Abstract
Purpose of Review
To describe vulnerable plaque pathobiology and summarize potential targets for molecular imaging with a focus on intravascular near-infrared fluorescence (NIRF) and its translatable applications.
Recent Findings
Structural imaging alone is unable to precisely identify high-risk plaques in patients with coronary artery disease (CAD). Intravascular near-infrared fluorescence (NIRF) imaging is an emerging translational approach that can image specific in vivo molecular processes and cells that characterize vulnerable plaques. High-priority NIRF targets imaged by intravascular NIRF imaging thus far include macrophages, cathepsin protease activity, oxidized low-density lipoprotein (oxLDL), and abnormal endothelial permeability. The newest generation of NIRF catheters is multimodal in nature and combines NIRF with either IVUS or OCT, providing simultaneous co-registered morphological and pathobiological assessment of atherosclerotic plaques. While most intravascular NIRF studies have been performed in a preclinical environment, a first-in-human NIR autofluorescence-OCT trial has recently been performed. These developments suggest that clinical intravascular NIRF molecular imaging will be available within the next 3 years.
Summary
Molecular imaging is a powerful approach to enhance our understanding of atherosclerosis pathophysiology. Intravascular NIRF/OCT and NIRF/IVUS molecular imaging is nearing its use in atherosclerosis patients and will initially leverage indocyanine green (ICG) as an FDA-approved NIRF agent that reports on abnormal plaque permeability. Clinical trials are needed to assess the value of intravascular NIRF imaging using ICG as well as other novel NIRF imaging agents to better understand vulnerable plaque pathobiology, event prediction, and to enable personalized pharmacotherapy of high-risk plaques and patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The voyage of discovery consists, not in seeking new landscapes, but in having new eyes.
--Marcel Proust
Introduction
Cardiovascular disease (CVD) due to atherosclerosis remains the leading cause of death worldwide, accounting for 15.2 million fatalities in 2016 [1]. It is well known now that atherosclerosis is a chronic inflammatory disease that involves multiple interconnected biological processes including endothelial dysfunction, monocyte infiltration, LDL oxidation, inflammatory protease activity, smooth muscle migration, neovascularization, intraplaque hemorrhage, and calcification [2]. Atheroma may be further classified as stable and unstable based on their underlying structural and/or molecular phenotype. Unstable plaque phenotypes, such as plaque rupture or erosion, are commonly present in plaques underlying ACS, as demonstrated by high-resolution intravascular imaging methods such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) [3]. In addition, certain IVUS-derived structural measures (high plaque burden > 70%, minimal luminal area < 4 square mm, or IVUS-virtual histology demarcated thin-capped fibroatheroma (TCFA)) may identify vulnerable plaques that will progress to cause ACS [4]. However, even when incorporating advanced plaque measures such as endothelial shear stress [5, 6], accurate prediction of events arising from vulnerable plaques is insufficient. As plaque pathobiology such as inflammation is a major driver of coronary events [7,8,9], an imaging method to specifically report on coronary arterial inflammation or other pathobiology could improve the detection of high-risk plaques.
Basis for Molecular Imaging
Cardiovascular molecular imaging has emerged as an important research and evolving clinical tool that allows in vivo visualization of key molecules and cells underlying plaque destabilization and progression [10,11,12]. Molecular imaging requires two components: an imaging hardware detection system (e.g., catheter and console) and an injectable molecular imaging agent (e.g., peptide, antibody, small molecule conjugated to a signal generating moiety) that targets a specific molecule or cell and provides a target-specific signal detectable by the desired hardware system. Although non-invasive molecular imaging via positron emission tomography (PET) has witnessed exciting advances in terms of coronary artery visualization using PET tracers, such as 18-fluorodeoxyglucose or 18-sodium fluoride [13, 14], PET imaging is still of unclear application to CAD patients, as the coronary arteries reside deep within the chest, are of small caliber (3–4 mm in diameter, approximately the resolution of PET), overlie the metabolically active heart, and are subject to cardiorespiratory motion and pulsatile blood flow [15, 16].
To enable high-resolution molecular imaging of CAD, where plaques of interest are of 1–5 mm thickness, intravascular near-infrared fluorescence (NIRF) had emerged as a translational intracoronary optical imaging modality that allows high-resolution detection of plaque molecules and cells [17]. More recently, hybrid imaging systems that fully integrate NIRF optical fibers with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) have been developed [18•, 19, 20•, 21•], enabling co-registered and quantitative molecular and structural imaging. In addition, a first human study using NIR autofluorescence (NIRAF)-OCT has already been performed [22••], demonstrating that NIR fluorescence imaging is feasible in CAD patients. These developments offer the potential to open a new horizon for personalized medical therapy of patients harboring high-risk coronary plaques [10, 23]. The focus of this review is to showcase recent advances in intravascular molecular imaging, with a focus on intravascular NIRF imaging.
Intravascular Imaging of High-Risk, Vulnerable Plaques
While our initial understanding of vulnerable coronary artery plaques derived from autopsy studies, over the past decades, multiple intracoronary imaging techniques have emerged, including IVUS, OCT, and near-infrared spectroscopy (NIRS). These methods have enabled in vivo clinical assessment of plaque structure and lipid-rich necrotic cores. It is important to note NIRS measures plaque lipid solely and is distinct from NIRF (fluorescence) molecular imaging, the focus of this review (Table 1). For more in-depth reviews on standalone IVUS, OCT, and NIRS, please refer to a recent intravascular imaging topical collection [24, 25].
Foundations of Near-Infrared Fluorescence High-Resolution Molecular Imaging
Near-infrared fluorescence (NIRF) molecular imaging is a clinically emerging optical imaging modality due to its high sensitivity, ability to deliver light down flexible fibers, relatively low cost, and the availability of chemical engineered NIR fluorophores that can report on a wide range of in vivo molecular processes [26]. NIRF imaging involves administering targeted or activatable fluorophores, injectable imaging agents that localize and bind to or interact with molecular or cellular target of interest in atheroma [23]. Excitingly, a number of NIRF molecular imaging agents are undergoing testing in patients with cancer (e.g., bevacizumab-IRDye800CW) and should provide an opportunity for further translation to the cardiovascular domain [27••, 28]. Illumination light from the near-infrared spectrum (650–900 nm) is focused on to the arterial wall via optical fibers and is used to excite fluorophores from their ground state (S0) to an excited state (S1, S2). The excited fluorophores then may re-emit light at a longer wavelength (fluorescence emission) and return to the ground state, available for another round of excitation. The emitted NIR fluorescence is collected by intravascular optical fibers housing the appropriate emission filters that exclude illumination light. Emission light (fluorescence) is then captured using a high sensitivity charge-coupled device (CCD) camera [17, 23]. Compared with visible light, near-infrared light-based imaging possesses several features that render it favorable for in vivo imaging: (A) lower absorption by hemoglobin allowing NIR light penetration through blood; (B) reduced scattering allowing deeper tissue penetration of light; and (C) reduced tissue autofluorescence, allowing more sensitive detection of injectable NIRF molecular imaging agents [29, 30].
The first intravascular, real-time in vivo NIRF catheter-based molecular imaging approach was described by Jaffer et al. in 2008 [31]. After injecting a cysteine protease-activatable NIRF molecular imaging agent (ProSense750/VM110), the NIRF fiber was percutaneously inserted into rabbit iliac arteries (1.5–2.5 mm in diameter) and was able to detect plaque inflammation through blood [31]. This non-rotational prototype, however, operated in a one-dimensional fashion, sensing the NIRF signal from a limited section of the circumferential arterial wall in a spectroscopic-type mode. To overcome these limitations, our group next developed a two-dimensional rotational NIRF intravascular catheter with automated pullback, to enhance the axial/longitudinal anatomical accuracy of the generated images. This allowed the construction of real-time, in vivo images of stented rabbit aortas [32••]. The group found increased stent-induced inflammatory activity around the edges of implanted stents, enabling a new in vivo to approach to assess stent-induced vascular injury and healing. However, this 2D NIRF approach employed a standalone imaging catheter, and required a second intravascular imaging catheter (i.e. IVUS) to provide anatomical information. This limitation provided the motivation to develop multimodal NIRF imaging systems.
Hybrid Intravascular Near-Infrared Fluorescence Molecular Imaging
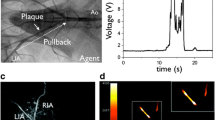
To enable simultaneous molecular and structural imaging via a single catheter, intravascular imaging systems combining NIRF and OCT have been developed [18•, 33]. This has enabled exact co-registration of molecular and structural imaging signals and furthermore allows for distance correction of NIRF signal based on the catheter distance to the lumen-wall border (Fig. 1) [11].
Serial in vivo dual-modality optical coherence tomographic-near-infrared fluorescence (OCT-NIRF) and intravascular ultrasound (IVUS) imaging was completed at weeks 8 and 12 after balloon injury to rabbit aorta. ProSenseVM110, a fluorophore that detects cathepsin protease activity, was injected 24 h prior to imaging. (A) Longitudinal co-registered NIRF-IVUS imaging at 8 and 12 weeks. (B) Axial NIRF-OCT fusion image (yellow/white: high near-infrared fluorescence; blue/black: low near-infrared fluorescence) at the location of the white dotted line in (A), second row (near-infrared fluorescence, 12 weeks). Matched cross-sectional fluorescence microscopy (red: ProSense VM110; green: autofluorescence) and histopathology demonstrates increased ProSense VM110 NIRF signal within a moderate fibrofatty atheroma (H&E) associated with cathepsin B immunostain. Scale bars, 1 mm (reprinted from Bourantas et al. [11], with permission from the European Society of Cardiology)
Hybrid NIRF-OCT Molecular-Structural Imaging
OCT provides exquisite spatial resolution, and two hybrid-dual modal OCT-NIRF systems (Fig. 1) have been developed and validated in vivo. The first one utilized a 2.4 Fr dual-clad fiber with single-mode OCT core and an inner NIRF cladding. This system enabled co-illumination of 750 nm NIRF and 1320 nm OCT light, and exact co-registration of the returned NIR fluorescence and OCT backscatter signals [18•]. This NIRF-OCT catheter system enabled molecular imaging of fibrin of a stent implanted in a cadaveric human coronary artery, and imaging of plaque inflammation in vivo in the rabbit iliac artery [18•]. Similarly, Lee et al. [33] developed a high-speed intravascular OCT-NIRF platform with a probe diameter of 2.5 Fr and image acquisition rate of 100 frames/s. Using this hybrid system, it was possible to detect ICG deposition in macrophage-rich plaques using an atheroma rabbit model [33], and later specific detection of macrophages was demonstrated [34]. This system was then further validated in pig coronary arteries, demonstrating the ability to image ICG leakage into atheroma and behind implanted DES [35•].
Hybrid NIRF-IVUS Imaging
For the past three decades, IVUS has been the worldwide dominant intravascular imaging approach. Compared with OCT, IVUS has greater depth penetration (5–6 mm) but lower spatial resolution (100–250 μm), lower contrast for discriminating plaque components, and is able to detect moderate backscatter from blood with 40–60 MHz transducers. While backscatter may complicate detection of the luminal border, IVUS does not require blood clearance. [36]. This feature makes an NIRF-IVUS combination appealing as both modalities can be used through blood. In a first proof-of-principle, Abran et al. [19•] engineered and validated a bimodal NIRF-IVUS catheter system in ex vivo rabbit atherosclerosis, demonstrating the ability to image ICG uptake in plaques. Next, Dixon and Hossack [37] designed a hybrid platform using side by side NIRF and IVUS imaging design with a larger probe size of 4.2 Fr, and performed ex vivo coronary artery imaging and NIRF signal correction. In the first in vivo demonstrations of NIRF-IVUS, Bozhko et al. demonstrated the ability to image ICG endothelial leakage and stent fibrin in vivo. More recently, Bertrand et al. demonstrated that NIRF-IVUS could detect locally administered NIRF inflammation agents in atheroma [38]. Future NIRF-IVUS designs are under development aiming to reduce the NIRF-IVUS catheter diameter to < 3 Fr, suitable for intracoronary use.
Hybrid Tri-Modality NIRF-OCT-IVUS Imaging
In 2017, Li et al. [39] designed a tri-modal catheter using IVUS, OCT, and NIRF technologies. The tri-modal catheter diameter of the probe is 1.0 mm (3.9 Fr), small enough to fit in 5 Fr guide, but likely too large for routine clinical use in its present form. The system is able to obtain all three IVUS, OCT, and NIRF imaging simultaneously. The group conducted phantom and ex vivo experiments using pig and rabbit arteries. This integrated tri-modal system has a great potential to identify vulnerable plaques through NIRF molecular imaging and provide the joint advantages of OCT and IVUS in a single system.
Applications of NIRF Molecular Imaging to Detect High-Risk Plaques
NIRF Molecular Imaging of Inflammatory Protease Activity
Inflammatory proteases, secreted by macrophages or activated smooth muscle cells, promote atheroma progression and plaque rupture. Two widely studied classes of proteases are cathepsins and matrix metalloproteinases (MMP). Cysteine cathepsins are a group of proteases that are mainly found in lysosomes. There are 11 human cysteine cathepsins (B, C, F, H, K, L, O, S, V, X, and W) that vary in their distribution and specific function [40]. In atherosclerosis, the elastolytic and collagenolytic activity of cysteine cathepsins especially S, K, B, L, and F have been linked to extracellular matrix degradation and hence, both positive and negative remodeling. It was shown that early human atherosclerotic lesions expressed cathepsins K and S [41], and deficiency in cathepsins K and S in a mouse model reduced atherosclerosis [42, 43]. ProSense/VM110 is one of the most widely investigated NIRF protease-activatable imaging agents in animal models. At baseline, ProSense/VM110 is optically silent. Once ProSense/VM110 circulates and localizes to the tissue of interest (in this case, plaques with cathepsin proteases), it is cleaved by cathepsins yielding highly fluorescent fragments [44]. ProSense activatable fluorophores are available in two forms: ProSense 680, activated by Cathepsin B, L, and S with emitted light of 700 nm, and ProSense 750/VM110, activated by Cathepsins B, L, S, K, V, and D with emitted light of 780 nm [44]. Another potential protease target for NIRF imaging is the class of metalloproteases (MMPs), which are linked to unstable/vulnerable atherosclerotic plaques [45].
Intravascular NIRF imaging has been able to detect plaque protease activity in a number of scenarios. An initial series of studies demonstrated the ability to image plaque inflammation at high resolution in vivo, using new NIRF catheter systems [18•, 31, 32••]. Thereafter, Calfon Press et al. [46•] demonstrated that everolimus-eluting stents, the most prevalent type of DES, can suppress plaque inflammation in vivo and reduce plaque macrophages, strengthening the potential of DES to serve as local therapy for vulnerable plaque. In a more recent application using serial NIRF-OCT and IVUS imaging, Osborn et al. [47] have demonstrated preliminarily that in rabbit atheroma, the presence of plaque inflammation independently predicts plaque progression. These preclinical studies on plaque inflammation using NIRF imaging will provide a framework for clinical studies once a NIRF catheter is approved by the FDA.
Oxidized Low-Density Lipoprotein
The presence of oxidized LDL (oxLDL) indicates oxidative stress, a high-risk plaque feature that localize within fibroatheroma lipid-rich core. LO1 is an auto-antibody that binds to copper-oxidized LDL in mice, rabbits, and humans [48]. By labeling the LO1 antibody with a NIRF dye AF750, Khamis et al. [49••] synthesized a NIRF agent called LO1-750 (excitation light of 750 nm). This enabled in vivo molecular imaging in mice using fluorescence molecular tomography (FMT), which is a non-intravascular NIRF imaging apparatus, combined with micro-computed tomography (CT). The group found increased accumulation of LO1-750 in the aorta and subclavian arteries of LDLr−/− mice when compared with the wild type (WT). When compared with MMPSense signal, LO1-750 showed higher NIRF signal ratio. Using ex vivo intravascular NIRF imaging, oxLDL was imaged in rabbit plaques [49••]. This is a step forward to be able image oxidative plaque stress in living subjects.
Cellular Inflammation in Areas of Impaired Endothelial Permeability
Impaired endothelial permeability has been linked to superficial plaque erosion and atherothrombosis. A macrophage-targeted NIRF iron oxide nanoparticle CLIO-CyAm7 was developed to investigate in vivo mechanisms of impaired endothelial function in an atheroma rabbit model [50•]. Using NIRF imaging, Stein-Merlob et al. showed that CLIO-CyAm7 nanoparticles deposited in plaque cells in areas of impaired endothelial barrier function [50•]. It was also shown that iron oxide nanoparticles had high concentration in thrombosed plaques when compared with non-thrombosed plaques [50•].
Molecular Imaging of Fibrin Deposition
Fibrin is the end product of coagulation cascade and is involved in both venous and arterial thrombosis [51]. FTP11-Cy7 is a NIRF fluorophore developed to specifically bind to fibrin in living subjects [52]. In 2012, Hara et al. [52] demonstrated that FTP11 binds to human in vitro thrombi and in vivo murine thrombi using non-invasive NIRF imaging. Later, the same group used FTP11 to detect fibrin deposition in unhealed stents and demonstrated increased fibrin deposition on DES compared with BMS [53•]. The group concluded that a substantial percentage of stent struts that appear to be covered by OCT imaging are actually covered by fibrin (Fig. 2). This may help better identify stents more prone to stent thrombosis. Additional applications of fibrin imaging are anticipated in elucidating culprit plaques in ACS patients, and in subjects with myocardial infarction and non-obstructive arteries (MINOCA), and identify high-risk plaques with subclinical fibrin-based thrombosis.
In rabbit aortas and at day 7, drug-eluting stents (DES) displayed greater fibrin deposition than bare metal stents (BMS), using the FTP11-CyAm7 fibrin NIRF molecular imaging agent. (A) In vivo 2D near-infrared fluorescence map with BMS (left) and DES (right), (B) corresponding fluorescence microscopy after longitudinally opening stents, (C) fluorescence reflectance imaging (FRI), and (D) fluorescence microscopy from the outside. In vivo axial NIRF-OCT images of day 7 (E) bare metal stent and (F) day 7 drug-eluting stent at the distal edge (arrowhead in A). Carstairs’ stain shows that some areas of BMS surface areas are fibrin negative (G), but almost all areas of DES tissue coverage are fibrin positive (H). (I) In vivo NIRF-fibrin signal from the proximal to distal stent edge of BMS and DES (n = 7 BMS, n = 7 DES). Scale bars = 1 mm (reprinted from Hara et al. [53], with permission from European Society of Cardiology)
NIRF Imaging of Atherosclerosis and Human Studies
NIRF Imaging of Impaired Plaque Endothelial Function Using Indocyanine Green in Patients
At present, intravascular NIRF molecular imaging has not yet been evaluated in patients, pending the availability of a clinically approved catheter. However, clinical NIRF molecular imaging will be facilitated by the availability of the targeted NIRF imaging agent, indocyanine green (ICG). ICG was first introduced in the 1950s as a blood flow dye and currently serves as a routine clinical retinal imaging agent. Unexpectedly, in 2011, ICG was demonstrated to function as a targeted molecular imaging agent, where it accumulated in lipid-rich macrophages in vitro, and was detectable rapidly by intravascular NIRF sensing in vivo in rabbit atheroma [54••] and subsequently in pig coronary atheroma [35•, 55••]. This paved the way for the first human trial testing whether ICG could be injected into living subjects and target human atheroma. The BRIGHT-CEA trial (Indocyanine Green Fluorescence Uptake in Human Carotid Artery Plaque) in 2016 injected ICG into patients undergoing carotid endarterectomy, and then, plaques were resected 99 min after ICG injection [55••]. Resected carotid plaques were then imaging using ex vivo NIRF macroscopic imaging and intravascular NIRF-OCT. The group demonstrated that ICG accumulated in plaque areas with impaired endothelial integrity, disrupted fibrous cap, and areas of neovascularization and intraplaque hemorrhage. The availability of ICG as an FDA-approved agent will accelerate first-in-human targeted NIRF imaging studies.
NIRAF-OCT Imaging of Intraplaque Hemorrhage
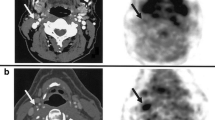
Concurrently with the development of targeted NIRF molecular imaging approaches, it was recognized that plaque NIR autofluorescence (NIRAF) might represent a new measure of high-risk plaques [56]. NIRAF is distinct from NIRF molecular imaging, in that NIRAF detects endogenous autofluorescence and does not require the administration of imaging agents. In addition, the excitation wavelength of light (633 nm) is significantly lower than that used for targeted NIRF molecular imaging (750–800 nm). NIRAF is detected after NIR light (633 -nm excitation in clinical subjects) illuminates plaques that possess certain endogenous NIR fluorophores, making its adoption into clinical use faster in the near future. Recognizing the possibility to advance the clinical potential of NIRAF imaging, a recent clinical trial explored the use of intracoronary NIRAF-OCT in CAD patients [22••]. Ughi et al. [22••] conducted the first human study on 12 patients with coronary artery disease undergoing PCI using a hybrid NIRAF-OCT catheter, designed to detect NIRAF in non-culprit lesions. This catheter received an investigational device exemption (IDE) from the FDA prior to clinical study. NIRAF was detected in 1–2 plaques per coronary artery, a relatively specific signal pattern. Elevated NIRAF signal was associated with high-risk morphologic phenotype like fibroatheroma, plaque rupture, and in-stent restenosis (Fig. 3) [22••]. While detection of NIRAF indicates components of intraplaque hemorrhage (e.g., bilirubin, protoporphyrin IX) [57], the complete set of molecules and biological processes underlying 633 nm NIRAF remains to be elucidated. Nonetheless, this landmark NIRAF-OCT study demonstrated the safety and utility of NIRAF to detect advanced plaques in CAD patients and provides a foundation for future intracoronary NIRAF studies, as well as targeted NIRF molecular imaging at longer wavelengths (750–800 nm).
OCT-NIRAF imaging of thin-cap fibroatheroma (TCFA) rupture. (A) Coronary angiogram of left anterior descending (LAD) artery. (B) 2D NIRAF map presenting a focal region of high NIRAF signal in the ostial LAD. (C, D, E) OCT-NIRAF cross-sections from sites in (B) with increased NIRAF, revealing subclinical fibrous cap rupture on OCT imaging. (F) Magnification of a cholesterol crystal below the cap, co-localized with high NIRAF, and (G, H) magnified views of the rupture site. In (G), the rupture site (arrowhead) is covered by a small white luminal thrombus (arrow) and the arrow in (H) points to the site of the thin-cap rupture, representing co-localized and very high focal NIRAF signal. (I) 3D cutaway rendering showing that the highest NIRAF spot appears focally within a large lipid pool (arrow), and the remaining portion of the vessel shows diffuse disease that was negative for NIRAF. Scale bars on OCT images and magnifications are equal to 1 mm and 0.5 mm, respectively; scale bar in (B) is equal to 5 mm (reprinted from Ughi et al. [22], with permission from the American College of Cardiology)
Practical Use of Intracoronary NIRF Imaging in the Cardiac Catheterization Laboratory
Intravascular NIRF molecular imaging is likely to be applied to assess high-risk non-culprit plaques in patients already undergoing culprit-lesion PCI. As PCI patients are increasingly undergoing IVUS or OCT for stent optimization [24], we envision that patients would undergo injection of a NIRF molecular imaging agent at the start of PCI, with subsequent NIRF-IVUS or NIRF-OCT at the end of the PCI. This would enable at least single-vessel coronary arterial phenotyping of plaques proximal and distal to the culprit zones. The longer term goal of additional NIRF information [27••] would help to better predict patient-specific risk based on the degree of abnormal plaque pathobiology, and then to prescribe specific medications based on the risk and the specific abnormal biology, enabling personalized medical therapy of CAD patients.
Conclusion
NIRF intravascular molecular imaging is a clinically translatable powerful approach to visualize high-risk plaque molecular processes, including macrophages, inflammatory protease activity, oxidized LDL molecules, and impaired endothelial barrier function. Hybrid intravascular catheters including NIRF-OCT or NIRF-IVUS technologies can depict microstructural and molecular changes of the arterial wall with high resolution and high sensitivity. At present, ICG is the only FDA-approved fluorophore for use in humans, but additional clinical NIR fluorophores to be approved for human use are emerging from the field of cancer NIRF molecular imaging. Following validation human studies, future clinical trials will determine the value of intravascular NIRF molecular imaging in patients, to enable personalized assessments of risk and targeted prescription of CAD pharmacotherapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 1 Apr 2019.
Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–30.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–81.
Xie Y, Mintz GS, Yang J, Doi H, Iñiguez A, Dangas GD, et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc Imaging. 2014;7:397–405.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43.
Ridker PM, Everett BM, Thuren T, MacFadyen J, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Chowdhury MM, Tawakol A, Jaffer FA. Molecular imaging of atherosclerosis: a clinical focus. Curr Cardiovasc Imaging Rep. 2017;10:1–11. https://doi.org/10.1007/s12410-017-9397-1.
Bourantas CV, Jaffer FA, Gijsen FJ, van Soest G, Madden SP, Courtney BK, et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur Heart J. 2017;38:400–12.
Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–62.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388–97.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13.
Bucerius J, Dijkgraaf I, Mottaghy FM, Schurgers LJ. Target identification for the diagnosis and intervention of vulnerable atherosclerotic plaques beyond 18F-fluorodeoxyglucose positron emission tomography imaging: promising tracers on the horizon. Eur J Nucl Med Mol Imaging. 2019;46:251–65.
Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Høilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–97.
Calfon MA, Rosenthal A, Mallas G, Mauskapf A, Nudelman RN, Ntziachristos V, et al. In vivo near infrared fluorescence (NIRF) intravascular molecular imaging of inflammatory plaque, a multimodal approach to imaging of atherosclerosis. J Vis Exp. 2011. https://doi.org/10.3791/2257.
• Yoo H, Kim JW, Shishkov M, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17:1680–4 This study validated the first use of a hybrid OCT-NIRF microstructural-molecular catheter system to visualize fibrin in cadaveric human coronary arteries with implanted stents and in vivo plaque inflammation in rabbit iliac artery.
• Abran M, Stähli BE, Merlet N, Mihalache-Avram T, Mecteau M, Rhéaume E, et al. Validating a bimodal intravascular ultrasound (IVUS) and near-infrared fluorescence (NIRF) catheter for atherosclerotic plaque detection in rabbits. Biomed Opt Express. 2015;6:3989–99 This study validated the use of bimodal IVUS-NIRF catheter and ICG in rabbit aortas and right iliac arteries to detect atherosclerotic plaque areas.
• Bozhko D, Osborn EA, Rosenthal A, et al. Quantitative intravascular biological fluorescence-ultrasound imaging of coronary and peripheral arteries in vivo. Eur Heart J Cardiovasc Imaging. 2017;18:1253–61 This study detailed the first in vivo IVUS and NIRF molecular-structural imaging system, accounting for distance correction using IVUS data. Corrected NIRF-IVUS was used to image plaque structure and inflammation in swine peripheral arteries with angioplasty-induced vascular injury, coronary artery stents with fibrin deposition, and of rabbit aorta atheroma.
• Ughi GJ, Verjans J, Fard AM, Wang H, Osborn E, Hara T, et al. Dual modality intravascular optical coherence tomography (OCT) and near-infrared fluorescence (NIRF) imaging: a fully automated algorithm for the distance-calibration of NIRF signal intensity for quantitative molecular imaging. Int J Card Imaging. 2015;31:259–68 This study validated the use of automatic distance-corrected algorithm for NIRF images using OCT-NIRF hybrid catheter in atherosclerotic rabbit aortas, compared to manual segmentation.
•• Ughi GJ, Wang H, Gerbaud E, Gardecki JA, Fard AM, Hamidi E, et al. Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging. JACC Cardiovasc Imaging. 2016;9:1304–14 First-in-human intravascular NIR fluorescence study using NIRAF-OCT catheter to detect plaque NIR autofluorescence 633 nm-based signal in non-culprit lesions associated with fibroatheroma, plaque rupture and in-stent restenosis.
Celeng C, de Keizer B, Merkely B, de Jong P, Leiner T, Takx RAP. PET molecular targets and near-infrared fluorescence imaging of atherosclerosis. Curr Cardiol Rep. 2018;20:11.
Bode MF, Jaffer FA. IVUS and OCT: current state-of-the-art in intravascular coronary imaging. Curr Cardiovasc Imaging Rep. 2019;12:29.
Swamy PM, Mamas MA, Bharadwaj AS. Role of near-infrared spectroscopy (NIRS) in intracoronary imaging. Curr Cardiovasc Imaging Rep. 2019;12:34.
Calfon MA, Rosenthal A, Mallas G, Mauskapf A (2011) In vivo near infrared fluorescence (NIRF) intravascular molecular imaging of inflammatory plaque, a multimodal approach to imaging of atherosclerosis. JoVE (Journal of.
•• van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315–9 This study was a landmark fluorescence molecular imaging study in patients. Folate-fluorescein isothiocyanate (FITC) is a NIRF molecular imaging agent that binds to FR-α receptor on epithelial ovarian cancer. Folate-FITC-based molecular imaging enabled greater detection of tumors by cancer surgeons using widefield fluorescence imaging.
Harlaar NJ, Koller M, de Jongh SJ, van Leeuwen B, Hemmer PH, Kruijff S, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol Hepatol. 2016;1:283–90.
Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:0010.
Sevick-Muraca EM, Rasmussen JC. Molecular imaging with optics: primer and case for near-infrared fluorescence techniques in personalized medicine. J Biomed Opt. 2008;13:041303.
Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–9.
•• Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–26 Landmark study that used the first two-dimensional rotational NIRF intravascular catheter with automated pullback allowing in vivo molecular imaging of stented rabbit aortas.
Lee S, Lee MW, Cho HS, Song JW, Nam HS, Oh DJ, et al. Fully integrated high-speed intravascular optical coherence tomography/near-infrared fluorescence structural/molecular imaging in vivo using a clinically available near-infrared fluorescence-emitting indocyanine green to detect inflamed lipid-rich atheromata in coronary-sized vessels. Circ Cardiovasc Interv. 2014;7:560–9.
Kim JB, Park K, Ryu J, et al. Intravascular optical imaging of high-risk plaques in vivo by targeting macrophage mannose receptors. Sci Rep. 2016;6:22608.
• Kim S, Lee MW, Kim TS, et al. Intracoronary dual-modal optical coherence tomography-near-infrared fluorescence structural-molecular imaging with a clinical dose of indocyanine green for the assessment of high-risk plaques and stent-associated inflammation in a beating coronary artery. Eur Heart J. 2016;37:2833–44 Injecting clinical dose of ICG along with using OCT-NIRF hybrid catheter was shown to accurately assess plaque inflammation and DES-related inflammation in in vivo swine coronary arteries.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10:1487–503.
Dixon AJ, Hossack JA. Intravascular near-infrared fluorescence catheter with ultrasound guidance and blood attenuation correction. J Biomed Opt. 2013;18:56009.
Bertrand M-J, Abran M, Maafi F, et al. In vivo near-infrared fluorescence imaging of atherosclerosis using local delivery of novel targeted molecular probes. Sci Rep. 2019;9:2670.
Li Y, Jing J, Qu Y, Miao Y, Zhang B, Ma T, et al. Fully integrated optical coherence tomography, ultrasound, and indocyanine green-based fluorescence tri-modality system for intravascular imaging. Biomed Opt Express. 2017;8:1036–44.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88.
Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–83.
Lutgens E, Lutgens SPM, Faber BCG, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107.
Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, et al. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:851–6.
Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004;94:433–45.
Newby AC. Metalloproteinase production from macrophages—a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp Physiol. 2016;101:1327–37.
• Calfon Press MA, Mallas G, Rosenthal A, et al. Everolimus-eluting stents stabilize plaque inflammation in vivo: assessment by intravascular fluorescence molecular imaging. Eur Heart J Cardiovasc Imaging. 2017;18:510–8 This in vivo NIRF molecular imaging study demonstrated that everolimus-eluting stents can suppress plaque macrophages and, hence, inflammation.
Abstract 656: In Vivo Plaque Inflammation and Endothelial Permeability Independently Predict Atherosclerosis Progression: A Serial Multimodality Imaging Study | Arteriosclerosis, Thrombosis, and Vascular Biology. Arterioscler. Thromb. Vasc. Biol.
Chang S-H, Johns M, Boyle JJ, McConnell E, Kirkham PA, Bicknell C, et al. Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein. Hybridoma. 2012;31:87–98.
•• Khamis RY, Woollard KJ, Hyde GD, et al. Near infrared fluorescence (NIRF) molecular imaging of oxidized LDL with an autoantibody in experimental atherosclerosis. Sci Rep. 2016;6:21785 The group synthesized LO1-750, a NIRF agent that binds to oxidized LDL and demonstrated the ability to image oxidized LDL and thus oxidative stress using preclinical intravascular NIRF imaging.
• Stein-Merlob AF, Hara T, McCarthy JR, Mauskapf A, Hamilton JA, Ntziachristos V, et al. Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ Cardiovasc Imaging. 2017. https://doi.org/10.1161/CIRCIMAGING.116.005813 CLIO-CyAm7 nanoparticle was shown to deposit in plaque cells of areas of impaired endothelial barrier and thrombosed plaques, as detected by standalone intravascular NIRF imaging.
Ciesienski KL, Caravan P. Molecular MRI of thrombosis. Curr Cardiovasc Imaging Rep. 2010;4:77–84.
Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–15.
• Hara T, Ughi GJ, McCarthy JR, Erdem SS, Mauskapf A, Lyon SC, et al. Intravascular fibrin molecular imaging improves the detection of unhealed stents assessed by optical coherence tomography in vivo. Eur Heart J. 2017;38:447–55 Intravascular NIRF-OCT coupled to FTP11-Cy7, a fibrin specific NIRF agent, was demonstrated to detect fibrin in unhealed stents that otherwise was not detectable by standalone OCT imaging. These findings may help better understand stent healing and the development of new stents or scaffolds.
•• Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra45 An important study that showed that FDA-approved ICG could serve as a targeted NIRF vulnerable plaque imaging agent. ICG accumulated in lipid-rich macrophages and was detectable by intravascular NIRF in atherosclerotic rabbit model.
•• Verjans JW, Osborn EA, Ughi GJ, et al. Targeted near-infrared fluorescence imaging of atherosclerosis: clinical and intracoronary evaluation of indocyanine green. JACC Cardiovasc Imaging. 2016;9:1087–95 First targeted atherosclerosis NIRF human trial during which ICG was injected into patients undergoing carotid endartectomy. NIRF-OCT intravascular imaging was used in resected carotid plaques and demonstrated that ICG accumulated in areas of disrupted fibrous cap, neovascularization and intraplaque hemorrhage.
Wang H, Gardecki JA, Ughi GJ, Jacques PV, Hamidi E, Tearney GJ. Ex vivo catheter-based imaging of coronary atherosclerosis using multimodality OCT and NIRAF excited at 633 nm. Biomed Opt Express. 2015;6:1363–75.
Htun NM, Chen YC, Lim B, et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques. Nat Commun. 2017;8:75.
Funding
This work was supported by NIH 1R01HL137913 (F.A.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Khraishah has no conflict of interest to disclose. Dr. Jaffer has received sponsored research grants from Canon and Siemens; he is a consultant for Boston Scientific, Abbott Vascular, Siemens, Philips, and Acrostak. Massachusetts General Hospital has a patent licensing arrangement with Canon, and Dr. Jaffer has the right to receive royalties.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Khraishah, H., Jaffer, F.A. Intravascular Molecular Imaging to Detect High-Risk Vulnerable Plaques: Current Knowledge and Future Perspectives. Curr Cardiovasc Imaging Rep 13, 8 (2020). https://doi.org/10.1007/s12410-020-9527-z
Published:
DOI: https://doi.org/10.1007/s12410-020-9527-z