Algéro-Tunisiens]. Doctorate Thesis in Natural Sciences. University Pierre et Marie Curie, Paris 6
WHO (2006) World Health Organization. Guidelines for drinking water quality, 3rd edn, incorporating first addendum. www.who.int/water_sanitation_health/dwq/gdwq3rev/en/index.html
Acknowledgments
This work has been realized through the framework of CNPRU project G02920070001 under the thematic: Systèmes d’irrigation et risques de pollution saline et azoté. Construction d’un indicateur de risque et application sur les plaines de Tébessa, (El Ma El Abiod, La Merdja et Chéria) Algérie. We would like to thank Profs. Mudry J. and Vergotten G. (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouabhia, A., Baali, F. & Fehdi, C. Impact of agricultural activity and lithology on groundwater quality in the Merdja area, Tebessa, Algeria. Arab J Geosci 3, 307–318 (2010). https://doi.org/10.1007/s12517-009-0087-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-009-0087-4